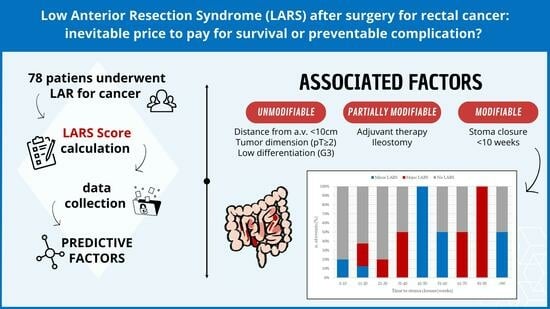
Low Anterior Resection Syndrome (LARS) after Surgery for Rectal Cancer: An Inevitable Price to Pay for Survival, or a Preventable Complication?
Abstract
:1. Introduction
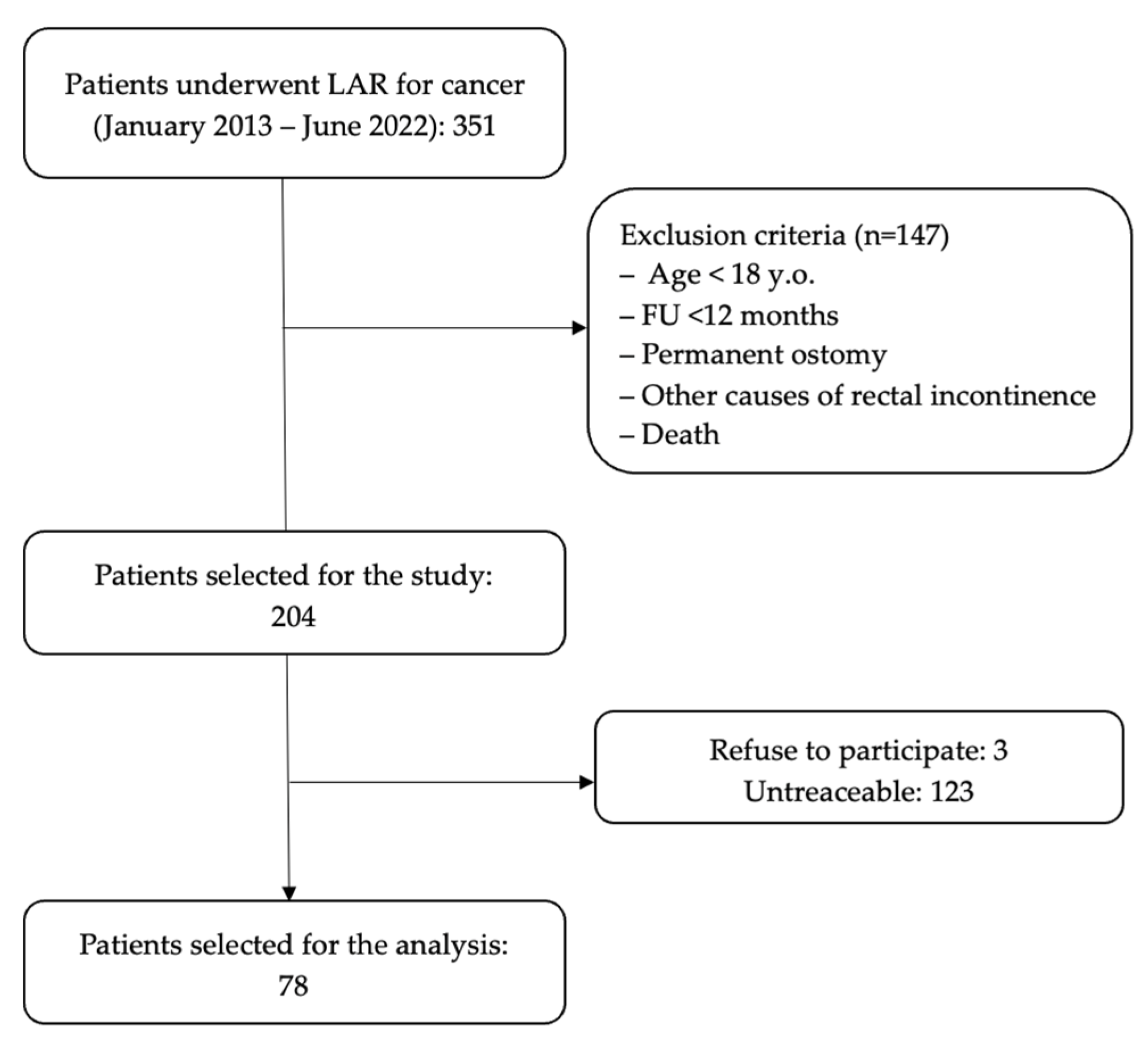
2. Materials and Methods
2.1. Statistical Analysis
2.2. LARS Score
- No LARS (L0): 0–20;
- Minor LARS (mL): 21–29;
- Major LARS (ML): 30–42.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Heald, R.J.; Ryall, R.D. Recurrence and Survival after Total Mesorectal Excision for Rectal Cancer. Lancet 1986, 1, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, W.; Marijnen, C.A.M.; Nagtegaal, I.D.; Kranenbarg, E.M.-K.; Putter, H.; Wiggers, T.; Rutten, H.J.T.; Påhlman, L.; Glimelius, B.; van de Velde, C.J.H.; et al. Preoperative Radiotherapy Combined with Total Mesorectal Excision for Resectable Rectal Cancer: 12-Year Follow-up of the Multicentre, Randomised Controlled TME Trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.L.C.; Lunniss, P.J.; Knowles, C.H.; Thaha, M.A.; Chan, C.L.H. Anterior Resection Syndrome. Lancet Oncol. 2012, 13, e403–e408. [Google Scholar] [CrossRef]
- Keane, C.; Wells, C.; O’Grady, G.; Bissett, I.P. Defining Low Anterior Resection Syndrome: A Systematic Review of the Literature. Color. Dis. 2017, 19, 713–722. [Google Scholar] [CrossRef]
- Keane, C.; Fearnhead, N.S.; Bordeianou, L.G.; Christensen, P.; Basany, E.E.; Laurberg, S.; Mellgren, A.; Messick, C.; Orangio, G.R.; Verjee, A.; et al. International Consensus Definition of Low Anterior Resection Syndrome. Color. Dis. 2020, 63, 274–284. [Google Scholar] [CrossRef]
- Denlinger, C.S.; Barsevick, A.M. The Challenges of Colorectal Cancer Survivorship. J. Natl. Compr. Cancer Netw. JNCCN 2009, 7, 883–893; quiz 894. [Google Scholar] [CrossRef]
- Varghese, C.; Wells, C.I.; Bissett, I.P.; O’Grady, G.; Keane, C. The Role of Colonic Motility in Low Anterior Resection Syndrome. Front. Oncol. 2022, 12, 975386. [Google Scholar] [CrossRef]
- Emmertsen, K.J.; Laurberg, S. Low Anterior Resection Syndrome Score: Development and Validation of a Symptom-Based Scoring System for Bowel Dysfunction After Low Anterior Resection for Rectal Cancer. Ann. Surg. 2012, 255, 922–928. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2020, 18, 806–815. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Croese, A.D.; Lonie, J.M.; Trollope, A.F.; Vangaveti, V.N.; Ho, Y.-H. A Meta-Analysis of the Prevalence of Low Anterior Resection Syndrome and Systematic Review of Risk Factors. Int. J. Surg. 2018, 56, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.L.; Cornish, J.; Morris, C.; on behalf of the LARRIS Trial Management Group. Functional Outcome following Rectal Surgery—Predisposing Factors for Low Anterior Resection Syndrome. Int. J. Color. Dis. 2017, 32, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Ekkarat, P.; Boonpipattanapong, T.; Tantiphlachiva, K.; Sangkhathat, S. Factors Determining Low Anterior Resection Syndrome after Rectal Cancer Resection: A Study in Thai Patients. Asian J. Surg. 2016, 39, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Bondeven, P.; Emmertsen, K.J.; Laurberg, S.; Pedersen, B.G. Neoadjuvant Therapy Abolishes the Functional Benefits of a Larger Rectal Remnant, as Measured by Magnetic Resonance Imaging after Restorative Rectal Cancer Surgery. Eur. J. Surg. Oncol. EJSO 2015, 41, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Hain, E.; Manceau, G.; Maggiori, L.; Mongin, C.; Prost À La Denise, J.; Panis, Y. Bowel Dysfunction after Anastomotic Leakage in Laparoscopic Sphincter-Saving Operative Intervention for Rectal Cancer: A Case-Matched Study in 46 Patients Using the Low Anterior Resection Score. Surgery 2017, 161, 1028–1039. [Google Scholar] [CrossRef]
- Carrillo, A.; Enríquez-Navascués, J.M.; Rodríguez, A.; Placer, C.; Múgica, J.A.; Saralegui, Y.; Timoteo, A.; Borda, N. Incidencia y caracterización del síndrome de resección anterior de recto mediante la utilización de la escala LARS (low anterior resection score). Cir. Esp. 2016, 94, 137–143. [Google Scholar] [CrossRef]
- Sturiale, A.; Martellucci, J.; Zurli, L.; Vaccaro, C.; Brusciano, L.; Limongelli, P.; Docimo, L.; Valeri, A. Long-Term Functional Follow-up after Anterior Rectal Resection for Cancer. Int. J. Color. Dis. 2017, 32, 83–88. [Google Scholar] [CrossRef]
- Li, X.; Fu, R.; Ni, H.; Du, N.; Wei, M.; Zhang, M.; Shi, Y.; He, Y.; Du, L. Effect of Neoadjuvant Therapy on the Functional Outcome of Patients with Rectal Cancer: A Systematic Review and Meta-Analysis. Clin. Oncol. 2023, 35, e121–e134. [Google Scholar] [CrossRef]
- Sun, R.; Dai, Z.; Zhang, Y.; Lu, J.; Zhang, Y.; Xiao, Y. The Incidence and Risk Factors of Low Anterior Resection Syndrome (LARS) after Sphincter-Preserving Surgery of Rectal Cancer: A Systematic Review and Meta-Analysis. Support. Care Cancer 2021, 29, 7249–7258. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Huang, M.; Huang, Y.; Yu, K.; Wang, X. Risk Factors of Postoperative Low Anterior Resection Syndrome for Colorectal Cancer: A Meta-Analysis. Asian J. Surg. 2022, 45, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Nicotera, A.; Falletto, E.; Arezzo, A.; Mistrangelo, M.; Passera, R.; Morino, M. Risk Factors for Low Anterior Resection Syndrome (LARS) in Patients Undergoing Laparoscopic Surgery for Rectal Cancer. Surg. Endosc. 2022, 36, 6059–6066. [Google Scholar] [CrossRef] [PubMed]
- Kauff, D.W.; Koch, K.P.; Somerlik, K.H.; Heimann, A.; Hoffmann, K.P.; Lang, H.; Kneist, W. Online Signal Processing of Internal Anal Sphincter Activity during Pelvic Autonomic Nerve Stimulation: A New Method to Improve the Reliability of Intra-Operative Neuromonitoring Signals: Online Signal Processing of Internal Anal Sphincter Activity. Color. Dis. 2011, 13, 1422–1427. [Google Scholar] [CrossRef]
- Filips, A.; Haltmeier, T.; Kohler, A.; Candinas, D.; Brügger, L.; Studer, P. LARS Is Associated with Lower Anastomoses, but Not with the Transanal Approach in Patients Undergoing Rectal Cancer Resection. World J. Surg. 2021, 45, 873–879. [Google Scholar] [CrossRef]
- Yan, M.; Lin, Z.; Wu, Z.; Zheng, H.; Shi, M. A Predictive Nomogram Model for Low Anterior Resection Syndrome after Rectal Cancer Resection. ANZ J. Surg. 2022, 92, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Brescia, A.; Muttillo, E.M.; Angelicone, I.; Madaffari, I.; Maggi, F.; Sperduti, I.; Gasparrini, M.; Osti, M.F. The Role of Indocyanine Green in Laparoscopic Low Anterior Resections for Rectal Cancer Previously Treated with Chemo-Radiotherapy: A Single-Center Retrospective Analysis. Anticancer Res. 2022, 42, 211–216. [Google Scholar] [CrossRef]
- Ahmad, N.Z.; Abbas, M.H.; Khan, S.U.; Parvaiz, A. A Meta-Analysis of the Role of Diverting Ileostomy after Rectal Cancer Surgery. Int. J. Color. Dis. 2021, 36, 445–455. [Google Scholar] [CrossRef]
- Niu, L.; Wang, J.; Zhang, P.; Zhao, X. Protective Ileostomy Does Not Prevent Anastomotic Leakage after Anterior Resection of Rectal Cancer. J. Int. Med. Res. 2020, 48, 030006052094652. [Google Scholar] [CrossRef]
- Mu, Y.; Zhao, L.; He, H.; Zhao, H.; Li, J. The Efficacy of Ileostomy after Laparoscopic Rectal Cancer Surgery: A Meta-Analysis. World J. Surg. Oncol. 2021, 19, 318. [Google Scholar] [CrossRef]
- Vogel, I.; Reeves, N.; Tanis, P.J.; Bemelman, W.A.; Torkington, J.; Hompes, R.; Cornish, J.A. Impact of a Defunctioning Ileostomy and Time to Stoma Closure on Bowel Function after Low Anterior Resection for Rectal Cancer: A Systematic Review and Meta-Analysis. Tech. Coloproctology 2021, 25, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Parnasa, S.Y.; Chill, H.; Helou, B.; Cohen, A.; Alter, R.; Shveiky, D.; Mizrahi, M.I.; Abu-Gazala, A.; Pikarsky, J.; Shussman, N. Low anterior resection syndrome following rectal cancer surgery: Are incidence and severity lower with long-term follow-up? Tech. Coloproctology 2022, 26, 981–989. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, J.; Wang, R.; Li, L.; Shi, L.; Ren, D.; Wang, J.; Deng, Y.; Dou, R. Impact of long-course neoadjuvant radiation on postoperative low anterior resection syndrome and stoma status in rectal cancer: Long-term functional follow-up of a randomized clinical trial. BJS Open 2022, 6, zrac127. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Category | Value (n = 78) Median (IQR) or n (%) |
|---|---|---|
| Age, years | 65 (57, 73) | |
| Sex | Female | 30 (38%) |
| Male | 48 (62%) | |
| ASA | I | 4 (5%) |
| II | 46 (59%) | |
| III | 28 (36%) | |
| IV | 0 (0%) | |
| Distance from anal verge | 0–5 cm | 10 (13%) |
| 5–10 cm | 30 (38%) | |
| 10–15 cm | 38 (49%) | |
| TNM Stage | 0 | 5 (6.4%) |
| 1 | 20 (26%) | |
| 2a | 14 (18%) | |
| 2b | 3 (3.8%) | |
| 3a | 6 (7.7%) | |
| 3b | 21 (27%) | |
| 3c | 6 (7.7%) | |
| 4a | 3 (3.8%) |
| Parameter | Category | Value (n = 78) Mean (IQR) or n (%) |
|---|---|---|
| Surgical technique | Open | 53 (68%) |
| VLS | 25 (32%) | |
| Operative time, min | Median (IQR) | 210 (180, 248) |
| Ileostomy | Yes | 29 (37%) |
| No | 49 (63%) | |
| Stoma closure, weeks | Median (IQR) | 25 (13, 51) |
| nRT | LCRT | 24 (31%) |
| SCRT | 4 (5%) | |
| No | 50 (64%) | |
| Adjuvant therapy | CHT | 6 (8%) |
| RT | 1 (1%) | |
| No | 69 (90%) | |
| Morbidity | Yes | 11 (17%) |
| No | 58 (83%) | |
| Clavien-Dindo Score | 1 | 3 (4%) |
| 2 | 4 (6%) | |
| 3a | 1 (1%) | |
| 3b | 4 (6%) | |
| LARS Score | No LARS | 54 (69%) |
| Minor LARS | 13 (17%) | |
| Major LARS | 11 (14%) |
| Parameter | Total LARS | Minor LARS | Major LARS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L0 (n = 54) | TL (n = 24) | p Value | L0 (n = 54) | mL (n = 13) | p Value | L0 (n = 54) | ML (n = 11) | p Value | ||
| Age, years | Median (IQR) | 67(57, 75) | 65 (55, 68) | 0.159 | 67 (57, 75) | 62 (53, 66) | 0.172 | 67 (57, 75) | 65 (56, 69) | 0.446 |
| Sex, n (%) | Female | 18 (60%) | 12 (40%) | 0.163 | 18 (72%) | 7 (28%) | 0.209 | 18 (78%) | 5 (22%) | 0.500 |
| Male | 36 (75%) | 12 (25%) | 36 (86%) | 6 (14%) | 36 (86%) | 6 (14%) | ||||
| ASA, n (%) | 1 | 2 (50%) | 2 (50%) | 0.315 | 2 (67%) | 1 (33%) | 0.221 | 2 (67%) | 1 (33%) | 0.717 |
| 2 | 30 (65%) | 16 (35%) | 30 (75%) | 10 (25%) | 30 (83%) | 6 (17%) | ||||
| 3 | 22 (79%) | 6 (21%) | 22 (92%) | 2 (8%) | 22 (85%) | 4 (15%) | ||||
| Distance from anal verge, n (%) | 0–5 cm | 6 (60%) | 4 (40%) | 0.180 | 6 (67%) | 3 (33%) | 0.515 | 6 (86%) | 1 (14%) | 0.041 |
| 5–10 cm | 18 (60%) | 12 (40%) | 18 (82%) | 4 (18%) | 18 (69%) | 8 (31%) | ||||
| 10–15 cm | 30 (79%) | 8 (21%) | 30 (83%) | 6 (17%) | 30 (94%) | 2 (6%) | ||||
| Tumor dimension, cm | Median (IQR) | 3.15 (2.20, 4.50) | 2.50 (1.85, 3.00) | 0.052 | 3.15 (2.20, 4.50) | 2.50 (2.00, 2.50) | 0.013 | 3.15 (2.20, 4.50) | 2.50 (1.78, 4.88) | 0.752 |
| pT, n (%) | T0 | 2 (67%) | 1 (33%) | 0.107 | 2 (100%) | 0 (0%) | 0.007 | 2 (67%) | 1 (33%) | 0.323 |
| Tis | 1 (50%) | 1 (50%) | 1 (50%) | 1 (50%) | 1 (100%) | 0 (0%) | ||||
| T1 | 6 (67%) | 3 (33%) | 6 (86%) | 1 (14%) | 6 (75%) | 2 (25%) | ||||
| T2 | 9 (50%) | 9 (50%) | 9 (53%) | 8 (47%) | 9 (90%) | 1 (10%) | ||||
| T3 | 31 (84%) | 6 (16%) | 31 (94%) | 2 (6.1%) | 31 (89%) | 4 (11%) | ||||
| T4a | 4 (57%) | 3 (43%) | 4 (100%) | 0 (0%) | 4 (57%) | 3 (43%) | ||||
| T4b | 1 (50%) | 1 (50%) | 1 (50%) | 1 (50%) | 1 (100%) | 0 (0%) | ||||
| pN, n (%) | N0 | 30 (68%) | 14 (32%) | 0.168 | 30 (79%) | 30 (79%) | 0.104 | 30 (83%) | 30 (83%) | 0.309 |
| N1 | 19 (79%) | 5 (21%) | 8 (21%) | 8 (21%) | 6 (17%) | 6 (17%) | ||||
| N2 | 5 (50%) | 5 (50%) | 19 (90%) | 19 (90%) | 19 (86%) | 19 (86%) | ||||
| G, n (%) | G0 | 2 (100%) | 0 (0%) | 0.189 | 2 (100%) | 0 (0%) | >0.999 | 2 (100%) | 0 (0%) | 0.038 |
| G1 | 3 (100%) | 0 (0%) | 3 (100%) | 0 (0%) | 3 (100%) | 0 (0%) | ||||
| G2 | 32 (78%) | 9 (22%) | 32 (82%) | 7 (18%) | 32 (94%) | 2 (5.9%) | ||||
| G3 | 8 (53%) | 7 (47%) | 8 (80%) | 2 (20%) | 8 (62%) | 5 (38%) | ||||
| Surgical Technique, n (%) | Open | 36 (68%) | 17 (32%) | 0.716 | 36 (78%) | 10 (22%) | 0.740 | 36 (84%) | 7 (16%) | >0.999 |
| VLS | 18 (72%) | 7 (28%) | 18 (86%) | 3 (14%) | 18 (82%) | 4 (18%) | ||||
| Operative time, min | Median (IQR) | 210 (180, 255) | 208 (170, 229) | 0.249 | 210 (180, 255) | 195 (155, 225) | 0.131 | 210 (180, 255) | 210 (193, 235) | 0.854 |
| Ileostomy | Yes | 17 (59%) | 12 (41%) | 0.118 | 17 (77%) | 5 (23%) | 0.745 | 17 (71%) | 7 (29%) | 0.083 |
| No | 37 (76%) | 12 (24%) | 37 (82%) | 8 (18%) | 37 (90%) | 4 (10%) | ||||
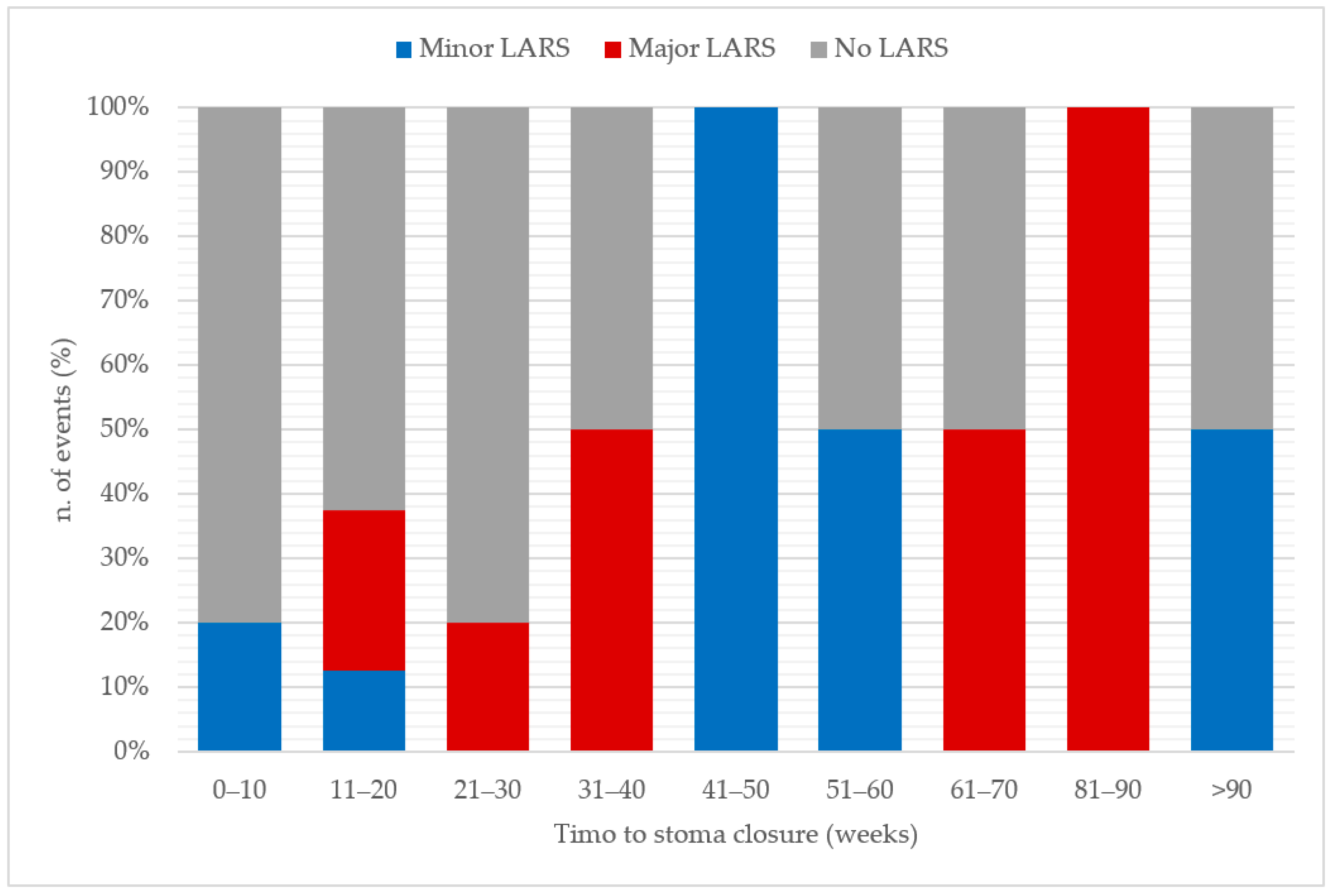
| Time to stoma closure, weeks | Median (IQR) | 19 (12, 26) | 43 (14, 71) | 0.106 | 19 (12, 26) | 46 (13, 51) | 0.410 | 19 (12, 26) | 39 (22, 74) | 0.105 |
| Neoadjuvant therapy | LCRT | 16 (67%) | 8 (33%) | 0.642 | 16 (80%) | 4 (20%) | 0.201 | 16 (80%) | 4 (20%) | 0.815 |
| SCRT | 2 (50%) | 2 (50%) | 2 (50%) | 2 (50%) | 2 (100%) | 0 (0%) | ||||
| No | 36 (72%) | 14 (28%) | 36 (84%) | 7 (16%) | 36 (84%) | 7 (16%) | ||||
| Adjuvant therapy | CHT | 4 (67%) | 2 (33%) | 0.608 | 4 (67%) | 2 (33%) | 0.672 | 4 (100%) | 0 (0%) | 0.327 |
| RT | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | ||||
| CHT + RT | 0 (0%) | 1 (100%) | 0 | 0 | 0 (0%) | 1 (100%) | ||||
| No | 48 (70%) | 21 (30%) | 48 (81%) | 11 (19%) | 48 (83%) | 10 (17%) | ||||
| Parameter | Total LARS | Minor LARS | Major LARS | |||||||
| OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | ||
| Distance from anal verge | 0–5 cm | - | - | 0.006 | - | - | 0.9 | - | - | 0.002 |
| 5–10 cm | 5.58 | 0.65, 77.9 | 0.99 | 0.02, 52.0 | >1000 | 0.00, >1000 | ||||
| 10–15 cm | 0.19 | 0.01, 2.33 | 0.35 | 0.00, 23.4 | 0.46 | 0.00, >1000 | ||||
| Tumor dimension, cm | Median | 0.60 | 0.35, 0.94 | 0.025 | 0.13 | 0.01, 0.44 | <0.001 | 0.76 | 0.27, 1.90 | 0.5 |
| pT | T0 | - | - | 0.015 | - | - | 0.003 | - | - | 0.2 |
| T1 | 0.91 | 0.03, 40.2 | >1000 | 0.00, NA | 0.21 | 0.00, 16.5 | ||||
| T2 | 9.05 | 0.36, 464 | >1000 | 0.00, NA | 0.52 | 0.00, 71.1 | ||||
| T3 | 0.91 | 0.04, 33.8 | >1000 | 0.00, NA | 0.36 | 0.01, 20.5 | ||||
| T4a | 18.8 | 0.31, >1000 | 0.00 | 0.00, >1000 | >1000 | 0.00, >1000 | ||||
| T4b | >1000 | 0.00, NA | >1000 | 0.00, NA | >1000 | 0.00, >1000 | ||||
| Tis | 14.6 | 0.19, >1000 | >1000 | 0.00, NA | 0.57 | 0.00, >1000 | ||||
| pN | N0 | - | - | 0.13 | - | - | 0.6 | - | - | 0.3 |
| N1a | 0.82 | 0.08, 6.31 | 0.00 | 4.60 | 0.29, 102 | |||||
| N1b | 0.26 | 0.01, 2.88 | 8.02 | 0.02, >1000 | 0.00 | 0.00, >1000 | ||||
| N1c | 0.00 | - | 0.00 | - | 0.00 | - | ||||
| N2a | 9.74 | 0.46, 395 | 14.1 | 0.06, >1000 | 0.94 | 0.00, >1000 | ||||
| N2b | 0.00 | - | 0.00 | - | 0.00 | - | ||||
| Neoadjuvant therapy | No | - | - | 0.3 | - | - | 0.066 | - | - | 0.6 |
| LCRT | 0.19 | 0.02, 1.49 | 0.03 | 0.00, 2.90 | 0.28 | 0.02, 4.31 | ||||
| SCRT | 0.27 | 0.01, 8.25 | 13.4 | 0.01, >1000 | 0.51 | 0.00, >1000 | ||||
| Adjuvant Therapy | No | - | - | 0.004 | - | - | 0.012 | - | - | 0.007 |
| CHT | 0.57 | 0.03, 7.14 | >1000 | 4.21, >1000 | 0.00 | 0.00, >1000 | ||||
| RT | 0.00 | - | 0.00 | - | 0.05 | 0.00, >1000 | ||||
| CHT + RT | >1000 | 0.00, NA | - | - | >1000 | 0.00, >1000 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muttillo, E.M.; La Franca, A.; Coppola, A.; Li Causi, F.S.; Checchelani, M.; Ceccacci, A.; Castagnola, G.; Garbarino, G.M.; Osti, M.F.; Balducci, G.; et al. Low Anterior Resection Syndrome (LARS) after Surgery for Rectal Cancer: An Inevitable Price to Pay for Survival, or a Preventable Complication? J. Clin. Med. 2023, 12, 5962. https://doi.org/10.3390/jcm12185962
Muttillo EM, La Franca A, Coppola A, Li Causi FS, Checchelani M, Ceccacci A, Castagnola G, Garbarino GM, Osti MF, Balducci G, et al. Low Anterior Resection Syndrome (LARS) after Surgery for Rectal Cancer: An Inevitable Price to Pay for Survival, or a Preventable Complication? Journal of Clinical Medicine. 2023; 12(18):5962. https://doi.org/10.3390/jcm12185962
Chicago/Turabian StyleMuttillo, Edoardo Maria, Alice La Franca, Alessandro Coppola, Francesco Saverio Li Causi, Marzia Checchelani, Alice Ceccacci, Giorgio Castagnola, Giovanni Maria Garbarino, Mattia Falchetto Osti, Genoveffa Balducci, and et al. 2023. "Low Anterior Resection Syndrome (LARS) after Surgery for Rectal Cancer: An Inevitable Price to Pay for Survival, or a Preventable Complication?" Journal of Clinical Medicine 12, no. 18: 5962. https://doi.org/10.3390/jcm12185962
APA StyleMuttillo, E. M., La Franca, A., Coppola, A., Li Causi, F. S., Checchelani, M., Ceccacci, A., Castagnola, G., Garbarino, G. M., Osti, M. F., Balducci, G., & Mercantini, P. (2023). Low Anterior Resection Syndrome (LARS) after Surgery for Rectal Cancer: An Inevitable Price to Pay for Survival, or a Preventable Complication? Journal of Clinical Medicine, 12(18), 5962. https://doi.org/10.3390/jcm12185962