Applications of CAD/CAM Technology for Craniofacial Implants Placement and Manufacturing of Auricular Prostheses—Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Source of Information
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias in Individual Studies
| Publications | No. of Cases | Purpose of Software Planning | Pre-Op Data for Digital Planning | Software | Printer/Miller | Printing Materials | Navigation System (Yes/No) | Location & No. of Implants | Implants System |
|---|---|---|---|---|---|---|---|---|---|
| Ciocca L., Scotti R. 2004 [26] | 1 | Fabrication of ear model | Minolta VIVID 900 3D laser scanner (Konica Minolta, Osaka, Japan) | Polygone editing tool (Minolta Co, Osaka, Japan), Rapidform CAD software (INUS Technology, Seoul, South Korea) | Z Printer 310 (Z Corp, Cambridge, MA, USA) | Powder and sealant from Z Corp. (Z Corp., Cambridge, MA, USA) | No | No | No |
| Sykes et al., 2004 [27] | 1 | Fabrication of ear model | Breuckmann OptoTOP scanner system (Breuckmann OptoTOP, Germany) | Polyworks software (InnovMetric Software), Freeform software (Freeform, NC, USA) | Thermojet printer | Wax material | No | No | No |
| Jiao et al., 2004 [28] | 1 | Fabrication of ear model | CT scan | Magics RP image ware (Materialise, Leuven, Belgium), Freeform software (Freeform, NC, USA) | Zippy-I RP machine (Kinergy Mechatronics, Singapore) | NM | No | No | No |
| Ciocca L et al., 2007 [29] | 1 | Fabrication of mold for auricular prosthesis and acrylic substructure | Minolta VIVID 900 3D laser scanner (Konica Minolta, Osaka, Japan) | Rapidform CAD software (INUS Technology, Seoul, South Korea), Software, Polygone editing tool (Minolta Co., Osaka, Japan) | Z Printer 310 (Z Corp, Cambridge, MA, USA) | Powder and sealant from Z Corp. (Z Corp., Cambridge, MA, USA) | No | No | No |
| Kurtulmus et al., 2009 [17] | 1 | Virtual implant planning | CT Scan | Implant 3D, Media Lab Software, 3D-Doctor software (Able Coorporation, Lexington, MA, USA) | NM | NM | No | No | No |
| Ciocca L et al., 2009 [30] | 1 | Surgical template for implant placement | CT, NextEngine Desktop 3D Scanner (NextEngine, Santa Monica, CA, USA) | Rapidform CAD software (INUS Technology, Seoul, South Korea) | Rapid prototyping machine (Z310Plus; Z Corp., Burlington, MA, USA) | NM | No | Right mastoid bone; 3 implants | NM |
| Turgut et al., 2009 [31] | 10 | Fabrication of ear model | CT scan | Modeling software (FreeForm Modeling Plus System, SensAble, Boston, MA) | Selective laser sintering (SLS) system (DTM Corp., Austin, TX, USA) | NM | No | No | No |
| Ciocca. L et al., 2010 [32] | 1 | Fabrication of mold for auricular prosthesis | NextEngine Desktop 3D Scanner (NextEngine, Santa Monica, CA, USA) | NextEngine Scan studio software (NextEngine, CA, USA) | 3D printer (Stratasys, Eden Prairie, MN, USA) | ABS P400 jet (Stratasys, Eden Prairie, MN, USA) | No | No | No |
| Verma et al., 2010 [22] | 2 | Virtual planning and intraoperative navigation for implant placement | CT scan | Navigation system, Stryker iNtellect Cranial (Stryker Navigation system, MI, USA) | NM | NM | Yes | Left and Right mastoid regions; 4 implants | Vistafix implants (Cochlear, Lone Tree, USA) |
| De Crescenzio F et al., 2011 [33] | 1 | Fabrication of mold for auricular prosthesis | NextEngine Desktop 3D Scanner (NextEngine, Santa Monica, CA, USA) | Rapidform CAD software (INUS Technology, Seoul, South Korea), Rhinoceros Software v. 4.0 (Robert McNeel & Associates, USA) | 3D printer (Stratasys, Eden Prairie, MN, USA) | ABS P400 jet (Stratasys, Eden Prairie, MN, USA) | No | Right mastoid bone; 2 implants | Straumann implants (Institut Straumann AG, Basel, Switzerland |
| Liacouras et al., 2011 [34] | 1 | Designing and creation of digital model and mold fabrication | CT scan, 3D photography/imaging (3dMD cranial System; 3dMD, Atlanta, GA, USA) | Mimics Software (Mimics Innovation Suite, Materialise, Leuven, Belgium), Freeform software (Freeform, NC, USA) (14 cases), Geomagics Studio software (3D Systems, Rock Hill, SC, USA) | ZPrinter 450, using zp130 Powder and zb59 Binder; (Z Corp., Cambridge, MA, USA) | NM | No | No | No |
| Kolodney et al., 2011 [35] | 1 | Surgical template for implant placement | CT scan | Mimics Software (Mimics Innovation Suite, Materialise, Leuven, Belgium), SurgiCase software (Materialise LLC, Ann Arbor, MI, USA) | NM | Somos DMX 100 Resin material (Somos DSM, Desotech Inc., Elgin, Illinois, USA) | No | Right mastoid bone; 3 implants | NM |
| Karatas MO et al., 2011 [36] | 2 | Fabrication of ear models | CT scans | Mimics Software (Mimics Innovation Suite, Materialise, Leuven, Belgium) | 3D ink-jet FDM printer (Z Corp, Cambridge, MA, USA), Perfactory Standard SXGA+ stereolithography printer (Envisiontec Inc., Germany) | Acrylic material | No | No | No |
| Bai et al., 2012 [37] | 1 | Surgical template for implant placement | CT scan | Geomagics Studio software (3D Systems, Rock Hill, SC, USA), Mimics Software (Mimics Innovation Suite, Materialise, Leuven, Belgium) | Rapid Prototyping machine AFS-360 printer (Long yuan Technology Ltd., Beijing, China) | Resin material (Details NM) | No | Left mastoid region, 3 implants | NM |
| Reitemeier et al., 2012 [38] | 1 | Surgical template for implant placement | CT scan | Software (VoXim v6.1; IVS Solutions AG, Chemnitz, Germany) | FDM Vantage S; (Stratasys, Eden Prairie, MN, USA) | Acrylic resin template | No | Right mastoid region, 2 implants | Straumann implants (Straumann GmbH, Freiburg, Germany) |
| Hatamleh and Watson. 2013 [39] | 1 | Fabrication of ear model | 3Shape R700 scanner (3 Shape, Copenhagen, Denmark) | 3 Shape software (3 Shape, Copenhagen, Denmark) | Z-Corp Printer (Z Corp, Cambridge, MA, USA) | NM | No | 2 craniofacial implants | Vistafix craniofacial implants (Cochlear, Surrey, UK) |
| Bai et al., 2014 [40] | 6 | Fabrication of mold for auricular prosthesis | CT scan, structured-light 3D scanner (3DSS-STD-II, Digital Manu, Shanghai, China) | Mimics Software (Mimics Innovation Suite, Materialise, Leuven, Belgium) | Rapid Prototyping machine AFS-360 printer (Long yuan Technology Ltd., Beijing, China) | Resin material | No | No | No |
| Tam CK et al., 2014 [8] | 6 | Fabrication of ear model | CT scan, 3dMDFace (3dMD, Atlanta, USA) | Mimics Software (Mimics Innovation Suite, Materialise, Leuven, Belgium), Surgical navigation system (BrainLAB, Feldkirchen, Germany) | Fused Deposition Modeling (FDM) | NM | Surgical navigation system (BrainLAB, Feldkirchen, Germany) | 12 implants were placed in mastoid bone | Dental implants (Friadent, Dentsply, Mannheim, Germany) |
| Watson and Hatamleh 2014 [41] | 3 | Fabrication of ear model | 3Shape R700 scanner (3 Shape, Copenhagen, Denmark) | Software Z-Build (Z Corp, Cambridge, MA, USA) | 3D printer (Z Corp., Cambridge, MA, USA). | Gypsum (150 Powder) (Z Corp, Cambridge, MA, USA) | No | No | No |
| Wang et al., 2015 [42] | 1 | Fabrication of model for implant placement planning | EBCT scan | Geomagics Studio software (3D Systems, Rock Hill, SC, USA) | SLS machine (AFS-360; (Long yuan Technology Ltd., Beijing, China) | Resin material | No | 3 implants in right mastoid bone | Implants (MDIC; FMMU, China) |
| Nuseir et al., 2015 [43] | 1 | Surgical template for implant placement | CT scan | Mimics Software (Mimics Innovation Suite, Materialise, Leuven, Belgium) | 3D printer (Z Corp, Cambridge, MA, USA) | NM | No | Left mastoid region, 2 implants | Vistafix craniofacial implants (Cochlear, Surrey, UK |
| Choi et al., 2016 [44] | 2 | Planning for craniofacial implant placement | CT scan | BrainLAB software (BrainLAB AG, Munich, Germany) | No | No | Image guidance systems (IGS) (Brainlab AG, Munich, Germany). | 4 implants in mastoid bone | Vistafix craniofacial implants (Cochlear, Surrey, UK) |
| Weissler et al., 2017 [45] | 1 | Virtual planning and intraoperative navigation for implant placement | CT scan, Laser scan | iPlan Cranial 3.0 BrainLAB software (BrainLAB AG, Munich, Germany) | NM | NM | Yes | Left & Right mastoid region; 4 implants | Vistafix craniofacial implants (Cochlear, Surrey, UK) |
| Yadav et al., 2017 [46] | 1 | Fabrication of mold for auricular prosthesis | CT scan | 3D modeling software | NM | NM | No | No | No |
| Nafij Bin Jamayet et al., 2018 [47] | 1 | Fabrication of ear model | NextEngine Desktop 3D Scanner (NextEngine, Santa Monica, CA, USA) | NextEngine Scan studio software (NextEngine, CA, USA), Rapidworks 64 version 4.1.0. (3D system, Inc., Rock Hill, USA) | Objet30 Scholar 3D Printer (Stratasys, Eden Prairie, MN, USA) | NM | No | No | No |
| Unkovskiy et al., 2018 [48] | 1 | Fabrication of mold for auricular prosthesis | Artec Color 3D scanner (Artec 3D, Luxembourg) | Artec Studio Software (Artec 3D, Luxembourg), Z brush software (Pixologic, Inc., Los Angeles, CA, USA) | ProJet 3510 CPXPlus (3D Systems, Rock Hill, SC, USA), SPro 60 HD (3D Systems, Rock Hill, SC, USA) | VisiJet M3 Hi-Cast printer (3D Systems, Rock Hill, SC, USA) | No | No | No |
| Sanghavi, et al., 2018 [49] | 1 | Fabrication of ear model | CT scan | Freeform software (Freeform, NC, USA) | 3D printing technology (Stereolithography) | Acrylic photopolymeric material | No | No | No |
| Ferreira R, Vives P. 2019 [50] | 2 | Fabrication of custom titanium plate for locator attachments | CT scan | Materialise 3-matic software 9.0 (Materalise, Leuven, Belgium) | Selective laser melting | Titanium grade 2 | No | No | No |
| Vijverberg MA et al., 2019 [51] | 11 | Surgical template for implant placement | CT scan | 3 Shape software (3 Shape, Copenhagen, Denmark) | NM | Polyamide material (Oceanz BV, Ede, The Netherlands) | No | 31 VXI300 implants in mastoid bone | Vistafix implants (Cochlear Bone Anchored Solutions AB, Mölnlycke, Sweden) |
| Cevik and Kocacikli. 2020 [52] | 1 | Fabrication of mold for auricular prosthesis | Artec Color 3D scanner (Artec 3D, Luxembourg) | Artec studio 16 software (Artec 3D, Luxembourg) | FDM technology printer; MakerBot Replicator 2 (MakerBot Industries, Brooklyn, NY, USA) | Polylactic acid material (PLA) | No | No | No |
| McHutchion and Aalto. 2021 [53] | 5 | Fabrication of scan bodies and molds for auricular prosthesis | 3dMD flex System (3dMD LLC, Atlanta, Georgia, USA), 3Shape R700 scanner (3 Shape, Copenhagen, Denmark) | Geomagics Studio software (3D Systems, Rock Hill, SC, USA) | Stereolithography 3D printer Form2 (Formlabs Inc., Somerville, Massachusetts, USA) | Clear resin, white resin (Formlabs Inc., Somerville, Massachusetts, USA) | No | No | No |
| Domingue D. et al., 2021 [54] | 1 | Surgical template for implant placement | CBCT scan | Meshmixer (Autodesk Inc., USA), Blue Sky Plan software (Blue Sky Bio, LLC, USA) | CEL Robox 3D printer (CEL, Bristol, UK) | nGen colorFabb polymer material (Eastman Chemical Company, Belfeld, Netherlands) | No | 4 implants in right mastoid bone | Vistafix craniofacial implants (Cochlear, Surrey, UK) |
| Unkovskiy et al., 2021 [55] | 1 | Fabrication of ear model and substructure and printing of silicone auricular prosthesis | Pritiface 3D photogrammetry system (pritiface; pritidenta GmbH, Germany), 3Shape R700 scanner (3 Shape, Copenhagen, Denmark) | Exocad software (Exocad, GmbH, Darmstadt, Germany), Z brush software (Pixologic, Inc., USA) | Stereolithography (SLA) (Form 2; Formlabs) (Formlabs Inc., Somerville, Massachusetts, USA) | Resin material (Flexible; Formlabs) (Formlabs Inc., Somerville, Massachusetts, USA), ACEO silicone material (Drop-on-Demand ACEO; Wacker Chemie AG, Munich, Germany) | No | No | No |
| Dashti et al., 2022 [56] | 1 | Fabrication of working cast and bar | Artec Color 3D scanner (Artec 3D, Luxembourg), 3Shape R700 scanner (3 Shape, Copenhagen, Denmark) | Z brush software (Pixologic, Inc., USA), Exocad software (Exocad, GmbH, Darmstadt, Germany) | Stereolithography printer | PowerDent resin material (ProTech Transfer Co., Ltd., Bangkok, Thailand) | No | No | No |
| Hatamleh MM et al., 2022 [57] | 3 | Surgical template for implant placement | CT scan | CMF Pro Plan; Materialise | Form 2; Formlabs GmbH | NM | No | Patient 1: Right mastoid bone. 2 implants of 4 mm Patient 2: 2 implants on each side | Branemark; Cochlear Europe Ltd. |
| Heydenrych A et al., 2023 [58] | 1 | Surgical template for implant placement | CT scan | Materialise 3-matic software 9.0 (Materalise, Leuven, Belgium) | Selective laser sintering printer | Nylon PA 2200 | No | Right mastoid bone. 3 implants | No |
| (a) | |||||||||
| Assessment | Author and Year | ||||||||
| Ciocca L, Scotti R. 2004 [26] | Sykes et al., 2004 [27] | Jiao et al., 2004 [28] | Ciocca L et al., 2007 [29] | Kurtulmus et al., 2009 [17] | Ciocca L et al., 2009 [30] | Ciocca. L et al., 2010 [32] | De Crescenzio F et al., 2011 [33] | ||
| Were patient’s demographic characteristics clearly described? | No | No | Yes | No | Yes | No | Yes | No | |
| Was the patient’s history clearly described and presented as a timeline? | No | No | Yes | No | Yes | No | Yes | No | |
| Was the current clinical condition of the patient on presentation clearly described? | No | Yes | Yes | No | Yes | Yes | Yes | Yes | |
| Were diagnostic tests or assessment methods and the results clearly described? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Was the intervention(s) or treatment procedure(s) clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was the post-intervention clinical condition clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were adverse events (harms) or unanticipated events identified and described? | Yes | No | No | Yes | No | No | No | No | |
| Does the case report provide takeaway lessons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Overall appraisal | Included | Included | Included | Included | Included | Included | Included | Included | |
| (b) | |||||||||
| Assessment | Author and Year | ||||||||
| Liacouras et al., 2011 [34] | Kolodney et al., 2011 [35] | Bai et al., 2012 [37] | Reitemeier et al., 2012 [38] | Hatamleh and Watson. 2013 [39] | Wang et al., 2015 [42] | Nuseir et al., 2015 [43] | Weissler et al., 2017 [45] | ||
| Were patient’s demographic characteristics clearly described? | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | |
| Was the patient’s history clearly described and presented as a timeline? | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | |
| Was the current clinical condition of the patient on presentation clearly described? | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | |
| Were diagnostic tests or assessment methods and the results clearly described? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Was the intervention(s) or treatment procedure(s) clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was the post-intervention clinical condition clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were adverse events (harms) or unanticipated events identified and described? | No | No | Yes | No | No | No | No | No | |
| Does the case report provide takeaway lessons? | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | |
| Overall appraisal | Included | Included | Included | Included | Included | Included | Included | Included | |
| (c) | |||||||||
| Assessment | Author and Year | ||||||||
| Yadav et al., 2017 [46] | Nafij Bin Jamayet et al., 2018 [47] | Unkovskiy et al., 2018 [48] | Sanghavi, et al., 2018 [49] | Cevik and Kocacikli. 2020 [52] | Domingue D. et al., 2021 [54] | Unkovskiy et al., 2021 [55] | Dashti et al., 2022 [56] | Heydenrych A et al., 2023 [58] | |
| Were patient’s demographic characteristics clearly described? | Yes | No | Yes | Yes | Yes | Yes | No | No | No |
| Was the patient’s history clearly described and presented as a timeline? | Yes | No | Yes | Yes | Yes | Yes | No | No | No |
| Was the current clinical condition of the patient at presentation clearly described? | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Were diagnostic tests or assessment methods and the results clearly described? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
| Was the intervention(s) or treatment procedure(s) clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the post-intervention clinical condition clearly described? | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Were adverse events (harms) or unanticipated events identified and described? | No | Yes | No | No | No | No | Yes | Yes | No |
| Does the case report provide takeaway lessons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Overall appraisal | Included | Included | Included | Included | Included | Included | Included | Included | Included |
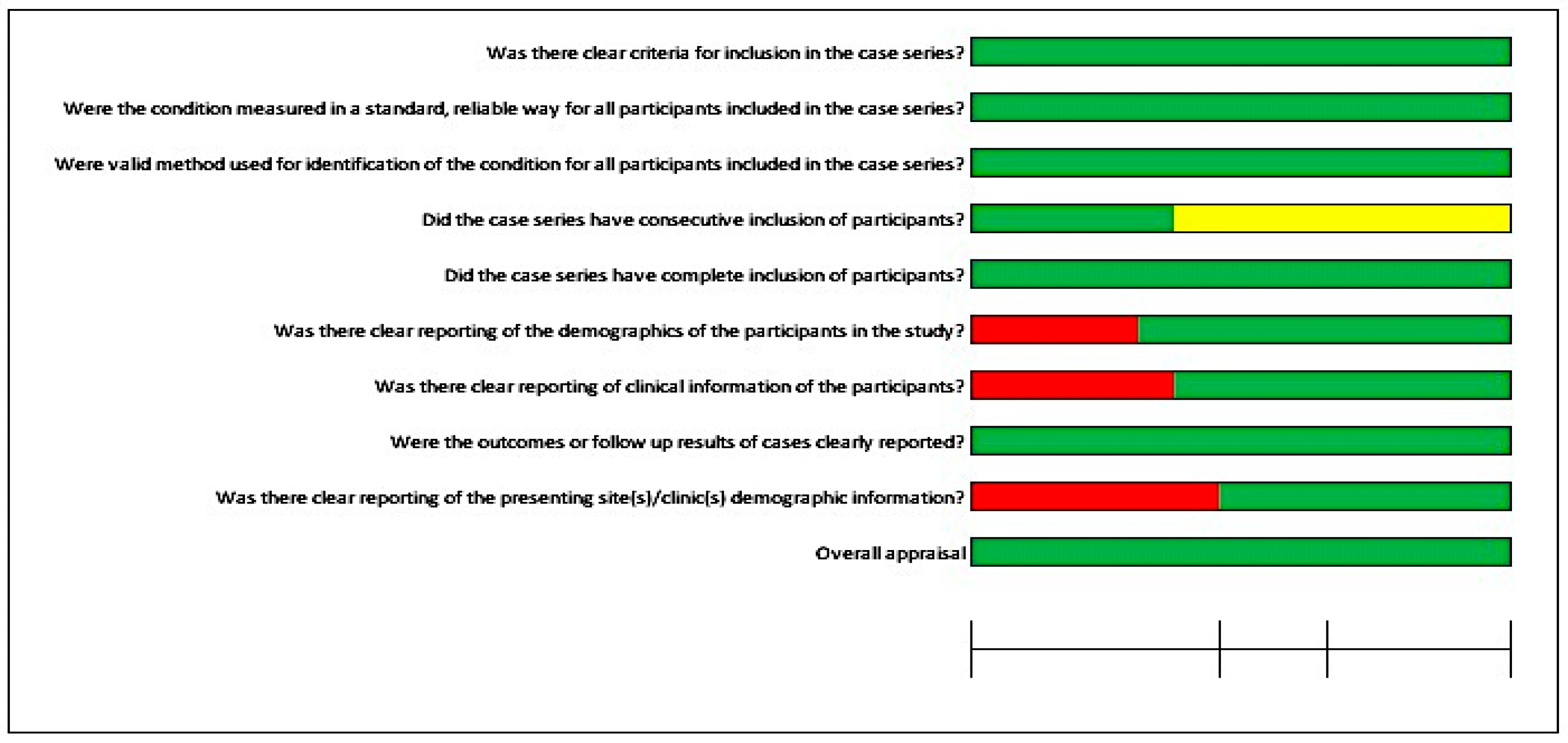
| (a) | |||||||
| Assessment | Author and Year | ||||||
| Turgut et al., 2009 [31] | Verma et al., 2010 [22] | Karatas MO et al., 2011 [36] | Bai et al., 2014 [40] | Tam CK et al., 2014 [8] | Watson and Hatamleh 2014 [41] | Choi et al., 2016 [44] | |
| Were there clear criteria for inclusion in the case series? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the condition measured in a standard, reliable way for all participants included in the case series? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were valid methods used for identification of the condition for all participants included in the case series? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Did the case series have consecutive inclusion of participants? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Did the case series have complete inclusion of participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was there clear reporting of the demographics of the participants in the study? | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Was there clear reporting of clinical information of the participants? | No | Yes | Yes | Yes | Yes | No | Yes |
| Were the outcomes or follow-up results of cases clearly reported? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | No | No | No | Yes | Yes | Yes | Yes |
| Overall appraisal | Included | Included | Included | Included | Included | Included | Included |
| (b) | |||||||
| Assessment | Author and Year | ||||||
| Ferreira R, Vives P. 2019 [50] | Vijverberg MA et al., 2019 [51] | McHutchion and Aalto. 2021 [53] | Hatamleh MM et al., 2022 [57] | ||||
| Were there clear criteria for inclusion in the case series? | Yes | Yes | Yes | Yes | |||
| Was the condition measured in a standard, reliable way for all participants included in the case series? | Yes | Yes | Yes | Yes | |||
| Were valid methods used for identification of the condition for all participants included in the case series? | Yes | Yes | Yes | Yes | |||
| Did the case series have consecutive inclusion of participants? | Unclear | Yes | Yes | Yes | |||
| Did the case series have complete inclusion of participants? | Yes | Yes | Yes | Yes | |||
| Was there clear reporting of the demographics of the participants in the study? | Yes | Yes | Yes | Yes | |||
| Was there clear reporting of clinical information of the participants? | Yes | No | Yes | Yes | |||
| Were the outcomes or follow up results of cases clearly reported? | Yes | Yes | Yes | Yes | |||
| Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | No | Yes | Yes | Yes | |||
| Overall appraisal | Included | Included | Included | Included | |||
3. Results
3.1. Study Selection
3.2. Study Characteristics
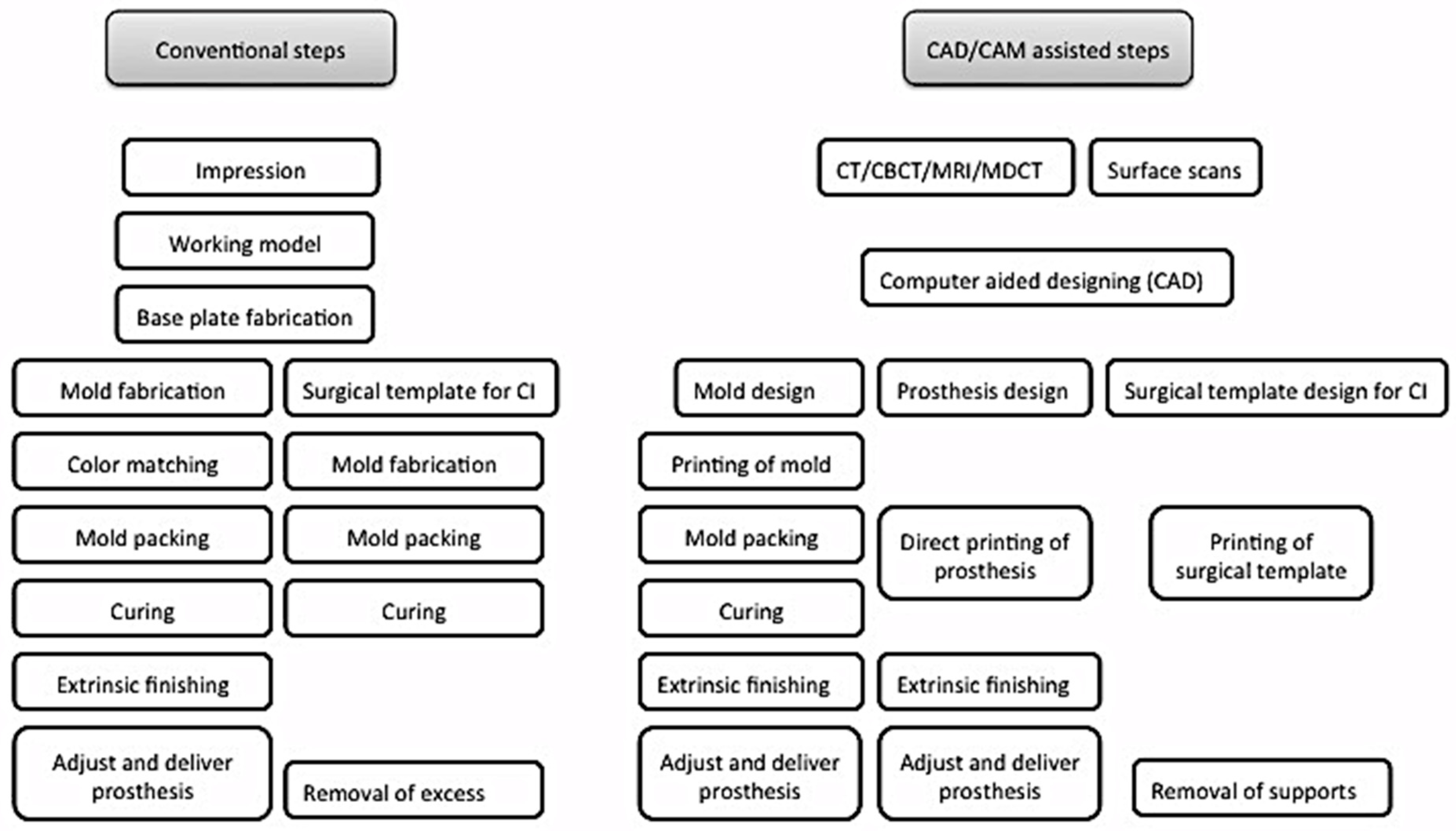
3.2.1. CAD/CAM Technology Applications for Prosthetic and Surgical Purposes
3.2.2. Preoperative Record for Digital Planning
3.2.3. Preoperative Record for Digital Designing
3.2.4. Printing Systems Utilized for Surgical and Prosthetic Phases
3.2.5. Guided Implant Surgery
3.3. Risks of Bias in Individual Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishimura, R.D.; Roumanas, E.; Sugai, T.; Moy, P.K. Auricular prostheses and osseointegrated implants: UCLA experience. J. Prosthet. Dent. 1995, 73, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Schwedtner, O.; Klein, M. A retrospective study of implant-retained auricular prostheses. Int. J. Oral Maxillofac. Implant. 2008, 23, 539–543. [Google Scholar]
- Visser, A.; Raghoebar, G.M.; Van Oort, R.P.; Vissink, A. Fate of implant-retained craniofacial prostheses: Life span and aftercare. Int. J. Oral Maxillofac. Implant. 2008, 23, 89–98. [Google Scholar]
- Aydin, C.; Karakoca, S.; Yilmaz, H.; Yilmaz, C. Implant-retained auricular prostheses: An assessment of implant success and prosthetic complications. Int. J. Prosthodont. 2008, 21, 241–244. [Google Scholar] [PubMed]
- Brent, B. Auricular repair with autogenous rib cartilage grafts: Two decades of experience with 600 cases. Plast Reconstr. Surg. 1992, 90, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, R.C. Total reconstruction of the external ear. Plast. Reconstr. Surg. Transplant Bull. 1959, 23, 1–15. [Google Scholar] [CrossRef]
- Thorne, C.H.M.; Brecht, L.E.D.; Bradley, J.P.M.; Levine, J.P.M.; Hammerschlag, P.M.; Longaker, M.T.M. Auricular Reconstruction: Indications for Autogenous and Prosthetic Techniques. Plast. Reconstr. Surg. 2001, 107, 1241–1251. [Google Scholar] [CrossRef]
- Tam, C.K.; McGrath, C.P.; Ho, S.M.Y.; Pow, E.H.N.; Luk, H.W.K.; Cheung, L.K. Psychosocial and Quality of Life Outcomes of Prosthetic Auricular Rehabilitation with CAD/CAM Technology. Int. J. Dent. 2014, 2014, 393571. [Google Scholar] [CrossRef]
- Gumieiro, E.H.; Dib, L.L.; Jahn, R.S.; Junior, J.F.d.S.; Nannmark, U.; Granström, G.; Abrahão, M. Bone-anchored titanium implants for auricular rehabilitation: Case report and review of literature. Sao Paulo Med. J. 2009, 127, 160–165. [Google Scholar] [CrossRef]
- Girod, S.C.; Rohlfing, T.; Maurer, C.R. Image-Guided Surgical Navigation in Implant-Based Auricular Reconstruction. J. Oral Maxillofac. Surg. 2008, 66, 1302–1306. [Google Scholar] [CrossRef]
- Williams, B.H.; Ochiai, K.T.; Baba, T.; A Caputo, A. Retention and load transfer characteristics of implant-retained auricular prostheses. Int. J. Oral Maxillofac. Implant. 2007, 22, 366–372. [Google Scholar]
- Lemon, J.C.; Chambers, M.S. Locking retentive attachment for an implant-retained auricular prosthesis. J. Prosthet. Dent. 2002, 87, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Waqas, T.S.; Shrestha, B.; Srithavaj, M.L.T.; Chotprasert, N. A two-step functional impression technique for the fabrication of an implant-retained silicone auricular prosthesis. J. Prosthet. Dent. 2017, 117, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meer, W.J.; Vissink, A.; Raghoebar, G.M.; Visser, A. Digitally designed surgical guides for placing extraoral implants in the mastoid area. Int. J. Oral Maxillofac. Implant. 2012, 27, 703–707. [Google Scholar]
- Datarkar, A.; Daware, S.; Dande, R.; Datarkar, U. Rehabilitation of unilateral congenital microtia by implant-retained prosthesis. Ann. Maxillofac. Surg. 2017, 7, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Ballo, A.M.; Nguyen, C.T.; Lee, V.S. Digital Workflow of Auricular Rehabilitation: A Technical Report Using an Intraoral Scanner. J. Prosthodont. 2019, 28, 596–600. [Google Scholar] [CrossRef]
- Kurtulmus, H.; Cotert, H.S.; Guneri, P. Computed tomography-based planning and three-dimensional modeling for craniofacial implant placement: A technical note. Int. J. Oral Maxillofac. Implant. 2009, 24, 943–946. [Google Scholar]
- Elbashti, M.; Sumita, Y.; Kelimu, S.; Aswehlee, A.; Awuti, S.; Hattori, M.; Taniguchi, H. Application of Digital Technologies in Maxillofacial Prosthetics Literature: A 10-Year Observation of Five Selected Prosthodontics Journals. Int. J. Prosthodont. 2019, 32, 45–50. [Google Scholar] [CrossRef]
- Kernen, F.; Kramer, J.; Wanner, L.; Wismeijer, D.; Nelson, K.; Flügge, T. A review of virtual planning software for guided implant surgery—Data import and visualization, drill guide design and manufacturing. BMC Oral Health 2020, 20, 251. [Google Scholar] [CrossRef]
- Ciocca, L.; Scotti, R. Oculo-facial rehabilitation after facial cancer removal: Updated CAD/CAM procedures: A pilot study. Prosthet. Orthot. Int. 2014, 38, 505–509. [Google Scholar] [CrossRef]
- Liu, H.; Bai, S.; Yu, X.; Zhao, Y. Combined use of a facial scanner and an intraoral scanner to acquire a digital scan for the fabrication of an orbital prosthesis. J. Prosthet. Dent. 2019, 121, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.N.; Schow, S.R.; Stone, B.H.; Triplett, R.G. Applications of surgical navigational systems for craniofacial bone-anchored implant placement. Int. J. Oral Maxillofac. Implants 2010, 25, 582–588. [Google Scholar] [PubMed]
- Nuseir, A.; Hatamleh, M.M.; Alnazzawi, A.; Al-Rabab’Ah, M.; Kamel, B.; Jaradat, E. Direct 3D Printing of Flexible Nasal Prosthesis: Optimized Digital Workflow from Scan to Fit. J. Prosthodont. 2019, 28, 10–14. [Google Scholar] [CrossRef]
- Moher DL, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Moola SA, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; Mu, P. Chapter 7: Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2017. [Google Scholar] [CrossRef]
- Ciocca, L.; Scotti, R. CAD-CAM generated ear cast by means of a laser scanner and rapid prototyping machine. J. Prosthet. Dent. 2004, 92, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Sykes, L.M.; Parrott, A.M.; Owen, C.P.; Snaddon, D.R. Applications of rapid prototyping technology in maxillofacial prosthetics. Int. J. Prosthodont. 2004, 17, 454–459. [Google Scholar] [PubMed]
- Jiao, T.; Zhang, F.; Huang, X.; Wang, C. Design and fabrication of auricular prostheses by CAD/CAM system. Int. J. Prosthodont. 2004, 17, 460–463. [Google Scholar] [PubMed]
- Ciocca, L.; Mingucci, R.; Gassino, G.; Scotti, R. CAD/CAM ear model and virtual construction of the mold. J. Prosthet. Dent. 2007, 98, 339–343. [Google Scholar] [CrossRef]
- Ciocca, L.; Mingucci, R.; Bacci, G.; Scotti, R. CAD-CAM construction of an auricular template for craniofacial implant positioning: A novel approach to diagnosis. Eur. J. Radiol. 2009, 71, 253–256. [Google Scholar] [CrossRef]
- Turgut, G.; Sacak, B.; Kiran, K.; Bas, L. Use of rapid prototyping in prosthetic auricular restoration. J. Craniofac. Surg. 2009, 20, 321–325. [Google Scholar] [CrossRef]
- Ciocca, L.; De Crescenzio, F.; Fantini, M.; Scotti, R. CAD/CAM bilateral ear prostheses construction for Treacher Collins syndrome patients using laser scanning and rapid prototyping. Comput. Methods Biomech. Biomed. Engin. 2010, 13, 379–386. [Google Scholar] [CrossRef]
- De Crescenzio, F.; Fantini, M.; Ciocca, L.; Persiani, F.; Scotti, R. Design and manufacturing of ear prosthesis by means of rapid prototyping technology. Proc. Inst. Mech. Eng. H. 2011, 225, 296–302. [Google Scholar] [PubMed]
- Liacouras, P.; Garnes, J.; Roman, N.; Petrich, A.; Grant, G.T. Designing and manufacturing an auricular prosthesis using computed tomography, 3-dimensional photographic imaging, and additive manufacturing: A clinical report. J. Prosthet. Dent. 2011, 105, 78–82. [Google Scholar] [CrossRef]
- Kolodney, H., Jr.; Swedenburg, G.; Taylor, S.S.; Carron, J.D.; Schlakman, B.N. The use of cephalometric landmarks with 3-dimensional volumetric computer modeling to position an auricular implant surgical template: A clinical report. J. Prosthet. Dent. 2011, 106, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Karatas, M.O.; Cifter, E.D.; Ozenen, D.O.; Balik, A.; Tuncer, E.B. Manufacturing implant supported auricular prostheses by rapid prototyping techniques. Eur. J. Dent. 2011, 5, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Bi, Y.; Dong, Y.; Feng, Z.; Zhao, Y. Computer-aided design/computer-aided manufacturing implant guide used in flapless surgery for auricular prosthesis. J. Oral Maxillofac. Surg. 2012, 70, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Reitemeier, B.; Schöne, C.; Schreiber, S.; Stockmann, F.; Ullmann, K.; Eckelt, U. Planning implant positions for an auricular prosthesis with digital data. J. Prosthet. Dent. 2012, 107, 128–131. [Google Scholar] [CrossRef]
- Hatamleh, M.M.; Watson, J. Construction of an implant-retained auricular prosthesis with the aid of contemporary digital technologies: A clinical report. J. Prosthodont. 2013, 22, 132–136. [Google Scholar] [CrossRef]
- Bai, S.-Z.; Feng, Z.-H.; Gao, R.; Dong, Y.; Bi, Y.-P.; Wu, G.-F.; Chen, X. Development and application of a rapid rehabilitation system for reconstruction of maxillofacial soft-tissue defects related to war and traumatic injuries. Mil. Med. Res. 2014, 1, 11. [Google Scholar] [CrossRef]
- Watson, J.; Hatamleh, M.M. Complete integration of technology for improved reproduction of auricular prostheses. J. Prosthet. Dent. 2014, 111, 430–436. [Google Scholar] [CrossRef]
- Wang, S.; Leng, X.; Zheng, Y.; Zhang, D.; Wu, G. Prosthesis-guided implant restoration of an auricular defect using computed tomography and 3-dimensional photographic imaging technologies: A clinical report. J. Prosthet. Dent. 2015, 113, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Nuseir, A.; Hatamleh, M.; Watson, J.; Al-Wahadni, A.M.; Alzoubi, F.; Murad, M. Improved Construction of Auricular Prosthesis by Digital Technologies. J. Craniofac. Surg. 2015, 26, e502–e505. [Google Scholar] [CrossRef]
- Choi, K.J.; Sajisevi, M.B.; McClennen, J.; Kaylie, D.M. Image-Guided Placement of Osseointegrated Implants for Challenging Auricular, Orbital, and Rhinectomy Defects. Ann. Otol. Rhinol. Laryngol. 2016, 125, 801–807. [Google Scholar] [CrossRef]
- Weissler, J.M.; Sosin, M.; Dorafshar, A.H.; Garcia, J.R. Combining Virtual Surgical Planning, Intraoperative Navigation, and 3-Dimensional Printing in Prosthetic-Based Bilateral Microtia Reconstruction. J. Oral. Maxillofac. Surg. 2017, 75, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Narayan, A.I.; Choudhry, A.; Balakrishnan, D. CAD/CAM-Assisted Auricular Prosthesis Fabrication for a Quick, Precise, and More Retentive Outcome: A Clinical Report. J. Prosthodont. 2017, 26, 616–621. [Google Scholar] [CrossRef]
- Jamayet, N.B.; Abdullah, J.Y.; Rahman, A.M.; Husein, A.; Alam, M.K. A fast and improved method of rapid prototyping for ear prosthesis using portable 3D laser scanner. J. Plast. Reconstr. Esthet. Surg. 2018, 71, 946–953. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Brom, J.; Huettig, F.; Keutel, C. Auricular Prostheses Produced by Means of Conventional and Digital Workflows: A Clinical Report on Esthetic Outcomes. Int. J. Prosthodont. 2018, 31, 63–66. [Google Scholar] [CrossRef]
- Sanghavi, R.V.; Shingote, S.D.; Abhang, T.N.; Thorat, P.R.; Vathare, A.S. An innovative technique for fabricating a mirror image wax pattern using three-dimensional printing technology for an auricular prosthesis. J. Res. Dent. Sci. 2018, 9, 91. [Google Scholar] [CrossRef]
- Ferreira, R.; Vives, P. Two auricular epithesis surgical cases retained by a custom titanium implant: Result at four years. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 147–151. [Google Scholar] [CrossRef]
- Vijverberg, M.A.; Verhamme, L.; van de Pol, P.; Kunst, H.P.M.; Mylanus, E.A.M.; Hol, M.K.S. Auricular prostheses attached to osseointegrated implants: Multidisciplinary work-up and clinical evaluation. Eur. Arch. Otorhinolaryngol. 2019, 276, 1017–1027. [Google Scholar] [CrossRef]
- Cevik, P.; Kocacikli, M. Three-dimensional printing technologies in the fabrication of maxillofacial prosthesis: A case report. Int. J. Artif. Organs. 2020, 43, 343–347. [Google Scholar] [CrossRef]
- McHutchion, L.; Aalto, D. Simulation of tissue-prosthesis margin interface by using surface scanning and digital design for auricular prostheses. J. Prosthet. Dent. 2021, 125, 361–372. [Google Scholar] [CrossRef]
- Domingue, D.; Glenn, N.C.; Vest, A.; White, J.R. Osseointegrated implant-retained auricular prosthesis constructed using cone-beam computed tomography and a prosthetically driven digital workflow: A case report. Clin. Case Rep. 2021, 9, 37–45. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Wahl, E.; Huettig, F.; Keutel, C.; Spintzyk, S. Multimaterial 3D printing of a definitive silicone auricular prosthesis: An improved technique. J. Prosthet. Dent. 2021, 125, 946–950. [Google Scholar] [CrossRef]
- Dashti, H.; Rajati Haghi, H.; Nakhaei, M.; Kiamanesh, E. A combined digital technique to fabricate an implant-retained auricular prosthesis for rehabilitation of hemifacial microsomia. J. Prosthet. Dent. 2022, 127, 807–810. [Google Scholar] [CrossRef]
- Hatamleh, M.M.; Watson, J.; Nuseir, A. Successful prosthetic salvage of a suboptimal autogenous auricular reconstruction with digital technologies: A report of 3 challenging treatments. J. Prosthet. Dent. 2022, 128, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Heydenrych, A.; van der Walt, J.G.; van den Heever, H.J. Auricular prosthesis positioning using virtual planning in combination with additive manufacturing. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101258. [Google Scholar] [CrossRef] [PubMed]
- Ewers, R.; Schicho, K.; Truppe, M.; Seemann, R.; Reichwein, A.; Figl, M.; Wagner, A. Computer-aided navigation in dental implantology: 7 years of clinical experience. J. Oral Maxillofac. Surg. 2004, 62, 329–334. [Google Scholar] [CrossRef]
- Mupparapu, M.; Singer, S.R. Implant imaging for the dentist. J. Can. Dent. Assoc. 2004, 70, 32. [Google Scholar] [PubMed]
- Tanveer, W.; Ridwan-Pramana, A.; Molinero-Mourelle, P.; Koolstra, J.H.; Forouzanfar, T. Systematic Review of Clinical Applications of CAD/CAM Technology for Craniofacial Implants Placement and Manufacturing of Nasal Prostheses. Int. J. Environ. Res. Public Health 2021, 18, 3756. [Google Scholar] [CrossRef]
- Tanveer, W.; Ridwan-Pramana, A.; Molinero-Mourelle, P.; Forouzanfar, T. Systematic Review of Clinical Applications of CAD/CAM Technology for Craniofacial Implants Placement and Manufacturing of Orbital Prostheses. Int. J. Environ. Res. Public Health 2021, 18, 11349. [Google Scholar] [CrossRef]
- Tahmaseb, A.; Wismeijer, D.; Coucke, W.; Derksen, W. Computer technology applications in surgical implant dentistry: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 25–42. [Google Scholar] [CrossRef]
- Jacobs, R.; Adriansens, A.; Verstreken, K.; Suetens, P.; van Steenberghe, D. Predictability of a three-dimensional planning system for oral implant surgery. Dentomaxillofac. Radiol. 1999, 28, 105–111. [Google Scholar] [CrossRef]
- Israelson, H.; Plemons, J.M.; Watkins, P.; Sory, C. Barium-coated surgical stents and computer-assisted tomography in the preoperative assessment of dental implant patients. Int. J. Periodontics Restor. Dent. 1992, 12, 52–61. [Google Scholar]
- Basten, C.H. The use of radiopaque templates for predictable implant placement. Quintessence Int. 1995, 26, 609–612. [Google Scholar]
- Mizrahi, B.; Thunthy, K.H.; Finger, I. Radiographic/surgical template incorporating metal telescopic tubes for accurate implant placement. Pract. Periodontics Aesthet. Dent. 1998, 10, 757–765. [Google Scholar]
- Whyms, B.J.; Vorperian, H.K.; Gentry, L.R.; Schimek, E.M.; Bersu, E.T.; Chung, M.K. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2013, 115, 682–691. [Google Scholar] [CrossRef]
- Taft, R.M.; Kondor, S.; Grant, G.T. Accuracy of rapid prototype models for head and neck reconstruction. J. Prosthet. Dent. 2011, 106, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Alauddin, M.S.; Baharuddin, A.S.; Mohd Ghazali, M.I. The Modern and Digital Transformation of Oral Health Care: A Mini Review. Healthcare 2021, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Miura, D.; Miyasaka, T.; Shinya, A. Dimensional Accuracy of Dental Casting Patterns Fabricated Using Consumer 3D Printers. Polymers 2020, 12, 2244. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.; Marti Marti, B.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Nayar, S.; Bhuminathan, S.; Bhat, W.M. Rapid prototyping and stereolithography in dentistry. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. 1), S216–S219. [Google Scholar] [CrossRef] [PubMed]


| Studies | Purpose | Digital Process | Convencional Process | |||
|---|---|---|---|---|---|---|
| Material | Cost | Time | No. of Appointments | Time | ||
| Sykes et al., 2004 [27] | Digitization of ear model and processing in Freeform software for rapid prototyping of mirrored ear model | Wax | NM | 4 h | NM | 6 h |
| Jiao et al., 2004 [28] | Printing and finishing of ear prosthesis | NM | NM | 1.5 h | NM | NM |
| Ciocca L et al., 2007 [29] | Computer-aided design and rapid prototyping of auricular mold and substructure | Resin | 15$ | NM | 3 | NM |
| Ciocca. L et al., 2010 [32] | Computer-aided design and rapid prototyping of auricular molds and substructures for bilateral auricular prostheses | —ABS | 36.58€ | 10 h 42 min | NM | NM |
| De Crescenzio F et al., 2011 [33] | Computer -aided design and rapid prototyping of auricular mold and substructure | ABS | 23.79€ | 6 h 39 min | 3 | NM |
| Watson and Hatamleh 2014 [41] | Scanning, manipulation, RP build, and finishing time for wax prototype | Wax | 58$ | 40 min | NM | 2 h |
| Bai et al., 2014 [40] | Clinical time spentComputer-aided design and rapid prototyping of auricular molds | Resin | NM | 4 h10 h | 2 | NM |
| Included Articles | Outcome | Recommendations | Limitations |
|---|---|---|---|
| Ciocca L, Scotti R. 2004 [26] | The stone cast of existing ears was scanned and mirrored with the help of software to the affected side. A 3D printer helped print the resin model of the ear for the affected side. | The technique utilized in this clinical case is faster; it takes about 8 h. Once the equipment is available, the cost of individual procedures can be less expensive as compared to the anaplastologist’s work. | The limitation of this clinical technique is the inability to reciprocate the color information, and the equipment cost can be expensive. |
| Sykes et al., 2004 [27] | Digital technology provided the way to obtain an accurate wax model in a reasonable short time as compared to conventional methodology for the rehabilitation of auricular defects. | External imaging methods can provide better accuracy than CT scan images, especially in cases of complex ear anatomy. Additionally, it does not require volumetric modeling interpolation between the slices. | High cost of equipment and a trained laboratory technician for digital designing and printing procedures. |
| Jiao et al., 2004 [28] | The shape, dimension, and anatomic contour of the ear prosthesis matched the normal side and fitted well on the defect side. | The Magics RP (Materilise) program permits the mirrored ear position to move anteroposterior superioinferior, projections from the surface to match the unaffected side precisely. | According to the authors, improvement of the technique is required. |
| Ciocca L et al., 2007 [29] | The described protocol enabled the fabrication of an ear prosthesis in three appointments through the construction of a virtual CAD/CAM model, a mold, and digitization of the implant location. | This protocol allows the scanning and replacement of a lost ear virtually without the need for a diagnostic wax pattern. | The limitations are the technical skills required to use CAD/CAM technology and the relevant costs of laboratory equipment, including 3D scanners and rapid prototyping machines. |
| Kurtulmus et al., 2009 [17] | The prosthetically driven implant’s location was determined by mirroring the normal ear to the defect side and the virtual construction of the model. The prosthesis was processed and fitted in a conventional way. | NM | NM |
| Ciocca L et al., 2009 [30] | The prosthetically driven implant’s locations were determined. A surgical template was printed to place the implants in the planned locations. | The location of landmarks during a CT scan and the duplication of the diagnostic template can induce errors; therefore, the authors recommend their protocol for the correct diagnosis of available bone and the precise transfer of planning to the surgical template. | NM |
| Turgut et al., 2009 [31] | Auricular prostheses were manufactured through mirroring of the contralateral normal ear and rapid prototyping of a 3D ear model. Digital technology helped complete the prosthetic rehabilitation with only one trial session. | The auricular prosthesis procedure was cost-effective, required fewer patient visits, and eliminated the chances of sculpturing errors by utilizing rapid prototyping methodologies. | The protocol described here is only applicable to patients with one missing ear. According to the authors, in the case of bilateral auricular atresia, esthetic ratios of facial features can be used as a guideline to design prostheses. |
| Ciocca. L et al., 2010 [32] | Bilateral auricular defects were rehabilitated by selecting a reference ear from the digital ear and nose library. The mold and substructure were designed virtually and rapidly prototyped for silicone packing to obtain bilateral auricular prostheses. | The Ear and Nose Digital Library is a useful tool if the patient has bilateral auricular defects. Mirroring a selected reference ear from a digital library can provide identical bilateral auricular prostheses. | The presence of a stair-case effect on the superficial surface is one limitation that can be minimized by using thin printing layers or using surface finishers conventionally, following construction of models or molds. |
| Verma et al., 2010 [22] | A surgical navigation system was used to plan and place the craniofacial implants in two patients without any reported complications. Prostheses were fabricated in a conventional way. | The authors recommend this technique as it is virtually possible to visualize future prostheses, which helps surgeons alter the soft tissues prior to or during surgery without the necessity to keep a soft tissue profile for soft tissue registered surgical templates. | NM |
| De Crescenzio F et al., 2011 [33] | A three-piece mold was designed and 3D-constructed by means of CAD/CAM technology. Precise positioning of the substructure was attained in the prototype mold. Molds are fitted precisely with silicone packing. | This technique reduced the time and cost considerably when compared to conventional procedures and anaplastologist services. To reduce the stair-case effect, mold parts were oriented along the vertical axis of the prosthesis (from bottom to top). | NM |
| Liacouras et al., 2011 [34] | A mold for an auricular prosthesis was designed and printed using rapid prototyping technology for silicone packing. A three-piece mold fitted well to the fabricated silicone auricular prosthesis. | Rapid prototyping material is usually devoid of color; therefore, it prevents contamination, which is the issue with colored stone molds. A three-piece mold is recommended for ease of recovery, positioning of the seam at a favorable spot, and ease of placement of the retentive substructure. | Laboratory technicians can induce very limited surface texture in 3D printed molds; therefore, it is difficult to produce that surface texture in a finished prosthesis. Furthermore, the cost and technical skills required to use CAD/CAM technology are the limiting factors. |
| Kolodney et al., 2011 [35] | Cephalometric analysis was used to plan the implant’s locations digitally and transferred into a physical ear model by rapid prototyping. The planned implant locations were then copied into a surgical template for craniofacial implant placement. | The authors recommend this technique of using cephalometric analysis to locate the implant’s location in patients who are facially symmetric. It is not applicable to craniofacial anomaly cases. | Despite the advantages of this technique, it is still necessary to place the physical wax pattern on the patient to verify the shape and position before proceeding to process it with silicone to fabricate a prosthesis. |
| Karatas MO et al., 2011 [36] | Intact ears were mirrored onto the defect side. Rapid prototyping technology was used to construct the ear models. Following the duplication of models, auricular prostheses were fabricated in a conventional way. | The authors suggest scanning and mirroring the intact side to the defect side, as these steps eliminate the potential errors from impression and wax carving. | The skilled laboratory technologist and cost of designing and printing equipment pose limitations to this technique. |
| Bai et al., 2012 [37] | The surgical template was digitally designed to place the auricular implants. A maxillary occlusal splint was connected to a surgical template to stabilize it, and implants were placed through flapless surgery. | The authors recommend this technique to design the surgical template for implant placement as it permits flapless surgery and precise implant placement, reducing the procedure time and morbidity. | This technique can only be used on dentate patients, as the surgical template needs to be stabilized with an occlusal splint. |
| Reitemeier et al., 2012 [38] | The surgical template for implant placement was designed by mirroring the intact ear to the defect side. The glabella and nose were used to stabilize the surgical template during the surgical procedure. | This approach facilitates the design of a surgical template for implant placement, which guides the symmetrical positioning of an auricular prosthesis. | The additional cost of a surgical template for implant placement can pose a limitation to this technique. |
| Hatamleh and Watson. 2013 [39] | The surgical template for implant placement was designed by mirroring the intact ear to the defect side. The ear model was printed and duplicated to adapt to the working cast for precise positioning during implant placement. Two implants were placed at the planned position in the mastoid bone. | Digital mirroring and printing the ear saved time, which is usually spent on wax carving. Mirrored images were saved in a computer system, which can be used to reprint the ear model for future prostheses, thus saving the patient’s visit and storage space. | NM |
| Bai et al., 2014 [40] | Intact ears were mirrored on the defect side and used to create a negative three-piece mold for silicone packing. Prostheses fitted well in all cases, and patients showed thorough satisfaction. | With this technique, the try-in step can be eliminated, thereby reducing the patient’s visits. Furthermore, by eliminating manual flasking and investing procedures. The overall time of fabrication was significantly reduced. | An objective investigation of patient satisfaction is required to evaluate the acceptance of prostheses following this digital technique. |
| Tam CK et al., 2014 [8] | The intact ear was mirrored on the defect side to obtain the prototyped model. A surgical navigation system was utilized to place the craniofacial implants. The delivered prostheses had good retention, stability, and a symmetrical position. Psychological assessment showed decreased depressive symptoms and positive emotions. | Computer-assisted planning and surgical navigation systems have been recommended by the authors. CAD software helps in preoperative planning, while surgical navigation systems improve intraoperative safety and prevent damage to critical anatomic structures. | The authors encountered problems with reduced retention of prostheses and discoloration or color mismatch of silicone auricular prostheses. |
| Watson and Hatamleh 2014 [41] | The intact ear was mirrored on the defect side and prototyped to obtain a 3D model. The obtained model was copied into a wax pattern and adjusted in a trial session on the patient. Mirroring the ear model produced an accurate shape and form, comparable to the intact normal ear. | The presented technique required less clinic time. Scanning, manipulation, rapid prototyping, and finishing the wax prototype took 40 min, while an average ear wax pattern requires 2 h. | The limitation of this technique is the cost of software and hardware use, maintenance, and trained staff to operate these digital systems. |
| Wang et al., 2015 [42] | The normal ear was mirrored on the defect side, and a surgical template was designed to place three implants on the defect side. The ear model was printed and duplicated into a wax pattern for trial and laboratory processing. | Remnants of the malformed ear should be removed to facilitate the correct position and enhance the stability of the prosthesis. | NM |
| Nuseir et al., 2015 [43] | The normal ear was mirrored on the defect side, and a surgical template was designed to place two implants on the defect side. The ear model was printed and duplicated into a wax pattern for trial and laboratory processing. | This technique saved time and reduced the patient’s visits. Additionally, current data would be useful for future fabrication of prostheses without the patient’s physical presence. | NM |
| Choi et al., 2016 [44] | An intraoperative navigation system was used to place two craniofacial implants at the auricular defect location. The trajectory of the implants was confirmed at the planned location by using a navigation probe. An implant-retained ear prosthesis was fabricated without complications. | The intraoperative navigation system provides real-time navigation into the localized anatomy on the backdrop of multiplanar views. This system is recommended by the authors for patients with altered anatomy and limited bone availability. | According to the authors, this study is limited by the number of cases and the study design. The goal of this study was to highlight management principles for patients with altered anatomy only. |
| Weissler et al., 2017 [45] | The father’s ear was scanned and mirrored on the defect side for implant planning. An intraoperative navigation system was used to place three implants bilaterally, while two implants on each side were used for prostheses retention without any reported complications. | The intraoperative navigation system helps to execute the planned cases precisely, especially in failed auricular reconstruction attempts or complex cases with limited bone availability. | NM |
| Yadav et al., 2017 [46] | The normal ear was mirrored on the defect side and used to design the mold for silicone packing. The prosthesis recovered from a 3D-printed mold was adjusted and delivered. The patient was satisfied with the final outcome. | The scanning of soft tissues prevents the distortion that is inevitable with conventional impression techniques. A digitally designed prosthesis is a more accurate procedure. The mold prepared by using this reported technique can be used multiple times to pack silicone. | The technique used in this case requires expensive equipment and a trained computer graphic designer, which ultimately increases the cost of a prosthesis. |
| Nafij Bin Jamayet et al., 2018 [47] | The intact ear was mirrored on the defect side and printed to obtain a 3D ear model. The model was duplicated into a wax pattern and processed with conventional techniques to fabricate an auricular prosthesis. | The digital system used in this study was portable, easy to use, saved clinic time, and produced an exact replica of a normal ear for the defect side. | According to the authors, this technique lacks the ability to procedure silicone prostheses with proper color match with adjacent skin. |
| Unkovskiy et al., 2018 [48] | The normal ear was mirrored on the defect side and processed with two different techniques: direct mold making (DMM) and indirect mold making (IMM). The authors concluded that IMM is the preferred technique, as the 3D-printed model, being an exact replica of a normal ear, can be duplicated for the trial step before laboratory processing. | According to the authors, the IMM technique is preferred over the DMM technique, as the 3D-printed model, being an exact replica of a normal ear, can be duplicated for a trial step before laboratory processing. Hence, a more predictable outcome can be achieved. | Anterior marginal fit was compromised following the DMM-derived prosthesis and the IMM-derived prototype model. However, following the IMM technique, the duplicated wax pattern was well adapted during the trial session, which improved the final outcome. |
| Sanghavi, et al., 2018 [49] | The intact ear was scanned and mirrored on the defect side to obtain the 3D-printed model. The model was duplicated into a wax pattern for the trial phase and processed conventionally. | This technique provides an accurate shape and form of the ear, which can be duplicated and processed in a conventional way, thereby making it an economical solution when compared to other 3D printing techniques. | According to the authors, the duplication step can induce errors such as distortion of the wax pattern, which would limit the accuracy and esthetics of the final prosthesis. |
| Ferreira R, Vives P. 2019 [50] | Custom plates with three locator attachment positions were designed digitally and printed with grade two titanium material. During the surgical procedure, the plates were screwed on the mastoid bone and used to retain ear prostheses without any reported complications. | The use of customized titanium plates can eliminate the problem of limited bone availability for craniofacial implants. This surgical procedure is safe and faster when compared to the placement of implants to retain facial prostheses. | NM |
| Vijverberg MA et al., 2019 [51] | A total of 31 implants were placed in 12 patients after digital planning and the design of a surgical template. None of these implants failed. GBI displayed positive scores from patients’ responses. | By digitally planning and designing surgical templates, higher accuracy and precise placement of craniofacial implants can be achieved. It further helps anaplastologists design the auricular prosthesis without compromising the retentive attachment position. | The study was from retrospective data; therefore, there might be missing data from patients’ records. Furthermore, Holger’s scores were not mentioned in two cases. |
| Cevik and Kocacikli. 2020 [52] | The intact ear was mirrored on the defect side. A negative mold was designed digitally and printed for silicone packing. This procedure reduced the fabrication steps, and the fabricated prosthesis fit well on the patient. | This technique saves a number of laboratory and clinical steps by mirroring the intact ear to the defect side and fabricating the mold without conventional wax and flasking procedures. | NM |
| McHutchion and Aalto. 2021 [53] | A total of five patients were scanned for ear defects, and scan bodies were designed and printed to fit on the implants. The intact ear model was scanned and mirrored on the defect side. Molds were designed and printed for silicone packing to obtain auricular prostheses. Two prostheses fit well, while the remaining prostheses had open margins against adjacent tissues. | This digital workflow can be used as the starting point for future planning and design of facial prostheses, following some improvements. | Two prostheses had a poor fit against the tissues. The third prosthesis fit well in some areas; however, a large gap was noticed along the upper edge of the prosthesis. Test prostheses exerted less pressure on underlying tissues, and surface details were lacking. |
| Domingue D. et al., 2021 [54] | Craniofacial implants were planned with digital planning software to be placed in the right mastoid location for an auricular prosthesis. Implants were precisely placed at the planned location with no clinically reported complications. | This technique prevents damage to the critical underlying tissues and optimizes the prosthetically driven approach to implant placement with precise angulation and depth. | NM |
| Unkovskiy et al., 2021 [55] | A silicone auricular prosthesis was printed with various shore A hardness of the silicone. External staining, sealing, and finishing were performed conventionally. The anterior margin was further adjusted with conventional silicone to blend with adjacent skin. | Through proper digital workflow and mirror imaging techniques, the precise size, shape, and position of the auricular prosthesis can be achieved. | The printed silicone prosthesis lacked surface details due to limited printing resolution. Furthermore, grinding with abrasive paper to create a staircase effect further made the silicone surface smooth. |
| Dashti et al., 2022 [56] | The intact ear was mirrored on the defect side to obtain the exact size and shape of the contralateral ear. Stereolithographic casts, substructures, and milled bars were obtained following virtual planning and design. The wax pattern was copied and processed conventionally to fabricate an auricular prosthesis. | This technique provides a stable working cast without the need for convectional impression steps. | The limitation of this technique was the scanner’s inability to record functional movements. It did not affect the outcome in the present study, but it can be a limitation in cases of excessive tissue movement during function. |
| Hatamleh MM et al., 2022 [57] | Implants were guided through surgical templates produced by using 3D planning software. | Symmetry is relatively easier to achieve using 3D planning software in cases of bilaterally missing ears. | NM |
| Heydenrych A et al., 2023 [58] | A surgical template to guide the implants and a template to orient the auricular prosthesis were designed and manufactured with rapid prototyping techniques. | The authors recommend this technique as it eases implant placement, saves time, and helps in the orientation of the auricular prosthesis in relation to the implants. | Implants were deviated in relation to the planned implant position by an average of 4.67 mm. The orientation guide helped overcome the discrepancy in implant position. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanveer, W.; Ridwan-Pramana, A.; Molinero-Mourelle, P.; Forouzanfar, T. Applications of CAD/CAM Technology for Craniofacial Implants Placement and Manufacturing of Auricular Prostheses—Systematic Review. J. Clin. Med. 2023, 12, 5950. https://doi.org/10.3390/jcm12185950
Tanveer W, Ridwan-Pramana A, Molinero-Mourelle P, Forouzanfar T. Applications of CAD/CAM Technology for Craniofacial Implants Placement and Manufacturing of Auricular Prostheses—Systematic Review. Journal of Clinical Medicine. 2023; 12(18):5950. https://doi.org/10.3390/jcm12185950
Chicago/Turabian StyleTanveer, Waqas, Angela Ridwan-Pramana, Pedro Molinero-Mourelle, and Tymour Forouzanfar. 2023. "Applications of CAD/CAM Technology for Craniofacial Implants Placement and Manufacturing of Auricular Prostheses—Systematic Review" Journal of Clinical Medicine 12, no. 18: 5950. https://doi.org/10.3390/jcm12185950
APA StyleTanveer, W., Ridwan-Pramana, A., Molinero-Mourelle, P., & Forouzanfar, T. (2023). Applications of CAD/CAM Technology for Craniofacial Implants Placement and Manufacturing of Auricular Prostheses—Systematic Review. Journal of Clinical Medicine, 12(18), 5950. https://doi.org/10.3390/jcm12185950








