Impact of Chronic Kidney Disease and Dialysis on Outcome after Surgery for Infective Endocarditis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Definition of Variables
2.4. Management of Patients
2.5. Statistical Analysis
3. Results
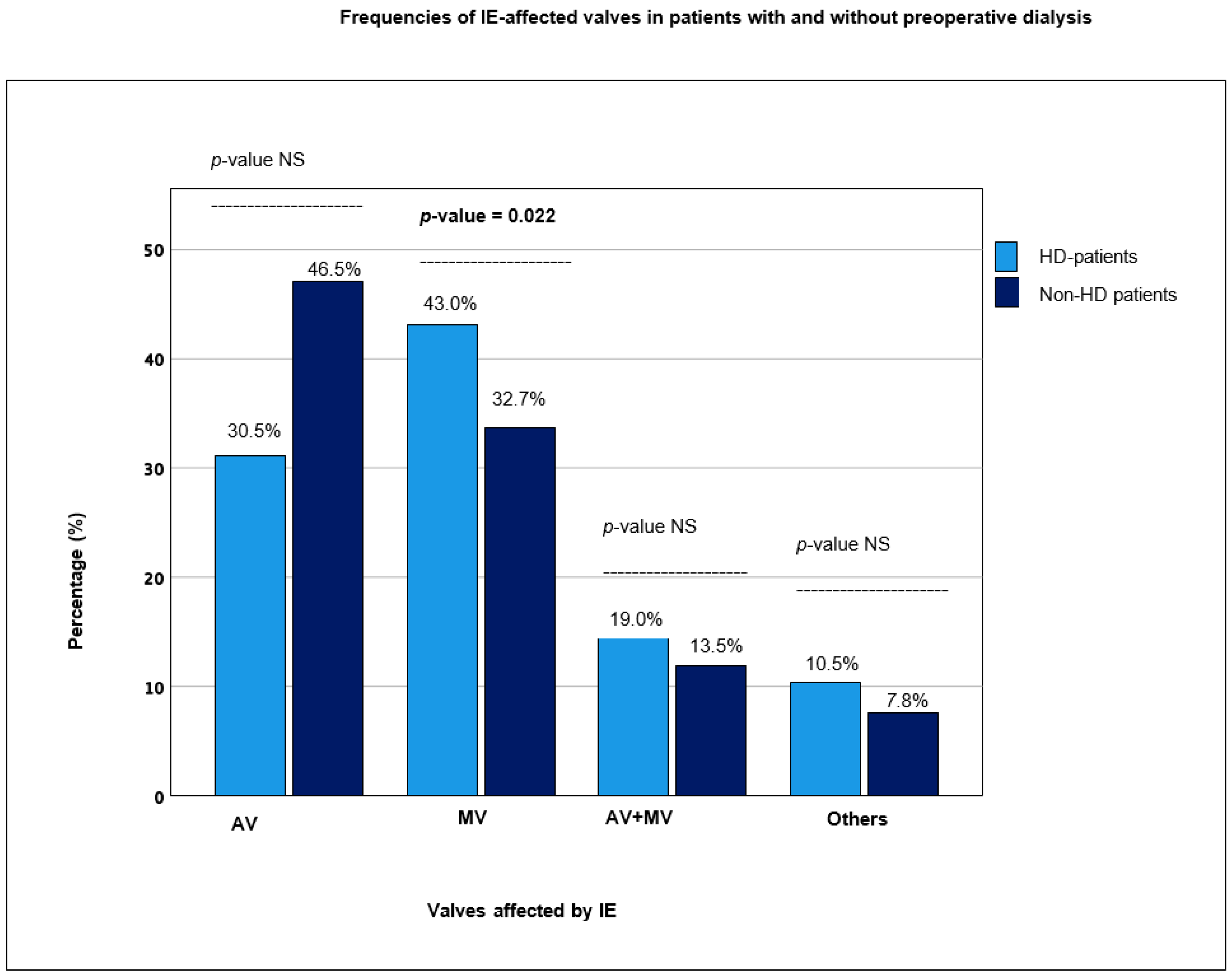
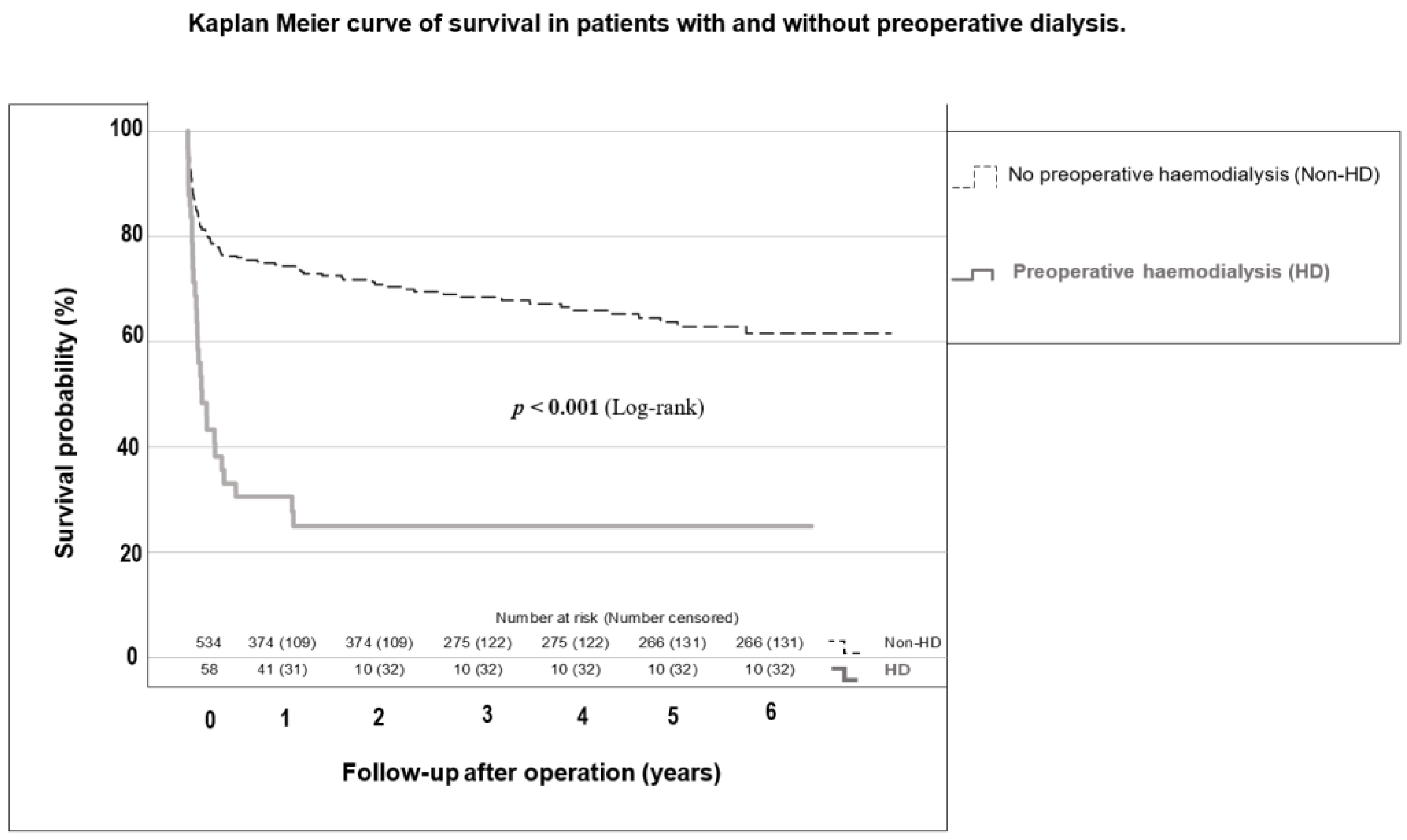
3.1. Comparison between Non-HD and HD Patients
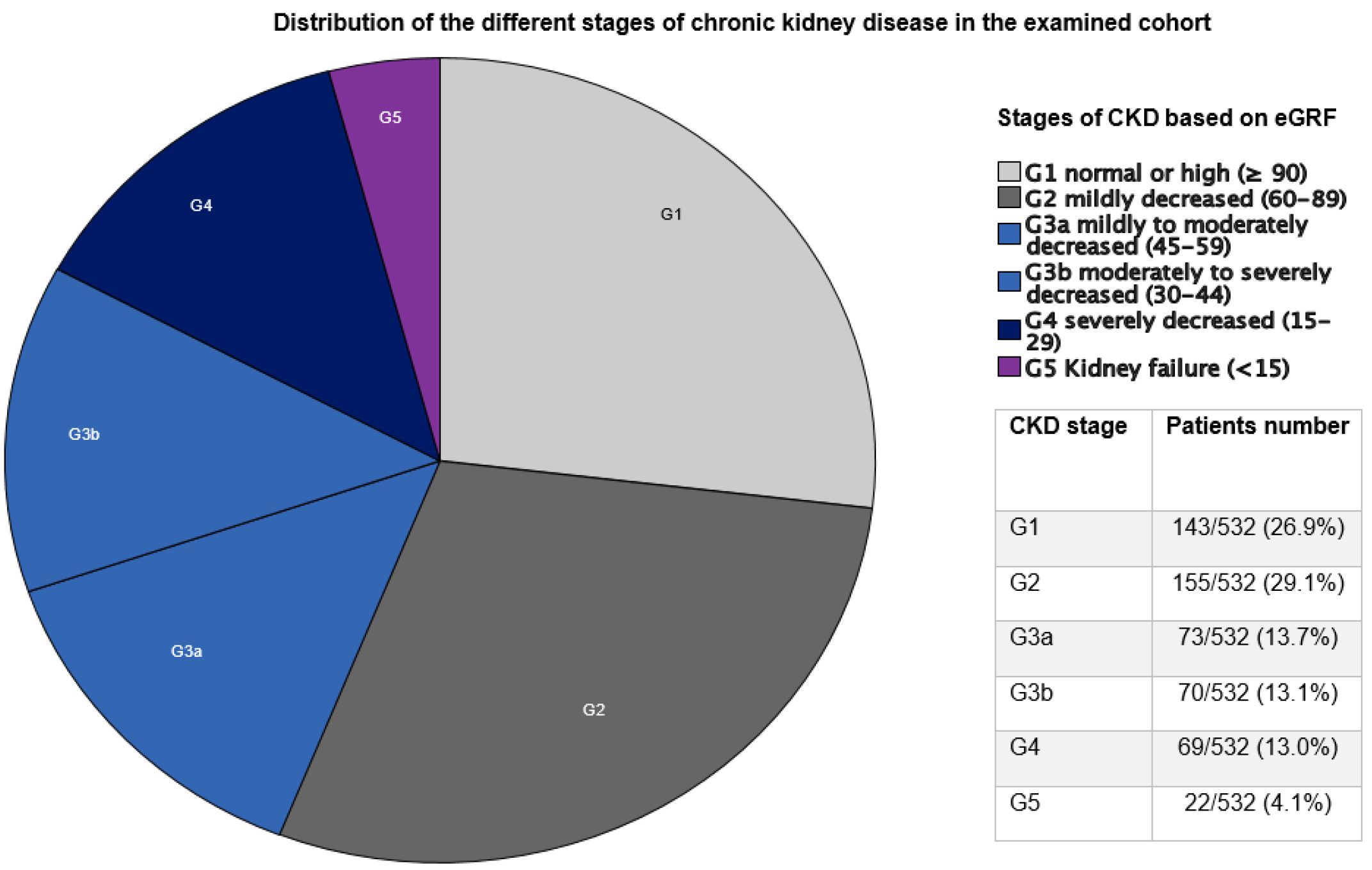
3.2. Results Based on CKD Stage
3.3. Predictors for 30-Day Mortality, 1-Year Mortality and New Postoperative Dialysis
4. Discussion
4.1. Demographics and Intraoperative Course
4.2. Spectrum of Pathogens
4.3. Postoperative Course and Complications
4.4. Independent Predictors for 30-Day and 1-Year Mortality and New-Onset Postoperative Dialysis
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| CI | Confidence interval |
| CKD | Chronic kidney disease |
| CPB | Cardiopulmonary bypass |
| CVEs | Cerebrovascular events |
| eGFR | Estimated glomerular filtration rate |
| EuroSCORE II | European System for Cardiac Operative Risk Evaluation |
| HD | Hemodialysis |
| IE | Infective endocarditis |
| IQR | Interquartile range |
| KDIGO | Kidney Disease—Improving Global Outcomes |
| OR | Odds ratio |
| NVE | Native valve endocarditis |
| PVE | Prosthetic valve endocarditis |
Appendix A

References
- Chang, C.-H.; Fan, P.-C.; Kuo, G.; Lin, Y.-S.; Tsai, T.-Y.; Chang, S.-W.; Tian, Y.-C.; Lee, C.-C. Infection in advanced chronic kidney disease and subsequent adverse outcomes after dialysis initiation: A nationwide cohort study. Sci. Rep. 2020, 10, 2938. [Google Scholar] [CrossRef] [PubMed]
- Bentata, Y. Physiopathological approach to infective endocarditis in chronic hemodialysis patients: Left heart versus right heart involvement. Ren. Fail. 2017, 39, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsen, L.U.P.; Dalgaard, L.S.; Wiggers, H.; Jensen-Fangel, S.; Jespersen, B.; Ellermann-Eriksen, S.; Østergaard, L.; Søgaard, O.S. Infective endocarditis in patients receiving chronic hemodialysis: A 21-year observational cohort study in Denmark. Am. Heart J. 2016, 182, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.; Bongiorni, M.; Casalta, J.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.; Iung, B. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Eknoyan, G.; Lameire, N.; Eckardt, K.; Kasiske, B.; Wheeler, D.; Levin, A.; Stevens, P.; Bilous, R.; Lamb, E.; Coresh, J. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013, 3, 5–14. [Google Scholar]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Bhatia, N.; Agrawal, S.; Garg, A.; Mohananey, D.; Sharma, A.; Agarwal, M.; Garg, L.; Agrawal, N.; Singh, A.; Nanda, S. Trends and outcomes of infective endocarditis in patients on dialysis. Clin. Cardiol. 2017, 40, 423–429. [Google Scholar] [CrossRef]
- Weber, C.; Gassa, A.; Rokohl, A.; Sabashnikov, A.; Deppe, A.-C.; Eghbalzadeh, K.; Merkle, J.; Hamacher, S.; Liakopoulos, O.J.; Wahlers, T. Severity of presentation, not sex, increases risk of surgery for infective endocarditis. Ann. Thorac. Surg. 2019, 107, 1111–1117. [Google Scholar] [CrossRef]
- Guo, M.; Pierre, E.S.; Clemence, J., Jr.; Wu, X.; Tang, P.; Romano, M.; Kim, K.M.; Yang, B. Impact of chronic renal failure on surgical outcomes in patients with infective endocarditis. Ann. Thorac. Surg. 2021, 111, 828–835. [Google Scholar] [CrossRef]
- Chaudry, M.S.; Gislason, G.H.; Kamper, A.-L.; Rix, M.; Dahl, A.; Østergaard, L.; Fosbøl, E.L.; Lauridsen, T.K.; Oestergaard, L.B.; Hassager, C. The impact of hemodialysis on mortality risk and cause of death in Staphylococcus aureus endocarditis. BMC Nephrol. 2018, 19, 216. [Google Scholar] [CrossRef]
- Omoto, T.; Aoki, A.; Maruta, K.; Masuda, T. Surgical outcome in hemodialysis patients with active-phase infective endocarditis. Ann. Thorac. Cardiovasc. Surg. 2016, 22, 181–185. [Google Scholar] [CrossRef]
- Nori, U.S.; Manoharan, A.; Thornby, J.I.; Yee, J.; Parasuraman, R.; Ramanathan, V. Mortality risk factors in chronic haemodialysis patients with infective endocarditis. Nephrol. Dial. Transplant. 2006, 21, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Kamalakannan, D.; Pai, R.M.; Johnson, L.B.; Gardin, J.M.; Saravolatz, L.D. Epidemiology and clinical outcomes of infective endocarditis in hemodialysis patients. Ann. Thorac. Surg. 2007, 83, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Leither, M.D.; Shroff, G.R.; Ding, S.; Gilbertson, D.T.; Herzog, C.A. Long-term survival of dialysis patients with bacterial endocarditis undergoing valvular replacement surgery in the United States. Circulation 2013, 128, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Luehr, M.; Weber, C.; Misfeld, M.; Lichtenberg, A.; Tugtekin, S.-M.; Diab, M.; Saha, S.; Li, Y.; Matsche, K.; Doenst, T. Virulence of Staphylococcus infection in surgically treated patients with endocarditis: A multicenter analysis. Ann. Surg. 2023, 277, e1364–e1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ju, P.; Liu, X.; Zhou, H.; Xue, F. Comparison of clinical characteristics and outcomes of infective endocarditis between haemodialysis and non-haemodialysis patients in China. J. Int. Med. Res. 2020, 48, 0300060520940435. [Google Scholar] [CrossRef]
- Sadeghi, M.; Behdad, S.; Shahsanaei, F. Infective endocarditis and its short and long-term prognosis in hemodialysis patients: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2021, 46, 100680. [Google Scholar] [CrossRef]
- Pericàs, J.M.; Llopis, J.; Jiménez-Exposito, M.J.; Kourany, W.M.; Almirante, B.; Carosi, G.; Durante-Mangoni, E.; Fortes, C.Q.; Giannitsioti, E.; Lerakis, S.; et al. Infective Endocarditis in Patients on Chronic Hemodialysis. J. Am. Coll. Cardiol. 2021, 77, 1629–1640. [Google Scholar] [CrossRef]
- Gallacher, P.J.; McAllister, D.A.; Mills, N.L.; Cruden, N.L.; Shah, A.S.V.; Dhaun, N. Infective Endocarditis Hospitalizations and Outcomes in Patients With End-Stage Kidney Disease: A Nationwide Data-Linkage Study. J. Am. Heart Assoc. 2021, 10, e022002, Erratum in J. Am. Heart Assoc. 2021, 10, e020806. [Google Scholar] [CrossRef]
- Raza, S.; Hussain, S.T.; Rajeswaran, J.; Ansari, A.; Trezzi, M.; Arafat, A.; Witten, J.; Ravichandren, K.; Riaz, H.; Javadikasgari, H. Value of surgery for infective endocarditis in dialysis patients. J. Thorac. Cardiovasc. Surg. 2017, 154, 61–70.e6. [Google Scholar] [CrossRef]
- Misfeld, M.; Girrbach, F.; Etz, C.D.; Binner, C.; Aspern, K.V.; Dohmen, P.M.; Davierwala, P.; Pfannmueller, B.; Borger, M.A.; Mohr, F.-W. Surgery for infective endocarditis complicated by cerebral embolism: A consecutive series of 375 patients. J. Thorac. Cardiovasc. Surg. 2014, 147, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis—Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef]
- Gagneux-Brunon, A.; Pouvaret, A.; Maillard, N.; Berthelot, P.; Lutz, M.; Cazorla, C.; Tulane, C.; Fuzellier, J.; Verhoeven, P.; Frésard, A. Acute kidney injury in infective endocarditis: A retrospective analysis. Med. Mal. Infect. 2019, 49, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Liu, Y.; Han, Q.; Tang, Y.; Zhao, L.; Qiao, F.; Xu, Z.; Yu, M.; Yuan, Z. Risk factors and short-term prognosis of preoperative renal insufficiency in infective endocarditis. J. Thorac. Dis. 2018, 10, 3679. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.K.; Jensen, A.D.; Bruun, N.E.; Kamper, A.-L.; Butt, J.H.; Havers-Borgersen, E.; Chaudry, M.S.; Torp-Pedersen, C.; Køber, L.; Fosbøl, E.L. Outcome of dialysis-requiring acute kidney injury in patients with infective endocarditis: A nationwide study. Clin. Infect. Dis. 2021, 72, e232–e239. [Google Scholar] [CrossRef]

| Variables | All Patients (n = 592) | Non-HD Group (n = 534) | HD Group (n = 58) | p-Value |
|---|---|---|---|---|
| Age (years) | 64.7 [50.9–73.5] a | 65.1 [50.6–73.6] a | 63.2 [53.4–72.8] a | 0.861 |
| Female | 149 (25.2%) | 132 (24.9%) | 17 (29.3%) | 0.464 |
| BMI (kg/m2) | 25.5 [23.3–28.7] a | 25.5 [23.3–28.7] a | 25.0 [23.5–28.8] a | 0.953 |
| COPD | 60 (10.2%) | 53 (10.0%) | 7 (12.1%) | 0.614 |
| Art. HTN | 374 (63.4%) | 331 (62.2%) | 43 (74.1%) | 0.074 |
| PHT | 54 (9.2%) | 46 (8.6%) | 8 (13.8%) | 0.197 |
| Hyperlipidemia | 176 (29.8%) | 159 (29.9%) | 17 (29.3%) | 0.927 |
| CAD | 169 (28.6%) | 147 (27.6%) | 22 (37.9%) | 0.099 |
| PVD | 55 (9.3%) | 46 (8.6%) | 9 (15.5%) | 0.087 |
| Diabetes mellitus | 153 (25.9%) | 127 (23.9%) | 26 (44.8%) | <0.001 |
| Known CVEs | 76 (12.9%) | 69 (13.0%) | 7 (12.1%) | 0.846 |
| Active smoker | 125 (21.2%) | 114 (21.4%) | 11 (19.0%) | 0.663 |
| LVEF < 30% | 15 (2.6%) | 14 (2.7%) | 1 (1.7%) | 0.666 |
| LVEF 30–50% | 108 (26.6%) | 92 (26.3%) | 16 (28.5%) | |
| LVEF > 50% | 274 (67.6%) | 236 (67.6%) | 38 (67.8%) | |
| EuroSCORE II | 8.0 [6.0–11.0] a | 8.0 [5.0–10.0] a | 9.5 [7.0–12.0] a | 0.004 |
| Variables | All Patients n = 592 | Non-HD Group n = 534 | HD Group n = 58 | p-Value |
|---|---|---|---|---|
| Re-thoracotomy | 93 (15.8%) | 83 (15.7%) | 10 (17.2%) | 0.754 |
| Tracheotomy | 91 (15.4%) | 73 (13.7%) | 18 (31.0%) | <0.001 |
| ICU stay (days) | 5.0 [2.0–10.0] a | 5.0 [2.0–9.0] a | 8.0 [4.0–14.0] a | <0.001 |
| In-hospital stay (days) | 13.0 [9.0–8.0] a | 13.0 [9.0–18.0] a | 11.0 [8.0–19.0] a | 0.193 |
| New pacemaker implantation | 59 (10.0%) | 56 (10.5%) | 3 (5.2%) | 0.196 |
| Myocardial infarction | 5 (0.8%) | 4 (0.8%) | 1 (1.7%) | 0.497 |
| Cerebrovascular events | 33 (5.6%) | 26 (4.9%) | 7 (12.1%) | 0.046 |
| Hospital re-admission | 176 (53.0%) | 158 (51.3%) | 18 (75.0%) | 0.022 |
| IE recurrence | 24 (7.3%) | 22 (7.2%) | 2 (8.7%) | 0.797 |
| 30-day mortality | 85 (19.4%) | 69 (17.4%) | 16 (38.1%) | 0.001 |
| 1-year mortality | 140 (33.7%) | 109 (29.1%) | 31 (75.6%) | <0.001 |
| Median survival (days) | 4.3 [4.0–4.6] a | 4.5 [4.2–4.8] a | 1.6 [0.8–2.4] a | <0.001 |
| Variables | G1 Group n = 143 (26.9%) | G2/G3a Groups n = 228 (42.8%) | p-Value | G3b/G4 Groups n = 139 (26.1%) | p-Value |
|---|---|---|---|---|---|
| Postoperative AKI | 21/143 (14.7%) | 70/226 (31.0%) | <0.001 | 70/139 (50.4%) | <0.001 |
| Re-thoracotomy | 11/143 (7.7%) | 38/226 (16.8%) | 0.012 | 31/139 (22.3%) | <0.001 |
| Tracheotomy | 10/143 (7.0%) | 33/227 (14.5%) | 0.027 | 33/138 (23.9%) | <0.001 |
| Postoperative coronary angiography | 1/142 (0.7%) | 2/227 (0.9%) | 0.854 | 0/138 (0.0%) | 0.243 |
| Myocardial infarction | 2/142 (1.4%) | 1/227 (0.4%) | 0.322 | 1/139 (0.7%) | 0.574 |
| New pacemaker implantation | 10/143 (7.0%) | 23/227 (10.1%) | 0.302 | 16/139 (11.5%) | 0.190 |
| CVEs | 5/143 (3.5%) | 11/226 (4.9%) | 0.529 | 12/138 (8.7%) | 0.068 |
| IE recurrence | 6/71 (8.5%) | 8/140 (5.7%) | 0.458 | 4/71 (5.6%) | 0.512 |
| Hospital re-admission | 33/72 (45.8%) | 72/140 (51.4%) | 0.440 | 39/73 (53.4%) | 0.361 |
| 30-Day Mortality | 1-Year Mortality | ||||
|---|---|---|---|---|---|
| Preoperative Risk Factor | OR [95% CI] | p-Value | Preoperative Risk Factor | OR [95% CI] | p-Value |
| Age > 65 years | 1.929 [1.192–3.121] | 0.007 | Age > 65 years | 1.955 [1.339–2.854] | <0.001 |
| Male gender | 1.924 [1.212–3.054] | 0.006 | Male gender | 1.809 [1.316–2.714] | <0.001 |
| Coronary artery disease | 1.655 [1.054–2.596] | 0.028 | Coronary artery disease | 1.590 [1.118–2.261] | 0.010 |
| Preoperative renal insufficiency | 2.729 [1.641–4.537] | <0.001 | Preoperative renal insufficiency | 2.493 [1.684–3.691] | <0.001 |
| Staphylococcus aureus infection | 1.747 [1.106–2.761] | 0.017 | Staphylococcus aureus infection | 1.567 [1.080–2.273] | 0.018 |
| Perivalvular abscess | 1.725 [1.108–2.684] | 0.016 | Prosthetic valve endocarditis | 1.578 [1.102–2.259] | 0.013 |
| Variables | OR [95% CI] | p-Value |
|---|---|---|
| Age > 65 years | 2.458 [1.346–4.490] | 0.003 |
| Peripheral vascular disease | 1.679 [1.034–2.728] | 0.036 |
| Preoperative renal insufficiency | 4.134 [2.245–7.612] | <0.001 |
| Prosthetic valve endocarditis | 2.062 [1.225–3.469] | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elderia, A.; Kiehn, E.; Djordjevic, I.; Gerfer, S.; Eghbalzadeh, K.; Gaisendrees, C.; Deppe, A.-C.; Kuhn, E.; Wahlers, T.; Weber, C. Impact of Chronic Kidney Disease and Dialysis on Outcome after Surgery for Infective Endocarditis. J. Clin. Med. 2023, 12, 5948. https://doi.org/10.3390/jcm12185948
Elderia A, Kiehn E, Djordjevic I, Gerfer S, Eghbalzadeh K, Gaisendrees C, Deppe A-C, Kuhn E, Wahlers T, Weber C. Impact of Chronic Kidney Disease and Dialysis on Outcome after Surgery for Infective Endocarditis. Journal of Clinical Medicine. 2023; 12(18):5948. https://doi.org/10.3390/jcm12185948
Chicago/Turabian StyleElderia, Ahmed, Ellen Kiehn, Ilija Djordjevic, Stephen Gerfer, Kaveh Eghbalzadeh, Christopher Gaisendrees, Antje-Christin Deppe, Elmar Kuhn, Thorsten Wahlers, and Carolyn Weber. 2023. "Impact of Chronic Kidney Disease and Dialysis on Outcome after Surgery for Infective Endocarditis" Journal of Clinical Medicine 12, no. 18: 5948. https://doi.org/10.3390/jcm12185948
APA StyleElderia, A., Kiehn, E., Djordjevic, I., Gerfer, S., Eghbalzadeh, K., Gaisendrees, C., Deppe, A.-C., Kuhn, E., Wahlers, T., & Weber, C. (2023). Impact of Chronic Kidney Disease and Dialysis on Outcome after Surgery for Infective Endocarditis. Journal of Clinical Medicine, 12(18), 5948. https://doi.org/10.3390/jcm12185948











