Predicting Treatment Response in Inflammatory Bowel Diseases: Cross-Sectional Imaging Markers
Abstract
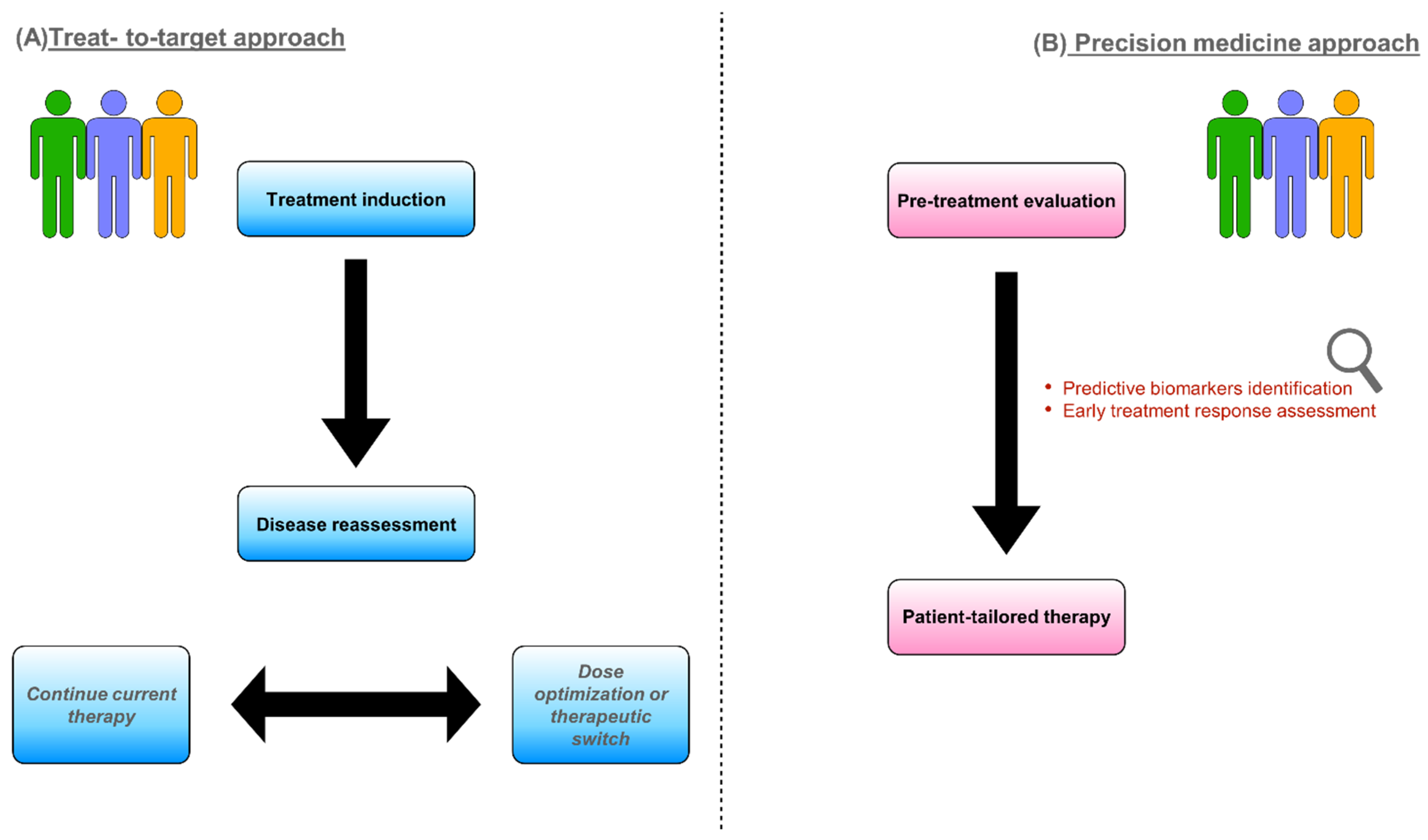
1. Introduction
2. Intestinal Ultrasound
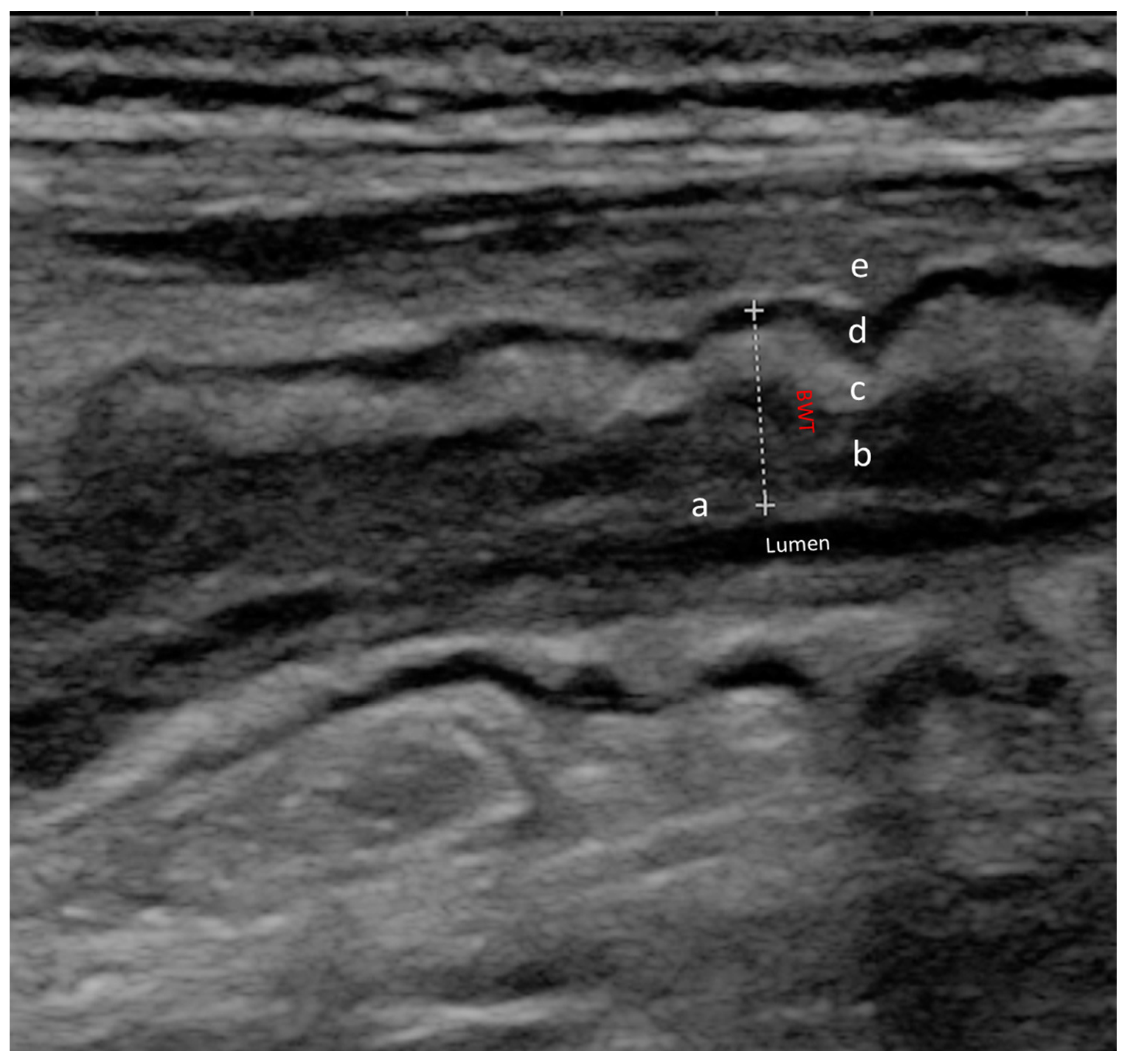
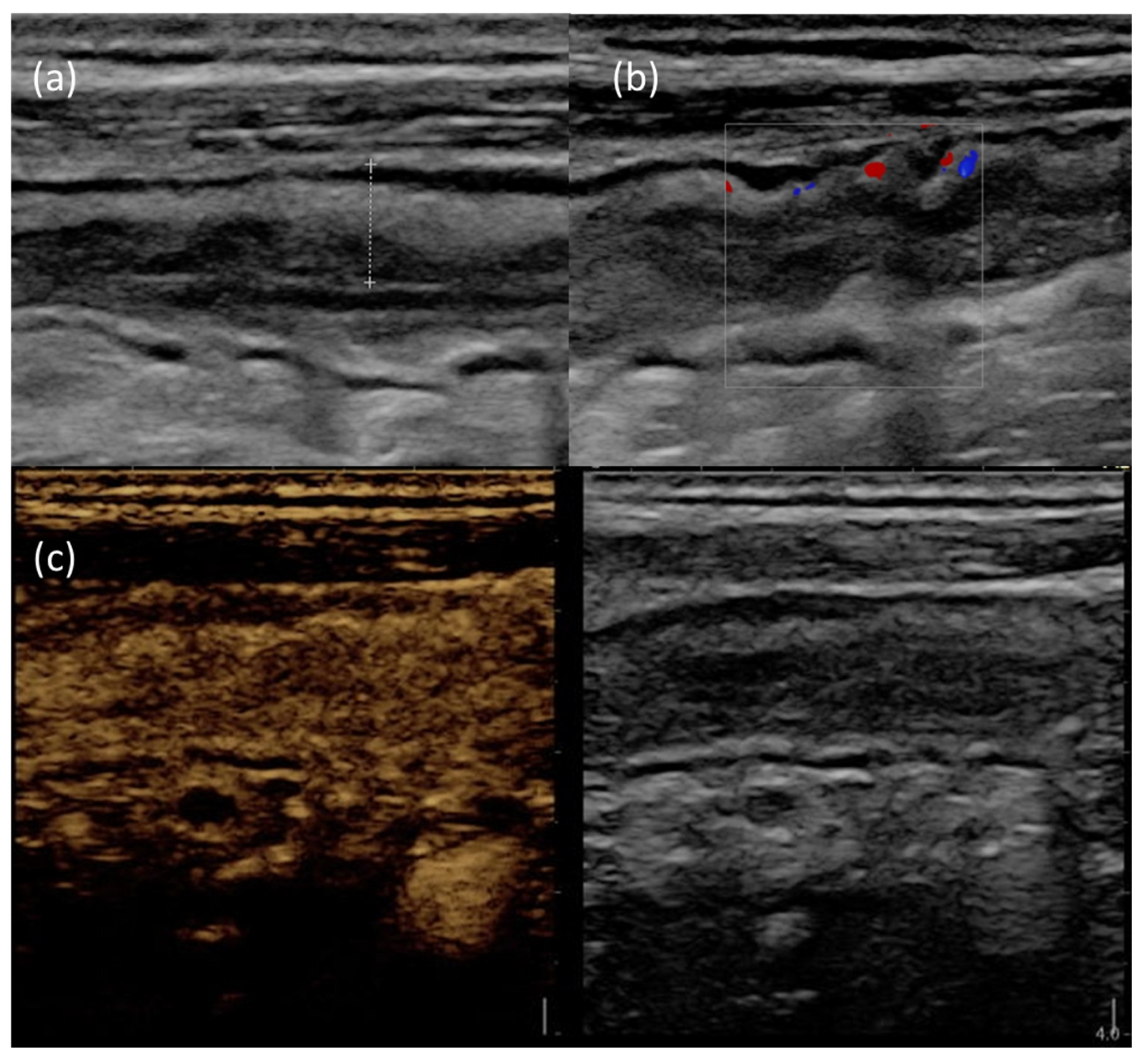
2.1. Role of IUS in CD
2.2. Role of IUS in UC
2.3. Role of IUS in Pediatric Setting
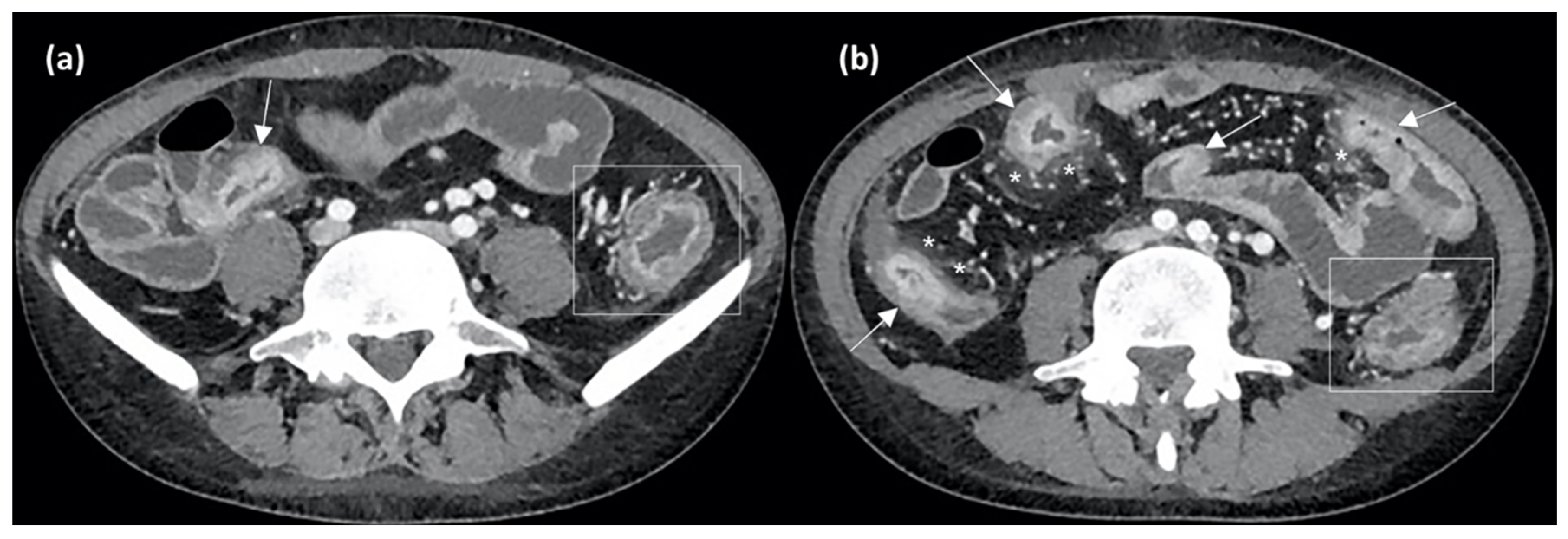
3. Magnetic Resonance Enterography
4. Computed Tomography Enterography and Radiomics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valle, E.; Gross, M.; Bickston, S.J. Infliximab. Expert Opin. Pharmacother. 2001, 2, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Vieujean, S.; Louis, E. Precision medicine and drug optimization in adult inflammatory bowel disease patients. Ther. Adv. Gastroenterol. 2023, 16, 17562848231173332. [Google Scholar] [CrossRef]
- Verstockt, B.; Noor, N.M.; Marigorta, U.M.; Pavlidis, P.; Deepak, P.; Ungaro, R.C. Scientific Workshop Steering Committee Results of the Seventh Scientific Workshop of ECCO: Precision Medicine in IBD-Disease Outcome and Response to Therapy. J. Crohns Colitis 2021, 15, 1431–1442. [Google Scholar] [CrossRef]
- Nancey, S.; Fumery, M.; Faure, M.; Boschetti, G.; Gay, C.; Milot, L.; Roblin, X. Use of imaging modalities for decision-making in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2023, 16, 17562848231151292. [Google Scholar] [CrossRef]
- Pecere, S.; Holleran, G.; Ainora, M.E.; Garcovich, M.; Scaldaferri, F.; Gasbarrini, A.; Zocco, M.A. Usefulness of contrast-enhanced ultrasound (CEUS) in Inflammatory Bowel Disease (IBD). Dig. Liver Dis. 2018, 50, 761–767. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Buisson, A.; Gonzalez, F.; Poullenot, F.; Nancey, S.; Sollellis, E.; Fumery, M.; Pariente, B.; Flamant, M.; Trang-Poisson, C.; Bonnaud, G.; et al. Comparative Acceptability and Perceived Clinical Utility of Monitoring Tools: A Nationwide Survey of Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1425–1433. [Google Scholar] [CrossRef]
- Miles, A.; Bhatnagar, G.; Halligan, S.; Gupta, A.; Tolan, D.; Zealley, I.; Taylor, S.A. METRIC investigators Magnetic resonance enterography, small bowel ultrasound and colonoscopy to diagnose and stage Crohn’s disease: Patient acceptability and perceived burden. Eur. Radiol. 2019, 29, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.A.; Mallett, S.; Bhatnagar, G.; Baldwin-Cleland, R.; Bloom, S.; Gupta, A.; Hamlin, P.J.; Hart, A.L.; Higginson, A.; Jacobs, I.; et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): A multicentre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Parente, F.; Greco, S.; Molteni, M.; Cucino, C.; Maconi, G.; Sampietro, G.M.; Danelli, P.G.; Cristaldi, M.; Bianco, R.; Gallus, S.; et al. Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment. Pharmacol. Ther. 2003, 18, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.; Maaser, C.; Zorzi, F.; Kannengiesser, K.; Hanauer, S.B.; Bruining, D.H.; Iacucci, M.; Maconi, G.; Novak, K.L.; Panaccione, R.; et al. Bowel Ultrasonography in the Management of Crohn’s Disease. A Review with Recommendations of an International Panel of Experts. Inflamm. Bowel Dis. 2016, 22, 1168–1183. [Google Scholar] [CrossRef] [PubMed]
- Alfarone, L.; Dal Buono, A.; Craviotto, V.; Zilli, A.; Fiorino, G.; Furfaro, F.; D’Amico, F.; Danese, S.; Allocca, M. Cross-Sectional Imaging Instead of Colonoscopy in Inflammatory Bowel Diseases: Lights and Shadows. J. Clin. Med. 2022, 11, 353. [Google Scholar] [CrossRef]
- Stenczel, N.D.; Purcarea, M.R.; Tribus, L.C.; Oniga, G.H. The role of the intestinal ultrasound in Crohn’s disease diagnosis and monitoring. J. Med. Life 2021, 14, 310–315. [Google Scholar] [CrossRef]
- Limberg, B. Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z. Gastroenterol. 1999, 37, 495–508. [Google Scholar]
- Kucharzik, T.; Tielbeek, J.; Carter, D.; Taylor, S.A.; Tolan, D.; Wilkens, R.; Bryant, R.V.; Hoeffel, C.; De Kock, I.; Maaser, C.; et al. ECCO-ESGAR Topical Review on Optimizing Reporting for Cross-Sectional Imaging in Inflammatory Bowel Disease. J. Crohns Colitis 2022, 16, 523–543. [Google Scholar] [CrossRef]
- Sagami, S.; Kobayashi, T.; Miyatani, Y.; Okabayashi, S.; Yamazaki, H.; Takada, T.; Kinoshita, K.; Allocca, M.; Kunisaki, R.; Ramaswamy, P.K.; et al. Accuracy of Ultrasound for Evaluation of Colorectal Segments in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 908–921.e6. [Google Scholar] [CrossRef]
- Nardone, O.M.; Calabrese, G.; Testa, A.; Caiazzo, A.; Fierro, G.; Rispo, A.; Castiglione, F. The Impact of Intestinal Ultrasound on the Management of Inflammatory Bowel Disease: From Established Facts Toward New Horizons. Front. Med. 2022, 9, 898092. [Google Scholar] [CrossRef]
- Ilvemark, J.F.K.F.; Hansen, T.; Goodsall, T.M.; Seidelin, J.B.; Al-Farhan, H.; Allocca, M.; Begun, J.; Bryant, R.V.; Carter, D.; Christensen, B.; et al. Defining Transabdominal Intestinal Ultrasound Treatment Response and Remission in Inflammatory Bowel Disease: Systematic Review and Expert Consensus Statement. J. Crohns Colitis 2022, 16, 554–580. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Imperatore, N.; Testa, A.; De Palma, G.D.; Nardone, O.M.; Pellegrini, L.; Caporaso, N.; Rispo, A. One-year clinical outcomes with biologics in Crohn’s disease: Transmural healing compared with mucosal or no healing. Aliment. Pharmacol. Ther. 2019, 49, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Novak, K.L.; Nylund, K.; Maaser, C.; Petersen, F.; Kucharzik, T.; Lu, C.; Allocca, M.; Maconi, G.; de Voogd, F.; Christensen, B.; et al. Expert Consensus on Optimal Acquisition and Development of the International Bowel Ultrasound Segmental Activity Score [IBUS-SAS]: A Reliability and Inter-rater Variability Study on Intestinal Ultrasonography in Crohn’s Disease. J. Crohns Colitis 2021, 15, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Sævik, F.; Eriksen, R.; Eide, G.E.; Gilja, O.H.; Nylund, K. Development and Validation of a Simple Ultrasound Activity Score for Crohn’s Disease. J. Crohns Colitis 2021, 15, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Freitas, M.; de Castro, F.D.; Macedo Silva, V.; Arieira, C.; Cúrdia Gonçalves, T.; Leite, S.; Moreira, M.J.; Cotter, J. Ultrasonographic scores for ileal Crohn’s disease assessment: Better, worse or the same as contrast-enhanced ultrasound? BMC Gastroenterol. 2022, 22, 252. [Google Scholar] [CrossRef]
- Wang, L.; Xu, C.; Zhang, Y.; Jiang, W.; Ma, J.; Zhang, H. External validation and comparison of simple ultrasound activity score and international bowel ultrasound segmental activity score for Crohn’s disease. Scand. J. Gastroenterol. 2023, 58, 883–889. [Google Scholar] [CrossRef]
- Allocca, M.; Craviotto, V.; Bonovas, S.; Furfaro, F.; Zilli, A.; Peyrin-Biroulet, L.; Fiorino, G.; Danese, S. Predictive Value of Bowel Ultrasound in Crohn’s Disease: A 12-Month Prospective Study. Clin. Gastroenterol. Hepatol. 2022, 20, e723–e740. [Google Scholar] [CrossRef]
- Allocca, M.; Craviotto, V.; Dell’Avalle, C.; Furfaro, F.; Zilli, A.; D’Amico, F.; Bonovas, S.; Peyrin-Biroulet, L.; Fiorino, G.; Danese, S. Bowel ultrasound score is accurate in assessing response to therapy in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2022, 55, 446–454. [Google Scholar] [CrossRef]
- Ripollés, T.; Paredes, J.M.; Martínez-Pérez, M.J.; Rimola, J.; Jauregui-Amezaga, A.; Bouzas, R.; Martin, G.; Moreno-Osset, E. Ultrasonographic Changes at 12 Weeks of Anti-TNF Drugs Predict 1-year Sonographic Response and Clinical Outcome in Crohn’s Disease: A Multicenter Study. Inflamm. Bowel Dis. 2016, 22, 2465–2473. [Google Scholar] [CrossRef]
- de Voogd, F.; Bots, S.; Gecse, K.; Gilja, O.H.; D’Haens, G.; Nylund, K. Intestinal Ultrasound Early on in Treatment Follow-up Predicts Endoscopic Response to Anti-TNFα Treatment in Crohn’s Disease. J. Crohns Colitis 2022, 16, 1598–1608. [Google Scholar] [CrossRef]
- Kucharzik, T.; Wilkens, R.; D’Agostino, M.-A.; Maconi, G.; Le Bars, M.; Lahaye, M.; Bravatà, I.; Nazar, M.; Ni, L.; Ercole, E.; et al. Early ultrasound response and progressive transmural remission after treatment with ustekinumab in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2022, 21, 153–163.e12. [Google Scholar] [CrossRef]
- Greis, C. Technology overview: SonoVue (Bracco, Milan). Eur. Radiol. 2004, 14 (Suppl. S8), P11–P15. [Google Scholar] [PubMed]
- Mocci, G.; Migaleddu, V.; Cabras, F.; Sirigu, D.; Scanu, D.; Virgilio, G.; Marzo, M. SICUS and CEUS imaging in Crohn’s disease: An update. J. Ultrasound 2017, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Saevik, F.; Nylund, K.; Hausken, T.; Ødegaard, S.; Gilja, O.H. Bowel perfusion measured with dynamic contrast-enhanced ultrasound predicts treatment outcome in patients with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; Gennari, A.G.; Cova, M.A. Early Predictors of the Long-term Response to Therapy in Patients With Crohn Disease Derived From a Time-Intensity Curve Analysis After Microbubble Contrast Agent Injection. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2019, 38, 947–958. [Google Scholar] [CrossRef]
- Laterza, L.; Ainora, M.E.; Garcovich, M.; Galasso, L.; Poscia, A.; Di Stasio, E.; Lupascu, A.; Riccardi, L.; Scaldaferri, F.; Armuzzi, A.; et al. Bowel contrast-enhanced ultrasound perfusion imaging in the evaluation of Crohn’s disease patients undergoing anti-TNFα therapy. Dig. Liver Dis. 2021, 53, 729–737. [Google Scholar] [CrossRef]
- Zorzi, F.; Ghosh, S.; Chiaramonte, C.; Lolli, E.; Ventura, M.; Onali, S.; De Cristofaro, E.; Fantini, M.C.; Biancone, L.; Monteleone, G.; et al. Response Assessed by Ultrasonography as Target of Biological Treatment for Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 2030–2037. [Google Scholar] [CrossRef]
- Maconi, G.; Ardizzone, S.; Parente, F.; Bianchi Porro, G. Ultrasonography in the evaluation of extension, activity, and follow-up of ulcerative colitis. Scand. J. Gastroenterol. 1999, 34, 1103–1107. [Google Scholar] [CrossRef]
- Parente, F.; Molteni, M.; Marino, B.; Colli, A.; Ardizzone, S.; Greco, S.; Sampietro, G.; Gallus, S. Bowel ultrasound and mucosal healing in ulcerative colitis. Dig. Dis. Basel Switz. 2009, 27, 285–290. [Google Scholar] [CrossRef]
- Maaser, C.; Petersen, F.; Helwig, U.; Fischer, I.; Roessler, A.; Rath, S.; Lang, D.; Kucharzik, T. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: Results from the TRUST&UC study. Gut 2020, 69, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Bots, S.; Nylund, K.; Gecse, K. Intestinal Ultrasound to Assess Disease Activity in Ulcerative Colitis: Development of a Novel UC-Ultrasound Index. J. Crohns Colitis 2022, 16, 337. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Taylor, K.M.; Friedman, A.B.; Swaine, A.P.; Gibson, D.J.; Gibson, P.R. Early Assessment With Gastrointestinal Ultrasound in Patients Hospitalised for a Flare of Ulcerative Colitis and Predicting the Need for Salvage Therapy: A Pilot Study. Ultrasound Med. Biol. 2021, 47, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Fiorino, G.; Bonovas, S.; Furfaro, F.; Gilardi, D.; Argollo, M.; Magnoni, P.; Peyrin-Biroulet, L.; Danese, S. Accuracy of Humanitas Ultrasound Criteria in Assessing Disease Activity and Severity in Ulcerative Colitis: A Prospective Study. J. Crohns Colitis 2018, 12, 1385–1391. [Google Scholar] [CrossRef]
- Allocca, M.; Filippi, E.; Costantino, A.; Bonovas, S.; Fiorino, G.; Furfaro, F.; Peyrin-Biroulet, L.; Fraquelli, M.; Caprioli, F.; Danese, S. Milan ultrasound criteria are accurate in assessing disease activity in ulcerative colitis: External validation. United Eur. Gastroenterol. J. 2021, 9, 438–442. [Google Scholar] [CrossRef]
- Girlich, C.; Schacherer, D.; Jung, E.M.; Klebl, F.; Huber, E. Comparison between quantitative assessment of bowel wall vascularization by contrast-enhanced ultrasound and results of histopathological scoring in ulcerative colitis. Int. J. Colorectal Dis. 2012, 27, 193–198. [Google Scholar] [CrossRef]
- Socaciu, M.; Ciobanu, L.; Diaconu, B.; Hagiu, C.; Seicean, A.; Badea, R. Non-Invasive Assessment of Inflammation and Treatment Response in Patients with Crohn’s Disease and Ulcerative Colitis using Contrast-Enhanced Ultrasonography Quantification. J. Gastrointest. Liver Dis. JGLD 2015, 24, 457–465. [Google Scholar] [CrossRef]
- Kellar, A.; Dolinger, M.; Novak, K.L.; Chavannes, M.; Dubinsky, M.; Huynh, H. Intestinal Ultrasound for the Pediatric Gastroenterologist: A Guide for Inflammatory Bowel Disease Monitoring in Children: Expert Consensus on Behalf of the International Bowel Ultrasound Group (IBUS) Pediatric Committee. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 142–148. [Google Scholar] [CrossRef]
- van Wassenaer, E.A.; van der Klift, R.R.; Staphorst, M.S.; van der Lee, J.H.; Benninga, M.A.; Koot, B.G.P. The child’s perception on monitoring inflammatory bowel disease activity. Eur. J. Pediatr. 2022, 181, 1143–1149. [Google Scholar] [CrossRef]
- Ho, S.S.C.; Keenan, J.I.; Day, A.S. Parent Perspectives of Diagnostic and Monitoring Tests Undertaken by Their Child with Inflammatory Bowel Disease. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 19–29. [Google Scholar] [CrossRef]
- Bremner, A.R.; Griffiths, M.; Argent, J.D.; Fairhurst, J.J.; Beattie, R.M. Sonographic evaluation of inflammatory bowel disease: A prospective, blinded, comparative study. Pediatr. Radiol. 2006, 36, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Dolinger, M.T.; Choi, J.J.; Phan, B.L.; Rosenberg, H.K.; Rowland, J.; Dubinsky, M.C. Use of Small Bowel Ultrasound to Predict Response to Infliximab Induction in Pediatric Crohn’s Disease. J. Clin. Gastroenterol. 2021, 55, 429–432. [Google Scholar] [CrossRef]
- Civitelli, F.; Di Nardo, G.; Oliva, S.; Nuti, F.; Ferrari, F.; Dilillo, A.; Viola, F.; Pallotta, N.; Cucchiara, S.; Aloi, M. Ultrasonography of the colon in pediatric ulcerative colitis: A prospective, blind, comparative study with colonoscopy. J. Pediatr. 2014, 165, 78–84.e2. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Torres, J.; Kumar, S.; Taylor, S.A.; Kucharzik, T. Recent advances in clinical practice: Advances in cross-sectional imaging in inflammatory bowel disease. Gut 2022, 71, 2587–2597. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Rodriguez, S.; García-Bosch, O.; Ordás, I.; Ayala, E.; Aceituno, M.; Pellisé, M.; Ayuso, C.; Ricart, E.; Donoso, L.; et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 2009, 58, 1113–1120. [Google Scholar] [CrossRef]
- Ordás, I.; Rimola, J.; Alfaro, I.; Rodríguez, S.; Castro-Poceiro, J.; Ramírez-Morros, A.; Gallego, M.; Giner, À.; Barastegui, R.; Fernández-Clotet, A.; et al. Development and Validation of a Simplified Magnetic Resonance Index of Activity for Crohn’s Disease. Gastroenterology 2019, 157, 432–439.e1. [Google Scholar] [CrossRef]
- Steward, M.J.; Punwani, S.; Proctor, I.; Adjei-Gyamfi, Y.; Chatterjee, F.; Bloom, S.; Novelli, M.; Halligan, S.; Rodriguez-Justo, M.; Taylor, S.A. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: Derivation and histopathological validation of an MR-based activity index. Eur. J. Radiol. 2012, 81, 2080–2088. [Google Scholar] [CrossRef]
- Oussalah, A.; Laurent, V.; Bruot, O.; Bressenot, A.; Bigard, M.-A.; Régent, D.; Peyrin-Biroulet, L. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut 2010, 59, 1056–1065. [Google Scholar] [CrossRef]
- Buisson, A.; Joubert, A.; Montoriol, P.-F.; Da Ines, D.; Hordonneau, C.; Pereira, B.; Garcier, J.-M.; Bommelaer, G.; Petitcolin, V. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment. Pharmacol. Ther. 2013, 37, 537–545. [Google Scholar] [CrossRef]
- Laurent, V.; Naudé, S.; Vuitton, L.; Zallot, C.; Baumann, C.; Girard-Gavanier, M.; Peyrin-Biroulet, L. Accuracy of Diffusion-weighted Magnetic Resonance Colonography in Assessing Mucosal Healing and the Treatment Response in Patients with Ulcerative Colitis. J. Crohns Colitis 2017, 11, 716–723. [Google Scholar] [CrossRef][Green Version]
- Xu, C.; Li, L.; Zhang, Y.; Wang, R.; Zhang, H. Diagnostic accuracy of different cross-sectional imaging techniques for disease location and activity in Crohn’s disease and external validation and comparison of MARIAs and IBUS-SAS. Abdom. Radiol. N. Y. 2023, 48, 821–832. [Google Scholar] [CrossRef]
- Eder, P.; Michalak, M.; Katulska, K.; Lykowska-Szuber, L.; Krela-Kazmierczak, I.; Stawczyk-Eder, K.; Klimczak, K.; Szymczak, A.; Linke, K. Magnetic resonance enterographic predictors of one-year outcome in ileal and ileocolonic Crohn’s disease treated with anti-tumor necrosis factor antibodies. Sci. Rep. 2015, 5, 10223. [Google Scholar] [CrossRef]
- Gibson, D.J.; Murphy, D.J.; Smyth, A.E.; McEvoy, S.H.; Keegan, D.; Byrne, K.; Mulcahy, H.E.; Cullen, G.; Malone, D.E.; Doherty, G.A. Magnetic resonance enterography findings as predictors of clinical outcome following antitumor necrosis factor treatment in small bowel Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Buisson, A.; Hordonneau, C.; Goutte, M.; Scanzi, J.; Goutorbe, F.; Klotz, T.; Boyer, L.; Pereira, B.; Bommelaer, G. Diffusion-weighted magnetic resonance enterocolonography in predicting remission after anti-TNF induction therapy in Crohn’s disease. Dig. Liver Dis. 2016, 48, 260–266. [Google Scholar] [CrossRef]
- Messadeg, L.; Hordonneau, C.; Bouguen, G.; Goutorbe, F.; Reimund, J.M.; Goutte, M.; Boucher, A.L.; Scanzi, J.; Reymond, M.; Allimant, C.; et al. Early Transmural Response Assessed Using Magnetic Resonance Imaging Could Predict Sustained Clinical Remission and Prevent Bowel Damage in Patients with Crohn’s Disease Treated with Anti-Tumour Necrosis Factor Therapy. J. Crohns Colitis 2020, 14, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Buisson, A.; Hordonneau, C.; Goutorbe, F.; Allimant, C.; Goutte, M.; Reymond, M.; Pereira, B.; Bommelaer, G. Bowel wall healing assessed using magnetic resonance imaging predicts sustained clinical remission and decreased risk of surgery in Crohn’s disease. J. Gastroenterol. 2019, 54, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, H.; Ishiguro, Y.; Hasui, K.; Hiraga, H.; Fukuda, S.; Shibutani, K.; Takai, Y. Prediction of maintained mucosal healing in patients with Crohn’s disease under treatment with infliximab using diffusion-weighted magnetic resonance imaging. Digestion 2014, 89, 49–54. [Google Scholar] [CrossRef]
- Thierry, M.-L.; Rousseau, H.; Pouillon, L.; Girard-Gavanier, M.; Baumann, C.; Lopez, A.; Danese, S.; Laurent, V.; Peyrin-Biroulet, L. Accuracy of Diffusion-weighted Magnetic Resonance Imaging in Detecting Mucosal Healing and Treatment Response, and in Predicting Surgery, in Crohn’s Disease. J. Crohns Colitis 2018, 12, 1180–1190. [Google Scholar] [CrossRef]
- Eder, P.; Łykowska-Szuber, L.; Katulska, K.; Stawczyk-Eder, K.; Krela-Kaźmierczak, I.; Klimczak, K.; Szymczak, A.; Stajgis, M.; Linke, K. Intestinal healing after anti-TNF induction therapy predicts long-term response to one-year treatment in patients with ileocolonic Crohn’s disease naive to anti-TNF agents. Przeglad Gastroenterol. 2016, 11, 187–193. [Google Scholar] [CrossRef]
- Rimola, J.; Fernàndez-Clotet, A.; Capozzi, N.; Rojas-Farreras, S.; Alfaro, I.; Rodríguez, S.; Masamunt, M.-C.; Ricart, E.; Ordás, I.; Panés, J. Pre-treatment magnetic resonance enterography findings predict the response to TNF-alpha inhibitors in Crohn’s disease. Aliment. Pharmacol. Ther. 2020, 52, 1563–1573. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, C.; Zhang, R.; Qiu, Y.; Wang, Y.; Liu, Z.; Chen, B.; He, Y.; Zeng, Z.; Li, X.; et al. Early transmural healing and its predictors assessed by magnetic resonance enterography in patients with Crohn’s disease receiving ustekinumab. Ther. Adv. Gastroenterol. 2023, 16, 17562848231170948. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Carbonnel, F.; Laharie, D.; Stefanescu, C.; Hébuterne, X.; Abitbol, V.; Nachury, M.; Brixi, H.; Bourreille, A.; Picon, L.; et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: A multicentre, prospective, observational cohort (CREOLE) study. Gut 2018, 67, 53–60. [Google Scholar] [CrossRef]
- Amitai, M.M.; Klang, E.; Levartovsky, A.; Rozendorn, N.; Soffer, S.; Taha, G.A.; Ungar, B.; Greener, T.; Ben-Horin, S.; Eliakim, R.; et al. Diffusion-weighted magnetic resonance enterography for prediction of response to tumor necrosis factor inhibitors in stricturing Crohn’s disease. Abdom. Radiol. N. Y. 2018, 43, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Mainenti, P.P.; Castiglione, F.; Rispo, A.; Laccetti, E.; Guarino, S.; Romeo, V.; Testa, A.; Pace, L.; Maurea, S. MR-enterography in Crohn’s disease: What MRE mural parameters are associated to one-year therapeutic management outcome? Br. J. Radiol. 2021, 94, 20200844. [Google Scholar] [CrossRef] [PubMed]
- Horsthuis, K.; Bipat, S.; Bennink, R.J.; Stoker, J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: Meta-analysis of prospective studies. Radiology 2008, 247, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, R.-N.; Mao, R.; Li, X.-H. Inflammatory bowel disease cross-sectional imaging: What’s new? United Eur. Gastroenterol. J. 2022, 10, 1179–1193. [Google Scholar] [CrossRef]
- Alyami, A.S. The Role of Radiomics in Fibrosis Crohn’s Disease: A Review. Diagn. Basel Switz. 2023, 13, 1623. [Google Scholar] [CrossRef]
- Hanžel, J.; Jairath, V.; Ma, C.; Guizzetti, L.; Zou, G.; Santillan, C.S.; Taylor, S.A.; van Viegen, T.; D’Haens, G.R.; Feagan, B.G.; et al. Responsiveness of Magnetic Resonance Enterography Indices for Evaluation of Luminal Disease Activity in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2022, 20, 2598–2606. [Google Scholar] [CrossRef]
- Meng, J.; Luo, Z.; Chen, Z.; Zhou, J.; Chen, Z.; Lu, B.; Zhang, M.; Wang, Y.; Yuan, C.; Shen, X.; et al. Intestinal fibrosis classification in patients with Crohn’s disease using CT enterography-based deep learning: Comparisons with radiomics and radiologists. Eur. Radiol. 2022, 32, 8692–8705. [Google Scholar] [CrossRef]
- Li, X.; Liang, D.; Meng, J.; Zhou, J.; Chen, Z.; Huang, S.; Lu, B.; Qiu, Y.; Baker, M.E.; Ye, Z.; et al. Development and Validation of a Novel Computed-Tomography Enterography Radiomic Approach for Characterization of Intestinal Fibrosis in Crohn’s Disease. Gastroenterology 2021, 160, 2303–2316.e11. [Google Scholar] [CrossRef]
- Laterza, L.; Boldrini, L.; Tran, H.E.; Votta, C.; Larosa, L.; Minordi, L.M.; Maresca, R.; Pugliese, D.; Zocco, M.A.; Ainora, M.E.; et al. Radiomics could predict surgery at 10 years in Crohn’s disease. Dig. Liver Dis 2022, 55, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Feng, J.; Suo, S.; Feng, Q.; Shen, J. A Novel Radiomics Nomogram for the Prediction of Secondary Loss of Response to Infliximab in Crohn’s Disease. J. Inflamm. Res. 2021, 14, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Hu, J.; Wang, X.; Li, C.; Gao, Y.; Li, J.; Ge, Y.; Wu, X. A novel clinical radiomics nomogram at baseline to predict mucosal healing in Crohn’s disease patients treated with infliximab. Eur. Radiol. 2022, 32, 6628–6636. [Google Scholar] [CrossRef] [PubMed]

| MRE | IUS | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| Disease presence | ||||
|
|
|
| |
| Disease extent | ||||
|
|
|
| |
| Disease activity | ||||
|
|
|
| |
| Disease complications | ||||
|
|
|
| |
| IUS | MRE |
|---|---|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mignini, I.; Maresca, R.; Ainora, M.E.; Larosa, L.; Scaldaferri, F.; Gasbarrini, A.; Zocco, M.A. Predicting Treatment Response in Inflammatory Bowel Diseases: Cross-Sectional Imaging Markers. J. Clin. Med. 2023, 12, 5933. https://doi.org/10.3390/jcm12185933
Mignini I, Maresca R, Ainora ME, Larosa L, Scaldaferri F, Gasbarrini A, Zocco MA. Predicting Treatment Response in Inflammatory Bowel Diseases: Cross-Sectional Imaging Markers. Journal of Clinical Medicine. 2023; 12(18):5933. https://doi.org/10.3390/jcm12185933
Chicago/Turabian StyleMignini, Irene, Rossella Maresca, Maria Elena Ainora, Luigi Larosa, Franco Scaldaferri, Antonio Gasbarrini, and Maria Assunta Zocco. 2023. "Predicting Treatment Response in Inflammatory Bowel Diseases: Cross-Sectional Imaging Markers" Journal of Clinical Medicine 12, no. 18: 5933. https://doi.org/10.3390/jcm12185933
APA StyleMignini, I., Maresca, R., Ainora, M. E., Larosa, L., Scaldaferri, F., Gasbarrini, A., & Zocco, M. A. (2023). Predicting Treatment Response in Inflammatory Bowel Diseases: Cross-Sectional Imaging Markers. Journal of Clinical Medicine, 12(18), 5933. https://doi.org/10.3390/jcm12185933







