Inverted U-Shaped Relationship between Obesity Parameters and Bone Mineral Density in Korean Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements
2.3. Statistical Analysis
- β1 × Age = linear effect of Age
- β2 × LM = linear effect of total-body LM
- β3 × BMI = linear effect of BMI
- β4 × BMI2 = quadratic effect of BMI
3. Results
3.1. Characteristics of Study Population
3.2. Age-Related Changes in TBLH BMD, Total-Body LM, and Total-Body FM
3.3. Crude Relationship between Obesity Parameters and TBLH BMD
3.4. Linear Relationship between Obesity Parameters and TBLH BMD
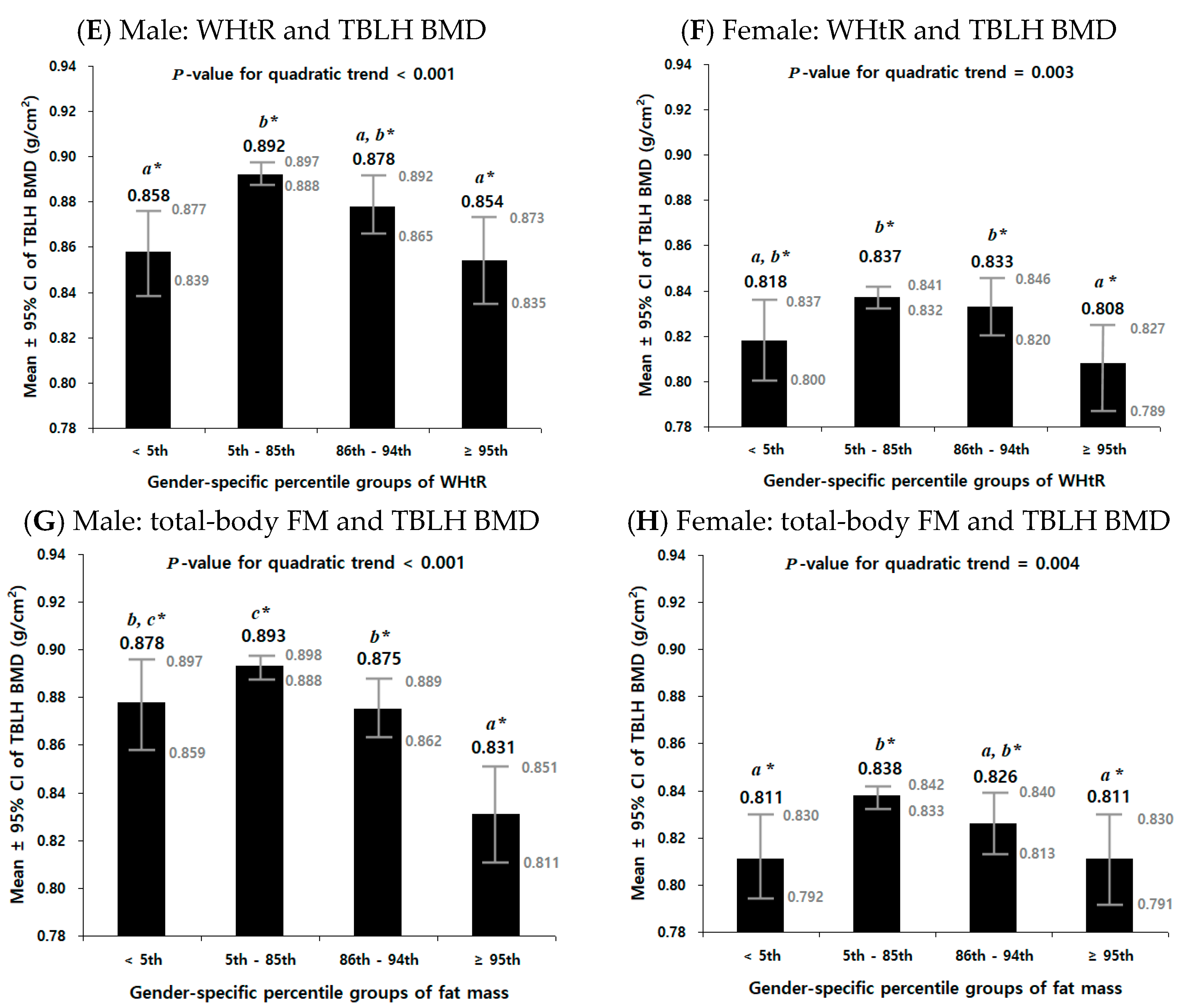
3.5. Non-Linear Relationship between Obesity Parameters and TBLH BMD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenberg, J.A. Obesity and early mortality in the United States. Obesity 2013, 21, 405–412. [Google Scholar] [CrossRef]
- Britton, K.A.; Massaro, J.M.; Murabito, J.M.; Kreger, B.E.; Hoffmann, U.; Fox, C.S. Body Fat Distribution, Incident Cardiovascular Disease, Cancer, and All-Cause Mortality. J. Am. Coll. Cardiol. 2013, 62, 921–925. [Google Scholar] [CrossRef]
- Rössner, S. Obesity: The disease of the twenty-first century. Int. J. Obes. 2002, 26, S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Verras, G.-I.; Mulita, F.; Pouwels, S.; Parmar, C.; Drakos, N.; Bouchagier, K.; Kaplanis, C.; Skroubis, G. Outcomes at 10-Year Follow-Up after Roux-en-Y Gastric Bypass, Biliopancreatic Diversion, and Sleeve Gastrectomy. J. Clin. Med. 2023, 12, 4973. [Google Scholar] [CrossRef] [PubMed]
- Mulita, F.; Lampropoulos, C.; Kehagias, D.; Verras, G.-I.; Tchabashvili, L.; Kaplanis, C.; Liolis, E.; Iliopoulos, F.; Perdikaris, I.; Kehagias, I. Long-term nutritional deficiencies following sleeve gastrectomy: A 6-year single-centre retrospective study. Menopause Rev. 2021, 20, 170–176. [Google Scholar] [CrossRef]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Qi, X.; Locke, J.; Rehman, S. Childhood and adolescent obesity in the United States: A public health concern. Glob. Pediatr. Health 2019, 6, 2333794X19891305. [Google Scholar] [CrossRef]
- Kim, J.H.; Moon, J.S. Secular trends in pediatric overweight and obesity in Korea. J. Obes. Metab. Syndr. 2020, 29, 12. [Google Scholar] [CrossRef] [PubMed]
- Pulgarón, E.R. Childhood obesity: A review of increased risk for physical and psychological comorbidities. Clin. Ther. 2013, 35, A18–A32. [Google Scholar] [CrossRef]
- Horesh, A.; Tsur, A.M.; Bardugo, A.; Twig, G. Adolescent and childhood obesity and excess morbidity and mortality in young adulthood—A systematic review. Curr. Obes. Rep. 2021, 10, 301–310. [Google Scholar] [CrossRef]
- Jebeile, H.; Cardel, M.I.; Kyle, T.K.; Jastreboff, A.M. Addressing psychosocial health in the treatment and care of adolescents with obesity. Obesity 2021, 29, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Paulis, W.; Silva, S.; Koes, B.; van Middelkoop, M. Overweight and obesity are associated with musculoskeletal complaints as early as childhood: A systematic review. Obes. Rev. 2014, 15, 52–67. [Google Scholar] [CrossRef]
- Dimitri, P.; Bishop, N.; Walsh, J.; Eastell, R. Obesity is a risk factor for fracture in children but is protective against fracture in adults: A paradox. Bone 2012, 50, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.L.; Slemenda, C.W.; Johnston, C.C. Age and bone mass as predictors of fracture in a prospective study. J. Clin. Investig. 1988, 81, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Bland, V.L.; Bea, J.W.; Blew, R.M.; Roe, D.J.; Lee, V.R.; Funk, J.L.; Going, S.B. Influence of changes in soft tissue composition on changes in bone strength in peripubertal girls: The STAR longitudinal study. J. Bone Miner. Res. 2021, 36, 123–132. [Google Scholar] [CrossRef]
- Rizzoli, R.; Bianchi, M.L.; Garabédian, M.; McKay, H.A.; Moreno, L.A. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 2010, 46, 294–305. [Google Scholar] [CrossRef]
- Leonard, M.B.; Shults, J.; Wilson, B.A.; Tershakovec, A.M.; Zemel, B.S. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am. J. Clin. Nutr. 2004, 80, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Ness, A.; Tobias, J.; Avon Longitudinal Study of Parents and Children Study Team. Adipose tissue stimulates bone growth in prepubertal children. J. Clin. Endocrinol. Metab. 2006, 91, 2534–2541. [Google Scholar] [CrossRef]
- Maggio, A.B.; Belli, D.C.; Puigdefabregas, J.W.B.; Rizzoli, R.; Farpour-Lambert, N.J.; Beghetti, M.; McLin, V.A. High bone density in adolescents with obesity is related to fat mass and serum leptin concentrations. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 723–728. [Google Scholar] [CrossRef]
- Kâ, K.; Rousseau, M.-C.; Lambert, M.; O’loughlin, J.; Henderson, M.; Tremblay, A.; Alos, N.; Nicolau, B. Association between lean and fat mass and indicators of bone health in prepubertal caucasian children. Horm. Res. Paediatr. 2013, 80, 154–162. [Google Scholar] [CrossRef]
- Cole, Z.; Harvey, N.C.; Kim, M.; Ntani, G.; Robinson, S.; Inskip, H.; Godfrey, K.; Cooper, C.; Dennison, E.; The Southampton Women’s Survey Study Group. Increased fat mass is associated with increased bone size but reduced volumetric density in pre pubertal children. Bone 2012, 50, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, P.N.; Blimkie, C.J.; Farpour-Lambert, N.; Briody, J.; Woodhead, H.; Cowell, C.; Howman-Giles, R. Correlates and determinants of bone mineral density in prepubertal girls. Pediatr. Exerc. Sci. 2002, 14, 345–357. [Google Scholar] [CrossRef]
- de Moraes, A.M.; Gonçalves, E.M.; de Oliveira Barbeta, V.J.; Guerra-Júnior, G. Cross-sectional study of the association of body composition and physical fitness with bone status in children and adolescents from 11 to 16 years old. BMC Pediatr. 2013, 13, 117. [Google Scholar] [CrossRef]
- Goulding, A.; Taylor, R.; Jones, I.; McAuley, K.; Manning, P.; Williams, S. Overweight and obese children have low bone mass and area for their weight. Int. J. Obes. 2000, 24, 627–632. [Google Scholar] [CrossRef]
- Ivuskans, A.; Lätt, E.; Mäestu, J.; Saar, M.; Purge, P.; Maasalu, K.; Jürimäe, T.; Jürimäe, J. Bone mineral density in 11–13-year-old boys: Relative importance of the weight status and body composition factors. Rheumatol. Int. 2013, 33, 1681–1687. [Google Scholar] [CrossRef]
- Ma, C.-M.; Lu, N.; Kong, F.-S.; Zhang, X.; Wang, R.; Yin, F.-Z. The relationship between metabolic syndrome and bone mineral density in adolescents: Analysis of the National Health and Nutrition Examination Survey. J. Pediatr. Endocrinol. Metab. 2022, 35, 901–912. [Google Scholar] [CrossRef]
- Petit, M.A.; Beck, T.J.; Shults, J.; Zemel, B.S.; Foster, B.J.; Leonard, M.B. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone 2005, 36, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.K.; Laing, E.M.; Baile, C.A.; Hamrick, M.W.; Hall, D.B.; Lewis, R.D. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am. J. Clin. Nutr. 2007, 86, 1530–1538. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Z.; Li, N.; Meng, X.; Wang, S.; Yu, C.; Leng, J.; Zhao, M.; Li, W.; Deng, Y. The association between overweight and obesity on bone mineral density in 12 to 15 years old adolescents in China. Medicine 2021, 100, e26872. [Google Scholar] [CrossRef]
- Manzoni, P.; Brambilla, P.; Pietrobelli, A.; Beccaria, L.; Bianchessi, A.; Mora, S.; Chiumello, G. Influence of body composition on bone mineral content in children and adolescents. Am. J. Clin. Nutr. 1996, 64, 603–607. [Google Scholar] [CrossRef]
- El Hage, Z.; Theunynck, D.; Jacob, C.; Moussa, E.; Baddoura, R.; Zunquin, G.; El Hage, R. Bone mineral content and density in obese, overweight and normal weight adolescent boys. Leban. Med. J. 2013, 61, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Valdimarsson, Ö.; Kristinsson, J.; Stefansson, S.; Valdimarsson, S.; Sigurdsson, G. Lean mass and physical activity as predictors of bone mineral density in 16–20-year old women. J. Intern. Med. 1999, 245, 489–496. [Google Scholar] [CrossRef]
- Witzke, K.A.; Snow, C.M. Lean body mass and leg power best predict bone mineral density in adolescent girls. Med. Sci. Sports Exerc. 1999, 31, 1558–1563. [Google Scholar] [CrossRef]
- Gracia-Marco, L.; Ortega, F.; Jimenez-Pavon, D.; Rodriguez, G.; Castillo, M.; Vicente-Rodriguez, G.; Moreno, L. Adiposity and bone health in Spanish adolescents. The HELENA study. Osteoporos. Int. 2012, 23, 937–947. [Google Scholar] [CrossRef]
- Brismar, T.; Ringertz, H. Effect of bone density of the head on total body DEXA measurements in 100 healthy Swedish women. Acta Radiol. 1996, 37, 101–106. [Google Scholar] [CrossRef]
- Taylor, A.; Konrad, P.T.; Norman, M.E.; Harcke, H.T. Total body bone mineral density in young children: Influence of head bone mineral density. J. Bone Miner. Res. 1997, 12, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, N.J.; Arabi, A.; Bachrach, L.K.; Fewtrell, M.; Fuleihan, G.E.-H.; Kecskemethy, H.H.; Jaworski, M.; Gordon, C.M. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: The revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 225–242. [Google Scholar] [CrossRef]
- Moon, J.-S.; Lee, S.-Y.; Nam, C.-M.; Choi, J.-M.; Choe, B.-K.; Seo, J.-W.; Oh, K.; Jang, M.-J.; Hwang, S.-S.; Yoo, M.-H. 2007 Korean National Growth Charts: Review of developmental process and an outlook. Korean J. Pediatr. 2008, 51, 1–25. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention National Center for Health Statistics. CDC Growth Charts. 2000. Available online: http://www.cdc.gov/growthcharts/ (accessed on 27 July 2023).
- De Laet, C.; Kanis, J.; Odén, A.; Johanson, H.; Johnell, O.; Delmas, P.; Eisman, J.; Kroger, H.; Fujiwara, S.; Garnero, P. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 1330–1338. [Google Scholar] [CrossRef]
- Valerio, G.; Gallè, F.; Mancusi, C.; Di Onofrio, V.; Guida, P.; Tramontano, A.; Ruotolo, E.; Liguori, G. Prevalence of overweight in children with bone fractures: A case control study. BMC Pediatr. 2012, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Koebnick, C.; Smith, N.; Adams, A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin. Orthop. Relat. Res. 2013, 471, 1199–1207. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.; Mendes, N.; Miller, K.K.; Rosen, C.J.; Lee, H.; Klibanski, A.; Misra, M. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J. Clin. Endocrinol. Metab. 2010, 95, 1247–1255. [Google Scholar] [CrossRef]
- Halade, G.V.; El Jamali, A.; Williams, P.J.; Fajardo, R.J.; Fernandes, G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp. Gerontol. 2011, 46, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Pirgon, O.; Bilgin, H.; Tolu, I.; Odabas, D. Correlation of insulin sensitivity with bone mineral status in obese adolescents with nonalcoholic fatty liver disease. Clin. Endocrinol. 2011, 75, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, R.; Ma, X.; Qin, Z.; Yang, Z.; Hong, H.; Lv, H.; Ye, K.; Wei, Y.; Zheng, W. Adiposity is not beneficial to bone mineral density in 0–5 year old Chinese children: The Jiangsu bone health study. Obes. Res. Clin. Pract. 2020, 14, 39–46. [Google Scholar] [CrossRef]
- Ferrer, F.S.; Castell, E.C.; Marco, F.C.; Ruiz, M.J.; Rico, J.A.Q.; Roca, A.P.N. Influence of weight status on bone mineral content measured by DXA in children. BMC Pediatr. 2021, 21, 185. [Google Scholar] [CrossRef]
- Ma, C.-M.; Lu, N.; Zhang, M.-M.; Kong, F.-S.; Lu, Q.; Yin, F.-Z.; Zhang, X.; Wang, R. The relationship between obesity and bone mineral density in children and adolescents: Analysis of the National Health and Nutrition Examination Survey. Arch. Osteoporos. 2023, 18, 25. [Google Scholar] [CrossRef]
- Klein, K.O.; Martha, P.M., Jr.; Blizzard, R.M.; Herbst, T.; Rogol, A.D. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. II. Estrogen levels as determined by an ultrasensitive bioassay. J. Clin. Endocrinol. Metab. 1996, 81, 3203–3207. [Google Scholar]
- Bailey, D.A.; Martin, A.D.; McKay, H.A.; Whiting, S.; Mirwald, R. Calcium accretion in girls and boys during puberty: A longitudinal analysis. J. Bone Miner. Res. 2000, 15, 2245–2250. [Google Scholar] [CrossRef] [PubMed]
- Binkovitz, L.A.; Henwood, M.J.; Sparke, P. Pediatric dual-energy X-ray absorptiometry: Technique, interpretation, and clinical applications. Semin. Nucl. Med. 2007, 37, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Barrile, G.C.; Cavioni, A.; Mansueto, F.; Mazzola, G.; Oberto, L.; Patelli, Z.; Pirola, M.; Tartara, A.; et al. Nutrition, Physical Activity, and Dietary Supplementation to Prevent Bone Mineral Density Loss: A Food Pyramid. Nutrients 2021, 14, 74. [Google Scholar] [CrossRef] [PubMed]




| Male (n = 1114) | Female (n = 946) | ||||
|---|---|---|---|---|---|
| Variable | Mean | (SD) | Mean | (SD) | p-Value * |
| Age (years) | 14.17 | (2.81) | 14.28 | (2.91) | 0.372 |
| Weight (kg) | 56.91 | (15.50) | 50.23 | (11.18) | <0.001 |
| Height (cm) | 163.78 | (12.96) | 157.21 | (8.13) | <0.001 |
| Obesity | |||||
| BMI (kg/m2) | 20.88 | (3.82) | 20.28 | (3.51) | <0.001 |
| Waist circumference (cm) | 71.09 | (10.60) | 67.23 | (8.66) | <0.001 |
| Waist-to-height ratio (cm/cm) | 0.434 | (0.057) | 0.427 | (0.049) | 0.003 |
| Body composition | |||||
| Lean mass (kg) | 40.95 | (10.73) | 32.06 | (5.62) | <0.001 |
| Fat mass (kg) | 13.42 | (6.87) | 16.27 | (6.13) | <0.001 |
| Bone mass (kg) | 1.98 | (0.58) | 1.77 | (0.39) | <0.001 |
| Percent lean mass (%) | 72.45 | (7.56) | 64.27 | (5.64) | <0.001 |
| Percent fat mass (%) | 23.12 | (8.08) | 31.40 | (5.92) | <0.001 |
| Percent bone mass (%) | 3.50 | (0.53) | 3.53 | (0.49) | 0.335 |
| TBLH bone | |||||
| Bone mineral density (g/cm2) | 0.887 | (0.138) | 0.834 | (0.100) | <0.001 |
| Bone mineral content (g) | 1497.30 | (490.56) | 1268.94 | (298.84) | <0.001 |
| Bone area (cm2) | 1646.94 | (340.93) | 1505.78 | (233.89) | <0.001 |
| Dependent Variable: TBLH BMD (g/cm2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n = 1114) | Female (n = 946) | ||||||||
| Parameter | Independent Variable | b | p-Value | Partial R | Ad-R2 | b | p-Value | Partial R | Ad-R2 |
| BMI | Intercept | 0.4091 | <0.001 | 0.736 | 0.3999 | <0.001 | 0.608 | ||
| Age (years) | 0.0080 | <0.001 | 0.180 | 0.0060 | <0.001 | 0.207 | |||
| Menarcheal status * | NA | 0.0396 | <0.001 | 0.184 | |||||
| Lean mass (kg) | 0.0096 | <0.001 | 0.562 | 0.0099 | <0.001 | 0.438 | |||
| BMI (kg/m2) | −0.0014 | 0.072 | −0.054 | 0.0000 | 0.990 | 0.000 | |||
| WC | Intercept | 0.4092 | <0.001 | 0.735 | 0.3903 | <0.001 | 0.608 | ||
| Age (years) | 0.0084 | <0.001 | 0.194 | 0.0061 | <0.001 | 0.209 | |||
| Menarcheal status * | NA | 0.0403 | <0.001 | 0.187 | |||||
| Lean mass (kg) | 0.0095 | <0.001 | 0.563 | 0.0095 | <0.001 | 0.422 | |||
| WC (cm) | −0.0004 | 0.137 | −0.045 | 0.0003 | 0.366 | 0.029 | |||
| WHtR | Intercept | 0.4155 | <0.001 | 0.735 | 0.3930 | <0.001 | 0.608 | ||
| Age (years) | 0.0083 | <0.001 | 0.189 | 0.0061 | <0.001 | 0.208 | |||
| Menarcheal status * | NA | 0.0402 | <0.001 | 0.185 | |||||
| Lean mass (kg) | 0.0093 | <0.001 | 0.631 | 0.0097 | <0.001 | 0.501 | |||
| WHtR (cm/cm) | −0.0632 | 0.125 | −0.046 | 0.0243 | 0.623 | 0.016 | |||
| Total-body FM | Intercept | 0.4012 | <0.001 | 0.738 | 0.3968 | <0.001 | 0.608 | ||
| Age (years) | 0.0073 | <0.001 | 0.167 | 0.0061 | <0.001 | 0.208 | |||
| Menarcheal status * | NA | 0.0395 | <0.001 | 0.184 | |||||
| Lean mass (kg) | 0.0098 | <0.001 | 0.628 | 0.0102 | <0.001 | 0.493 | |||
| Total-body FM (kg) | −0.0013 | <0.001 | −0.109 | −0.0004 | 0.436 | −0.025 | |||
| Dependent Variable: TBLH BMD (g/cm2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n = 1114) | Female (n = 946) | ||||||||
| Parameter | Independent Variable | b | p-Value | Partial R | Ad-R2 | b | p-Value | Partial R | Ad-R2 |
| BMI | Intercept | 0.1306 | 0.013 | 0.743 | 0.1723 | 0.001 | 0.616 | ||
| Age (years) | 0.0079 | <0.001 | 0.180 | 0.0061 | <0.001 | 0.211 | |||
| Menarcheal status * | NA | 0.0330 | <0.001 | 0.153 | |||||
| Lean mass (kg) | 0.0094 | <0.001 | 0.556 | 0.0097 | <0.001 | 0.436 | |||
| BMI (kg/m2) | 0.0251 | <0.001 | 0.155 | 0.0219 | <0.001 | 0.142 | |||
| [BMI]2 (kg/m2) | −0.0006 | <0.001 | −0.166 | −0.0005 | <0.001 | −0.144 | |||
| WC | Intercept | 0.0011 | 0.988 | 0.743 | −0.0635 | 0.456 | 0.621 | ||
| Age (years) | 0.0080 | <0.001 | 0.187 | 0.0059 | <0.001 | 0.207 | |||
| Menarcheal status * | NA | 0.0329 | <0.001 | 0.153 | |||||
| Lean mass (kg) | 0.0093 | <0.001 | 0.557 | 0.0095 | <0.001 | 0.430 | |||
| WC (cm) | 0.0110 | <0.001 | 0.160 | 0.0135 | <0.001 | 0.181 | |||
| [WC]2 (cm) | −0.0001 | <0.001 | −0.168 | −0.0001 | <0.001 | −0.179 | |||
| WHtR | Intercept | 0.0010 | 0.992 | 0.740 | −0.0896 | 0.402 | 0.616 | ||
| Age (years) | 0.0084 | <0.001 | 0.194 | 0.0061 | <0.001 | 0.212 | |||
| Menarcheal status * | NA | 0.0360 | <0.001 | 0.167 | |||||
| Lean mass (kg) | 0.0093 | <0.001 | 0.631 | 0.0100 | <0.001 | 0.514 | |||
| WHtR (cm/cm) | 1.7790 | <0.001 | 0.125 | 2.1793 | <0.001 | 0.149 | |||
| [WHtR]2 (cm/cm) | −2.0057 | <0.001 | −0.130 | −2.4083 | <0.001 | −0.148 | |||
| Total-body FM | Intercept | 0.3752 | <0.001 | 0.741 | 0.3536 | <0.001 | 0.616 | ||
| Age (years) | 0.0072 | <0.001 | 0.167 | 0.0059 | <0.001 | 0.206 | |||
| Menarcheal status * | NA | 0.0333 | <0.001 | 0.155 | |||||
| Lean mass (kg) | 0.0098 | <0.001 | 0.629 | 0.0101 | <0.001 | 0.494 | |||
| Total-body FM (kg) | 0.0027 | 0.021 | 0.069 | 0.0056 | <0.001 | 0.128 | |||
| [Total-body FM]2 (kg) | −0.0001 | <0.001 | −0.108 | −0.0001 | <0.001 | −0.144 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Yoon, I.; Cha, H.; Kim, H.-J.; Ryu, O.-H. Inverted U-Shaped Relationship between Obesity Parameters and Bone Mineral Density in Korean Adolescents. J. Clin. Med. 2023, 12, 5869. https://doi.org/10.3390/jcm12185869
Lee J, Yoon I, Cha H, Kim H-J, Ryu O-H. Inverted U-Shaped Relationship between Obesity Parameters and Bone Mineral Density in Korean Adolescents. Journal of Clinical Medicine. 2023; 12(18):5869. https://doi.org/10.3390/jcm12185869
Chicago/Turabian StyleLee, Jongseok, Insang Yoon, Hwajung Cha, Ho-Jung Kim, and Ohk-Hyun Ryu. 2023. "Inverted U-Shaped Relationship between Obesity Parameters and Bone Mineral Density in Korean Adolescents" Journal of Clinical Medicine 12, no. 18: 5869. https://doi.org/10.3390/jcm12185869
APA StyleLee, J., Yoon, I., Cha, H., Kim, H.-J., & Ryu, O.-H. (2023). Inverted U-Shaped Relationship between Obesity Parameters and Bone Mineral Density in Korean Adolescents. Journal of Clinical Medicine, 12(18), 5869. https://doi.org/10.3390/jcm12185869





