MRI-Based Quantification of Pan-Alimentary Function and Motility in Subjects with Diabetes and Gastrointestinal Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subject Selection
2.2. Study Design
2.3. Questionnaire Data
2.4. Image Acquisition
2.5. MRI
2.6. Quality Assessment
2.7. Image Processing
2.8. Patient Classification Using Machine Learning
2.9. Statistical Analysis
3. Results
3.1. Demography
3.2. Questionnaires
3.2.1. Symptoms Assessed at Baseline
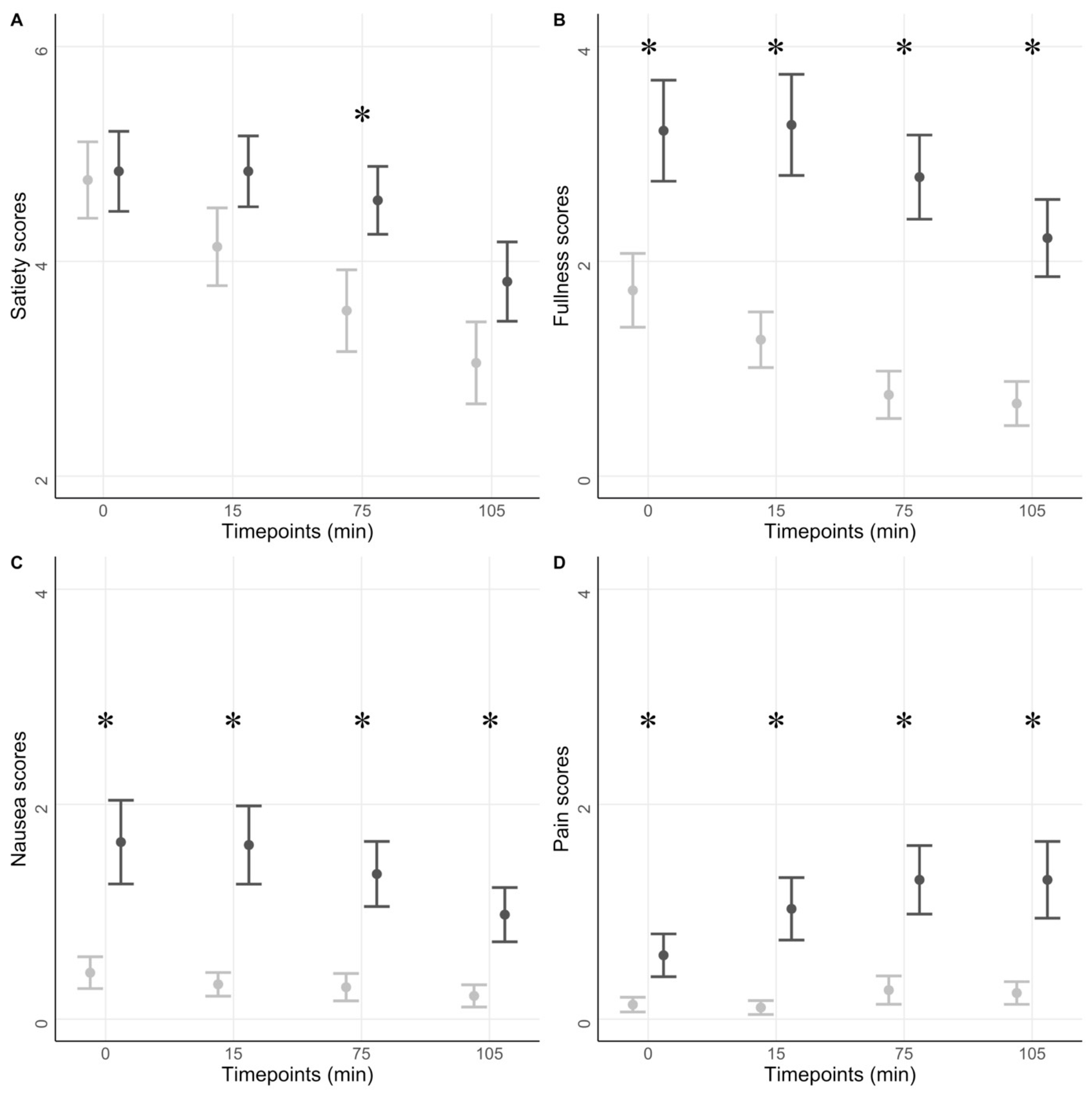
3.2.2. Symptom Scores in Response to a Test Meal
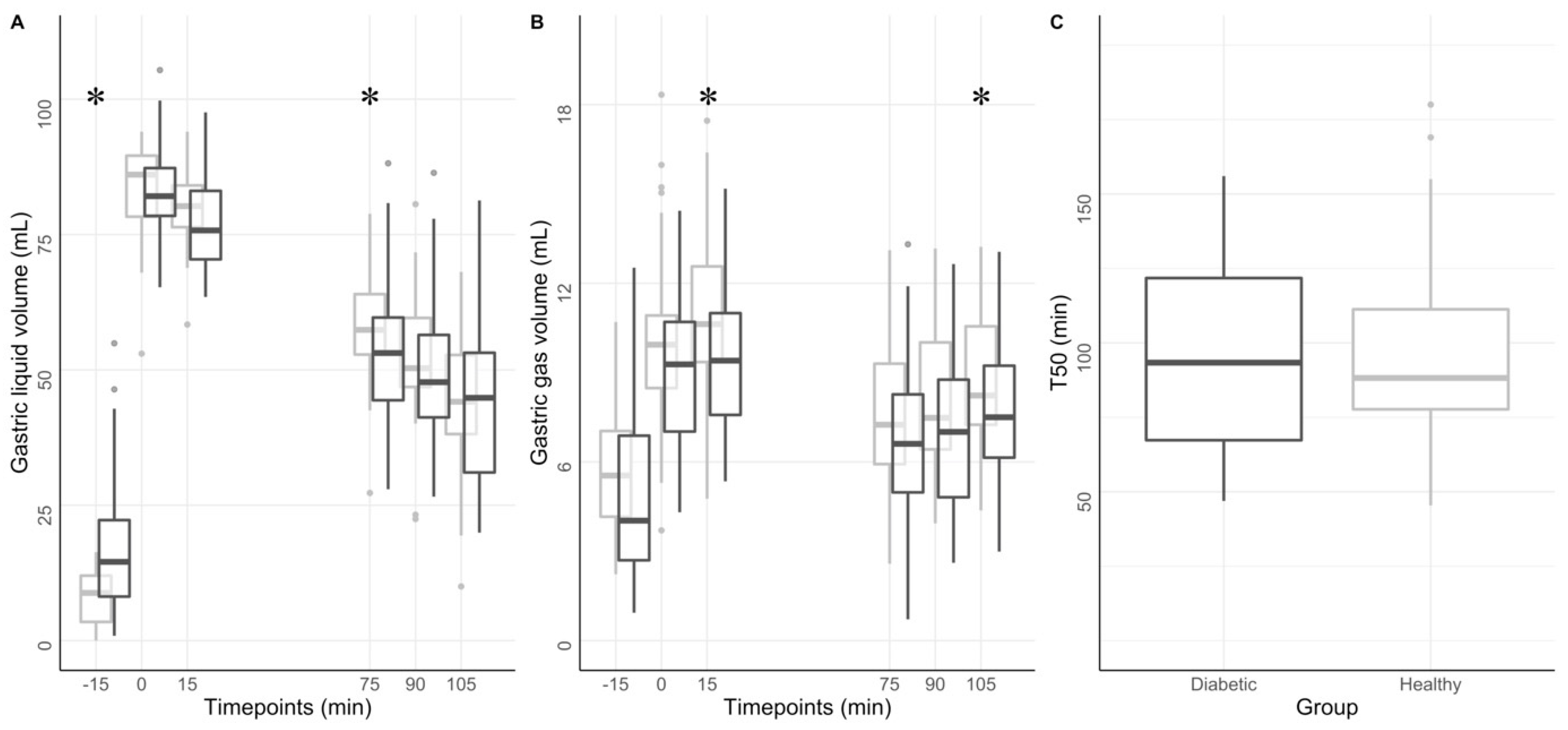
3.3. Gastric Volumes Distribution and Compartmental Analysis
3.4. Gastric Motility and Emptying
3.5. Small Bowel Volume and Motility
3.6. Colonic Volume and Motility
3.7. Colonic Water Content
3.8. Association between Symptoms Scores and Multi-Segmental MRI Measures
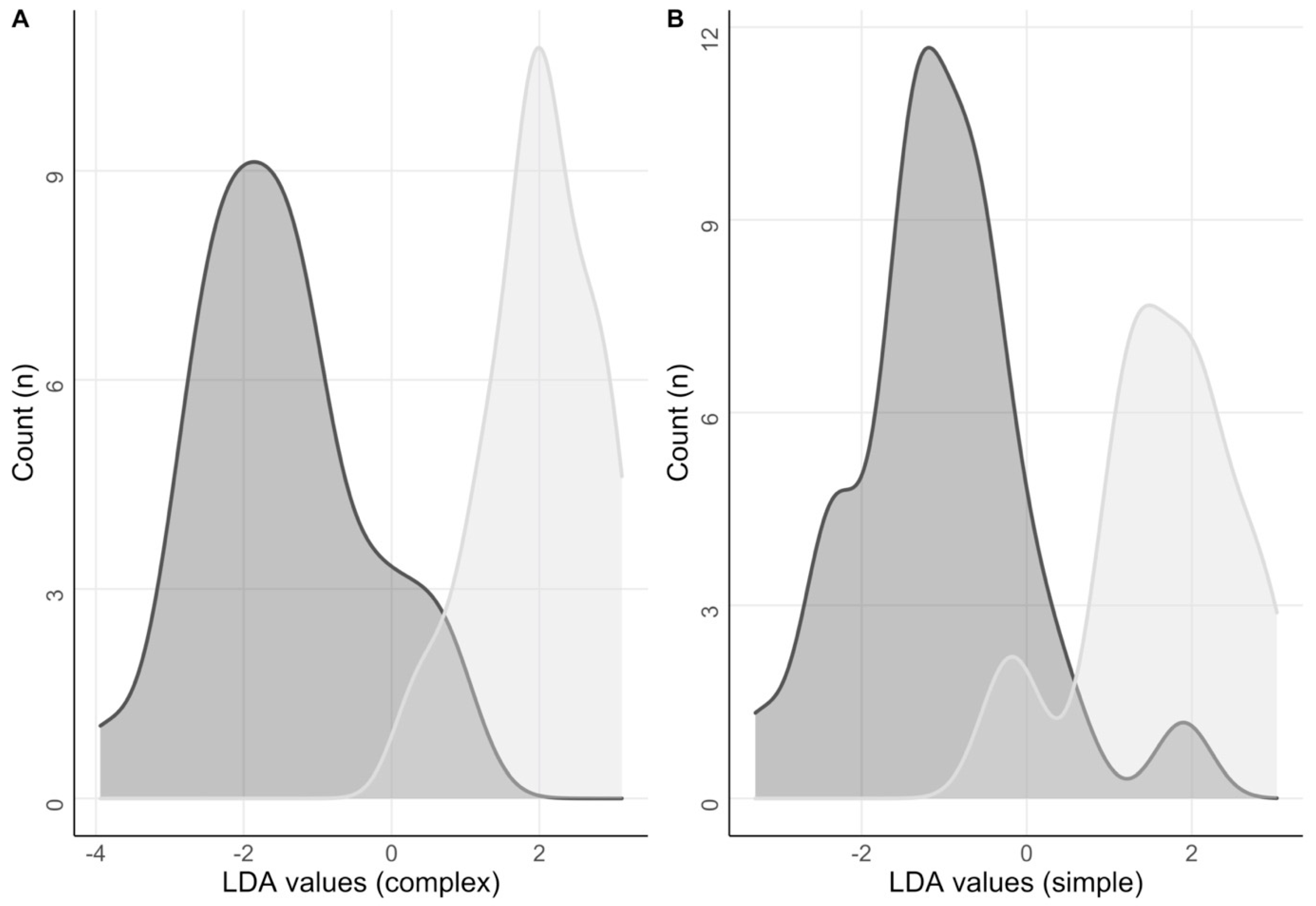
3.9. Elastic Net Regression and Linear Discriminant Analysis
4. Discussion
4.1. Overview of Findings and Comparison with Recent Literature
4.1.1. Stomach
4.1.2. Small Bowel
4.1.3. Colon
4.2. Limitations and Future Perspectives
4.3. New Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zawada, A.; Moszak, M.; Skrzypczak, D.; Grzymisławski, M. Gastrointestinal complications in patients with diabetes mellitus. Adv. Clin. Exp. Med. 2018, 27, 567–572. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Rayner, C.K.; Verhagen, M.A.M.T.; Hebbard, G.S.; DiMatteo, A.C.; Doran, S.M.; Horowitz, M. Proximal Gastric Compliance and Perception of Distension in Type 1 Diabetes Mellitus: Effects of Hyperglycemia. Am. J. Gastroenterol. 2000, 95, 1175–1183. [Google Scholar] [CrossRef]
- Peles, S.; Petersen, J.; Aviv, R.; Policker, S.; Abu-Hatoum, O.; Ben-Haim, S.A.; Gutterman, D.D.; Sengupta, J.N. Enhancement of antral contractions and vagal afferent signaling with synchronized electrical stimulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G577–G585. [Google Scholar] [CrossRef][Green Version]
- Horváth, V.J.; Putz, Z.; Izbéki, F.; Körei, A.E.; Gerő, L.; Lengyel, C.; Kempler, P.; Várkonyi, T. Diabetes-Related Dysfunction of the Small Intestine and the Colon: Focus on Motility. Curr. Diabetes Rep. 2015, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Buysschaert, M.; Donckier, J.; Dive, A.; Ketelslegers, J.-M.; Lambert, A.E. Gastric Acid and Pancreatic Polypeptide Responses to Sham Feeding Are Impaired in Diabetic Subjects with Autonomic Neuropathy. Diabetes 1985, 34, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Soykan, I.; Sivri, B.; Sarosiek, I.; Kiernan, B.; McCallum, R.W. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig. Dis. Sci. 1998, 43, 2398–2404. [Google Scholar] [CrossRef]
- Alcalá-Gonzalez, L.G.; Malagelada, C.; Livovsky, D.M.; Azpiroz, F. Effect of colonic distension on small bowel motility measured by jejunal high-resolution manometry. Neurogastroenterol. Motil. 2022, 34, e14351. [Google Scholar] [CrossRef]
- Khalaf, A.; Hoad, C.L.; Menys, A.; Nowak, A.; Taylor, S.A.; Paparo, S.; Lingaya, M.; Falcone, Y.; Singh, G.; Spiller, R.C.; et al. MRI assessment of the postprandial gastrointestinal motility and peptide response in healthy humans. Neurogastroenterol. Motil. 2018, 30, e13182. [Google Scholar] [CrossRef]
- de Zwart, I.M.; Haans, J.J.L.; Verbeek, P.; Eilers, P.H.C.; de Roos, A.; Masclee, A.A.M. Gastric accommodation and motility are influenced by the barostat device: Assessment with magnetic resonance imaging. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G208–G214. [Google Scholar] [CrossRef] [PubMed]
- Mariani, G.; Paglianiti, I. Joint SNMMI and EANM guideline for small-bowel and colon transit: An important step towards long-awaited standardization. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 405–407. [Google Scholar] [CrossRef][Green Version]
- Vijayvargiya, P.; Jameie-Oskooei, S.; Camilleri, M.; Chedid, V.; Erwin, P.J.; Murad, M.H. Association between delayed gastric emptying and upper gastrointestinal symptoms: A systematic review and meta-analysis. Gut 2019, 68, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Okdahl, T.; Bertoli, D.; Brock, B.; Krogh, K.; Krag Knop, F.; Brock, C.; Drewes, A.M. Study protocol for a multicentre, randomised, parallel group, sham-controlled clinical trial investigating the effect of transcutaneous vagal nerve stimulation on gastrointestinal symptoms in people with diabetes complicated with diabetic autonomic neurop. BMJ Open 2021, 11, e038677. [Google Scholar] [CrossRef]
- Bertoli, D.; Mark, E.B.; Liao, D.; Brock, C.; Brock, B.; Knop, F.K.; Krogh, K.; Frøkjær, J.B.; Drewes, A.M. Pan-alimentary assessment of motility, luminal content, and structures: An MRI-based framework. Scand. J. Gastroenterol. 2023, 1–12. [Google Scholar] [CrossRef]
- Revicki, D.A.; Camilleri, M.; Kuo, B.; Norton, N.J.; Murray, L.; Palsgrove, A.; Parkman, H.P. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment. Pharmacol. Ther. 2009, 30, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, H.A.; Cheung, W.-Y.; Russell, I.T.; Durai, D.; Alrubaiy, L.; Williams, J.G. Psychometric development of the Gastrointestinal Symptom Rating Questionnaire (GSRQ) demonstrated good validity. J. Clin. Epidemiol. 2015, 68, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Malagelada, C.; Barba, I.; Accarino, A.; Molne, L.; Mendez, S.; Campos, E.; Gonzalez, A.; Alonso-Cotoner, C.; Santos, J.; Malagelada, J.-R.; et al. Cognitive and hedonic responses to meal ingestion correlate with changes in circulating metabolites. Neurogastroenterol. Motil. 2016, 28, 1806–1814. [Google Scholar] [CrossRef]
- Livovsky, D.M.; Pribic, T.; Azpiroz, F. Food, Eating, and the Gastrointestinal Tract. Nutrients 2020, 12, 986. [Google Scholar] [CrossRef]
- Hoad, C.L.; Parker, H.; Hudders, N.; Costigan, C.; Cox, E.F.; Perkins, A.C.; Blackshaw, P.E.; Marciani, L.; Spiller, R.C.; Fox, M.R.; et al. Measurement of gastric meal and secretion volumes using magnetic resonance imaging. Phys. Med. Biol. 2015, 60, 1367–1383. [Google Scholar] [CrossRef]
- Sandberg, T.H.; Nilsson, M.; Poulsen, J.L.; Gram, M.; Frøkjær, J.B.; Østergaard, L.R.; Drewes, A.M. A novel semi-automatic segmentation method for volumetric assessment of the colon based on magnetic resonance imaging. Abdom. Imaging 2015, 40, 2232–2241. [Google Scholar] [CrossRef]
- Bertoli, D.; Mark, E.B.; Liao, D.; Brock, C.; Frøkjær, J.B.; Drewes, A.M. A novel MRI-based three-dimensional model of stomach volume, surface area, and geometry in response to gastric filling and emptying. Neurogastroenterol. Motil. 2022, 35, e14497. [Google Scholar] [CrossRef] [PubMed]
- Fruehauf, H.; Menne, D.; Kwiatek, M.A.; Forras-Kaufman, Z.; Kaufman, E.; Goetze, O.; Fried, M.; Schwizer, W.; Fox, M. Inter-observer reproducibility and analysis of gastric volume measurements and gastric emptying assessed with magnetic resonance imaging. Neurogastroenterol. Motil. 2011, 23, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Menys, A.; Taylor, S.A.; Emmanuel, A.; Ahmed, A.; Plumb, A.A.; Odille, F.; Alam, A.; Halligan, S.; Atkinson, D. Global Small Bowel Motility: Assessment with Dynamic MR Imaging. Radiology 2013, 269, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Odille, F.; Menys, A.; Ahmed, A.; Punwani, S.; Taylor, S.A.; Atkinson, D. Quantitative assessment of small bowel motility by nonrigid registration of dynamic MR images. Magn. Reson. Med. 2012, 68, 783–793. [Google Scholar] [CrossRef]
- Major, G.; Murray, K.; Singh, G.; Nowak, A.; Hoad, C.L.; Marciani, L.; Silos-Santiago, A.; Kurtz, C.B.; Johnston, J.M.; Gowland, P.; et al. Demonstration of differences in colonic volumes, transit, chyme consistency, and response to psyllium between healthy and constipated subjects using magnetic resonance imaging. Neurogastroenterol. Motil. 2018, 30, e13400. [Google Scholar] [CrossRef] [PubMed]
- Kung, B.; Chiang, M.; Perera, G.; Pritchard, M.; Stewart, R. Unsupervised Machine Learning to Identify Depressive Subtypes. Healthc. Inform. Res. 2022, 28, 256–266. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.; Liu, T.; Yang, Z.; Chen, W.; Zhang, S. A robust spike sorting method based on the joint optimization of linear discrimination analysis and density peaks. Sci. Rep. 2022, 12, 15504. [Google Scholar] [CrossRef]
- Nguyen, C.D.; Moreno-Betancur, M.; Rodwell, L.; Romaniuk, H.; Carlin, J.B.; Lee, K.J. Multiple imputation of semi-continuous exposure variables that are categorized for analysis. Stat. Med. 2021, 40, 6093–6106. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Armstrong, R.A. Recommendations for analysis of repeated-measures designs: Testing and correcting for sphericity and use of manova and mixed model analysis. Ophthalmic Physiol. Opt. 2017, 37, 585–593. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Sainani, K.L. Bonferroni, Holm, and Hochberg Corrections: Fun Names, Serious Changes to P Values. PMR 2014, 6, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Eichstaedt, K.E.; Kovatch, K.; Maroof, D.A. A less conservative method to adjust for familywise error rate in neuropsychological research: The Holm’s sequential Bonferroni procedure. NeuroRehabilitation 2013, 32, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Gollifer, R.M.; Taylor, S.A.; Menys, A.; Zarate-Lopez, N.; Chatoor, D.; Emmanuel, A.; Atkinson, D. Magnetic resonance imaging assessed enteric motility and luminal content analysis in patients with severe bloating and visible distension. Neurogastroenterol. Motil. 2022, 34, e14381. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-Smith, V.C.; Major, G.; Ashleigh, L.; Murray, K.; Hoad, C.L.; Marciani, L.; Gowland, P.A.; Spiller, R.C. Insights Into the Different Effects of Food on Intestinal Secretion Using Magnetic Resonance Imaging. J. Parenter. Enter. Nutr. 2018, 42, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, R.M.; Thomas, E.A.; Goh, S.M.; Sjövall, H.; Bornstein, J.C. Segmentation induced by intraluminal fatty acid in isolated guinea-pig duodenum and jejunum. J. Physiol. 2004, 556, 557–569. [Google Scholar] [CrossRef]
- Camilleri, M.; Colemont, L.J.; Phillips, S.F.; Brown, M.L.; Thomforde, G.M.; Chapman, N.; Zinsmeister, A.R. Human gastric emptying and colonic filling of solids characterized by a new method. Am. J. Physiol. Gastrointest. Liver Physiol. 1989, 257, G284–G290. [Google Scholar] [CrossRef]
- Freitas, D.; Boué, F.; Benallaoua, M.; Airinei, G.; Benamouzig, R.; Lutton, E.; Jourdain, L.; Dubuisson, R.-M.; Maître, X.; Darrasse, L.; et al. Glycemic response, satiety, gastric secretions and emptying after bread consumption with water, tea or lemon juice: A randomized crossover intervention using MRI. Eur. J. Nutr. 2022, 61, 1621–1636. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Karwoski, R.A.; Fidler, J.; Holmes, D.R.; Robb, R.A.; Riederer, S.J.; Zinsmeister, A.R. Comparison of manual and semiautomated techniques for analyzing gastric volumes with MRI in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G582–G587. [Google Scholar] [CrossRef][Green Version]
- Ramtoola, S.; Nyeland, M.E.; Jacobsen, J.; Ploug, U.J.; Kragh, N.; Zimmermann, E. Characteristics of newly diagnosed adults with type 1 diabetes in the UK and evolution of glycaemic control, body mass index and Charlson comorbidity index over the first 5 years after diagnosis. Prim. Care Diabetes 2020, 14, 349–355. [Google Scholar] [CrossRef]
- Garg, H.; Podder, S.; Bala, I.; Gulati, A. Comparison of fasting gastric volume using ultrasound in diabetic and non-diabetic patients in elective surgery: An observational study. Indian J. Anaesth. 2020, 64, 391. [Google Scholar] [CrossRef]
- Stanghellini, V.; Tosetti, C.; Paternicò, A.; Barbara, G.; Morselli-Labate, A.; Monetti, N.; Marengo, M.; Corinaldesi, R. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology 1996, 110, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Sarnelli, G.; Caenepeel, P.; Geypens, B.; Janssens, J.; Tack, J. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am. J. Gastroenterol. 2003, 98, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, V.; Tack, J. Gastroparesis: Separate entity or just a part of dyspepsia? Gut 2014, 63, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, D.J.; Lacy, B.E. Gastroparesis and functional dyspepsia: Different diseases or different ends of the spectrum? Curr. Opin. Gastroenterol. 2020, 36, 509–517. [Google Scholar] [CrossRef]
- Cogliandro, R.F.; Rizzoli, G.; Bellacosa, L.; De Giorgio, R.; Cremon, C.; Barbara, G.; Stanghellini, V. Is gastroparesis a gastric disease? Neurogastroenterol. Motil. 2019, 31, e13562. [Google Scholar] [CrossRef]
- Schol, J.; Wauters, L.; Dickman, R.; Drug, V.; Mulak, A.; Serra, J.; Enck, P.; Tack, J.; Accarino, A.; Barbara, G.; et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on gastroparesis. United Eur. Gastroenterol. J. 2021, 9, 287–306. [Google Scholar] [CrossRef]
- Brock, C.; Liao, D.; Wegeberg, A.-M.; Mohr Drewes, A. The antroduodenal transition time is prolonged in adults with type 1 diabetes. Neurogastroenterol. Motil. 2021, 33, e14144. [Google Scholar] [CrossRef]
- Surjanhata, B.; Brun, R.; Wilding, G.; Semler, J.; Kuo, B. Small bowel fed response as measured by wireless motility capsule: Comparative analysis in healthy, gastroparetic, and constipated subjects. Neurogastroenterol. Motil. 2018, 30, e13268. [Google Scholar] [CrossRef]
- Camilleri, M.; Malagelada, J.-R. Abnormal intestinal motility in diabetics with the gastroparesis syndrome. Eur. J. Clin. Investig. 1984, 14, 420–427. [Google Scholar] [CrossRef]
- Rana, S.; Bhansali, A.; Bhadada, S.; Sharma, S.; Kaur, J.; Singh, K. Orocecal transit time and small intestinal bacterial overgrowth in type 2 diabetes patients from North India. Diabetes Technol. Ther. 2011, 13, 1115–1120. [Google Scholar] [CrossRef]
- Rosa-e-Silva, L.; Troncon, L.E.; Oliveira, R.B.; Foss, M.C.; Braga, F.J.; Gallo Junior, L. Rapid distal small bowel transit associated with sympathetic denervation in type I diabetes mellitus. Gut 1996, 39, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Pavin, E.J.; Parisi, M.C.R.; Lorena, S.L.S.; Brunetto, S.Q.; Ramos, C.D.; Pavan, C.R.; Mesquita, M.A. Delayed Small Intestinal Transit in Patients with Long-Standing Type 1 Diabetes Mellitus: Investigation of the Relationships with Clinical Features, Gastric Emptying, Psychological Distress, and Nutritional Parameters. Diabetes Technol. Ther. 2013, 15, 32–38. [Google Scholar] [CrossRef]
- Tominaga, K.; Sato, H.; Yokomichi, H.; Tsuchiya, A.; Yoshida, T.; Kawata, Y.; Mizusawa, T.; Yokoyama, J.; Terai, S. Variation in small bowel transit time on capsule endoscopy. Ann. Transl. Med. 2020, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Klinge, M.W.; Sutter, N.; Mark, E.B.; Haase, A.-M.; Borghammer, P.; Schlageter, V.; Lund, S.; Fleischer, J.; Knudsen, K.; Drewes, A.M.; et al. Gastric Emptying Time and Volume of the Small Intestine as Objective Markers in Patients With Symptoms of Diabetic Enteropathy. J. Neurogastroenterol. Motil. 2021, 27, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Sangnes, D.A.; Lundervold, K.; Bekkelund, M.; Volkmann, H.L.; Berentsen, B.; Gilja, O.H.; Dimcevski, G.; Søfteland, E. Gastrointestinal transit and contractility in diabetic constipation: A wireless motility capsule study on diabetes patients and healthy controls. United Eur. Gastroenterol. J. 2021, 9, 1168–1177. [Google Scholar] [CrossRef]
- Cowlam, S.; Vinayagam, R.; Khan, U.; Marsden, S.; Minty, I.; Moncur, P.; Bain, I.; Yiannakou, Y.J. Blinded comparison of faecal loading on plain radiography versus radio-opaque marker transit studies in the assessment of constipation. Clin. Radiol. 2008, 63, 1326–1331. [Google Scholar] [CrossRef]
- Klinge, M.W.; Borghammer, P.; Lund, S.; Fedorova, T.; Knudsen, K.; Haase, A.; Christiansen, J.J.; Krogh, K. Enteric cholinergic neuropathy in patients with diabetes: Non-invasive assessment with positron emission tomography. Neurogastroenterol. Motil. 2020, 32, e13731. [Google Scholar] [CrossRef]
- Du, Y.T.; Rayner, C.K.; Jones, K.L.; Talley, N.J.; Horowitz, M. Gastrointestinal Symptoms in Diabetes: Prevalence, Assessment, Pathogenesis, and Management. Diabetes Care 2018, 41, 627–637. [Google Scholar] [CrossRef]
- Jackson, M.W.; Gordon, T.P.; Waterman, S.A. Disruption of intestinal motility by a calcium channel-stimulating autoantibody in type 1 diabetes. Gastroenterology 2004, 126, 819–828. [Google Scholar] [CrossRef]
- Dass, N.B.; John, A.K.; Bassil, A.K.; Crumbley, C.W.; Shehee, W.R.; Maurio, F.P.; Moore, G.B.T.; Taylor, C.M.; Sanger, G.J. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol. Motil. 2007, 19, 66–74. [Google Scholar] [CrossRef]
- Bódi, N.; Talapka, P.; Poles, M.Z.; Hermesz, E.; Jancsó, Z.; Katarova, Z.; Izbéki, F.; Wittmann, T.; Fekete, É.; Bagyánszki, M. Gut Region-Specific Diabetic Damage to the Capillary Endothelium Adjacent to the Myenteric Plexus: Capillary Damage in the Diabetic Intestine. Microcirculation 2012, 19, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Nezami, B.G.; Mwangi, S.M.; Lee, J.E.; Jeppsson, S.; Anitha, M.; Yarandi, S.S.; Farris, A.B.; Srinivasan, S. MicroRNA 375 Mediates Palmitate-Induced Enteric Neuronal Damage and High-Fat Diet-Induced Delayed Intestinal Transit in Mice. Gastroenterology 2014, 146, 473–483.e3. [Google Scholar] [CrossRef] [PubMed]


| Patients with DM and GI Symptoms (n = 46) | Healthy (n = 40) | |
|---|---|---|
| Age (years) | 62 ± 12 | 57 ± 10 |
| Gender (M/F) | 28:18 | 27:13 |
| BMI * (kg/m2) | 30 ± 6 | 27 ± 5 |
| Smokers (%) | 9.5 (n = 4) | 8.1 (n = 3) |
| Mean systolic pressure (mmHg) | 134 ± 15 | 141 ± 21 |
| Mean diastolic pressure * | 78 ± 9 | 85 ± 11 |
| Diabetes (type1/type2) | 9:36 | - |
| Diabetes duration (years) | 20 ± 14 | - |
| Charlson Comorbidity index | 1.8 ± 1.0 | - |
| Questionnaires-GCSI | ||
| Nausea score * | 0.7 ± 0.8 | 0 ± 0 |
| Satiety score * | 1.5 ± 0.9 | 0.3 ± 0.5 |
| Bloating score * | 1.9 ± 1.5 | 0.3 ± 0.6 |
| Total GCSI score * | 1.4 ± 0.9 | 0.3 ± 0.4 |
| Questionnaires-GSRS | ||
| Reflux score * | 2.1 ± 1.0 | 1.3 ± 0.6 |
| Abdominal pain score * | 2.5 ± 1.0 | 1.1 ± 0.2 |
| Indigestion score * | 3.2 ± 1.4 | 1.3 ± 0.4 |
| Diarrhea score * | 3.0 ± 1.6 | 1.1 ± 0.3 |
| Constipation score * | 3.3 ± 1.4 | 1.1 ± 0.2 |
| Total GSRS score * | 2.8 ± 0.8 | 1.2 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoli, D.; Mark, E.B.; Liao, D.; Okdahl, T.; Nauser, S.; Daugberg, L.H.; Brock, C.; Brock, B.; Knop, F.K.; Krogh, K.; et al. MRI-Based Quantification of Pan-Alimentary Function and Motility in Subjects with Diabetes and Gastrointestinal Symptoms. J. Clin. Med. 2023, 12, 5968. https://doi.org/10.3390/jcm12185968
Bertoli D, Mark EB, Liao D, Okdahl T, Nauser S, Daugberg LH, Brock C, Brock B, Knop FK, Krogh K, et al. MRI-Based Quantification of Pan-Alimentary Function and Motility in Subjects with Diabetes and Gastrointestinal Symptoms. Journal of Clinical Medicine. 2023; 12(18):5968. https://doi.org/10.3390/jcm12185968
Chicago/Turabian StyleBertoli, Davide, Esben Bolvig Mark, Donghua Liao, Tina Okdahl, Serena Nauser, Louise Hostrup Daugberg, Christina Brock, Birgitte Brock, Filip Krag Knop, Klaus Krogh, and et al. 2023. "MRI-Based Quantification of Pan-Alimentary Function and Motility in Subjects with Diabetes and Gastrointestinal Symptoms" Journal of Clinical Medicine 12, no. 18: 5968. https://doi.org/10.3390/jcm12185968
APA StyleBertoli, D., Mark, E. B., Liao, D., Okdahl, T., Nauser, S., Daugberg, L. H., Brock, C., Brock, B., Knop, F. K., Krogh, K., Brøndum Frøkjær, J., & Drewes, A. M. (2023). MRI-Based Quantification of Pan-Alimentary Function and Motility in Subjects with Diabetes and Gastrointestinal Symptoms. Journal of Clinical Medicine, 12(18), 5968. https://doi.org/10.3390/jcm12185968







