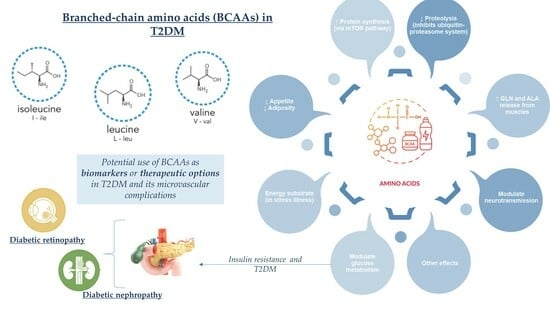
Depiction of Branched-Chain Amino Acids (BCAAs) in Diabetes with a Focus on Diabetic Microvascular Complications
Abstract
:1. Introduction
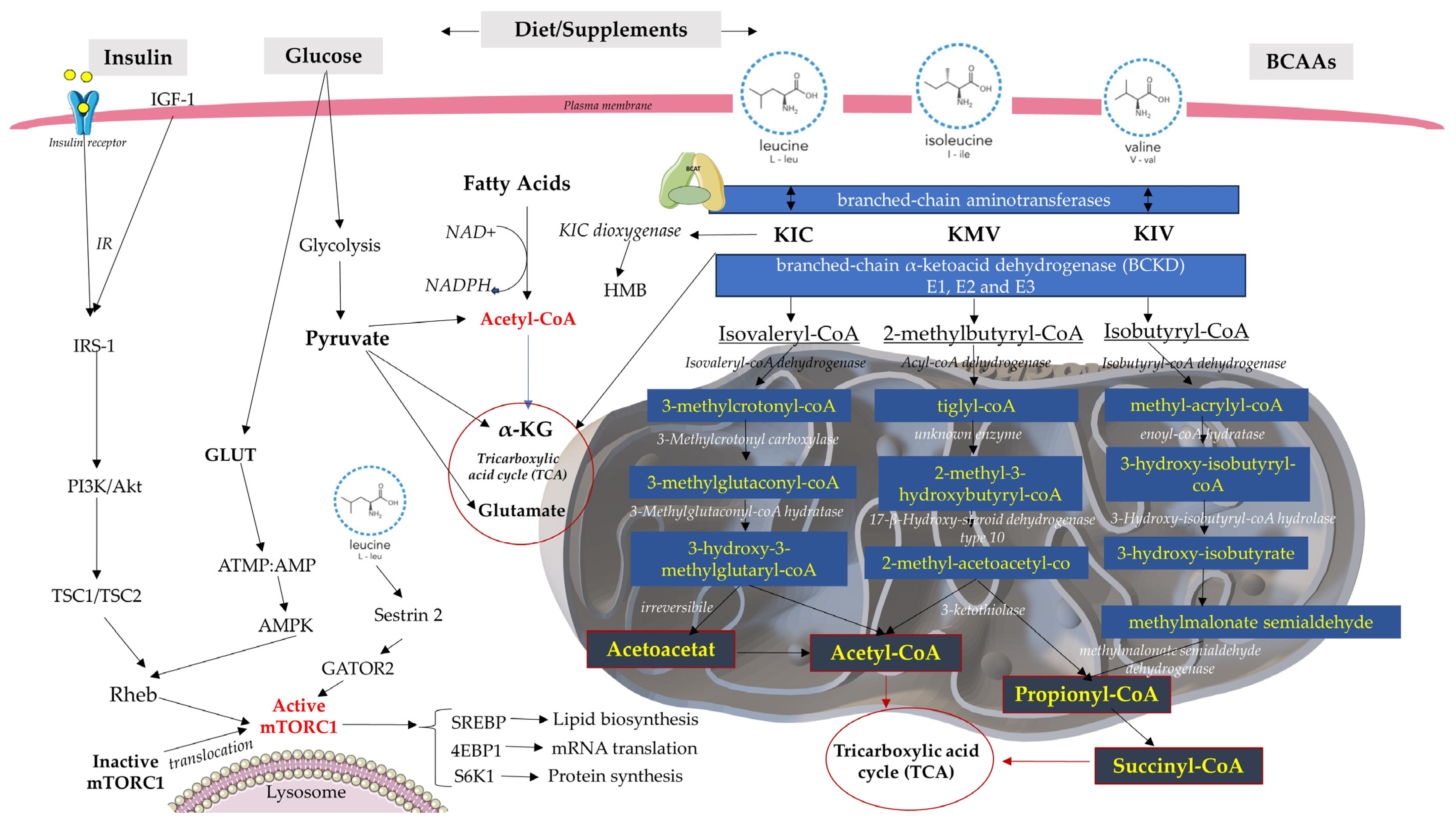
2. BCAA Catabolism and Metabolism
2.1. Insulin Resistance and Inflammation
2.2. BCAA Metabolic Gene Expression in Diabetes
3. Diabetic Retinopathy
4. Diabetic Nephropathy
5. Therapeutic and Biomarker Potential of BCAAs
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Das, S.R.; Gibbons, C.H.; et al. Introduction and Methodology: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. 1), S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wu, H.; Li, Z. The Pathogenesis of Diabetes. Int. J. Mol. Sci. 2023, 24, 6978. [Google Scholar] [CrossRef] [PubMed]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S.F.J. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, I.; Ardavani, A.; Vanweert, F.; Mellett, A.; Atherton, P.J.; Idris, I. The Association between Circulating Branched Chain Amino Acids and the Temporal Risk of Developing Type 2 Diabetes Mellitus: A Systematic Review & Meta-Analysis. Nutrients 2022, 14, 4411. [Google Scholar] [CrossRef]
- Kim, J.E.; Nam, H.; Park, J.I.; Cho, H.; Lee, J.; Kim, H.-E.; Kim, D.K.; Joo, K.W.; Kim, Y.S.; Kim, B.-S.; et al. Gut Microbial Genes and Metabolism for Methionine and Branched-Chain Amino Acids in Diabetic Nephropathy. Microbiol. Spectr. 2023, 11, e0234422. [Google Scholar] [CrossRef] [PubMed]
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of Branched-Chain Amino Acid Metabolism in the Pathogenesis of Obesity and Type 2 Diabetes-Related Metabolic Disturbances BCAA Metabolism in Type 2 Diabetes. Nutr. Diabetes 2022, 12, 35. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-Chain Amino Acids in Metabolic Signalling and Insulin Resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Tobias, D.K.; Clish, C.; Mora, S.; Li, J.; Liang, L.; Hu, F.B.; Manson, J.E.; Zhang, C. Dietary Intakes and Circulating Concentrations of Branched-Chain Amino Acids in Relation to Incident Type 2 Diabetes Risk Among High-Risk Women with a History of Gestational Diabetes Mellitus. Clin. Chem. 2018, 64, 1203–1210. [Google Scholar] [CrossRef]
- Dimou, A.; Tsimihodimos, V.; Bairaktari, E. The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology. Int. J. Mol. Sci. 2022, 23, 4022. [Google Scholar] [CrossRef]
- de Bandt, J.P.; Coumoul, X.; Barouki, R. Branched-Chain Amino Acids and Insulin Resistance, from Protein Supply to Diet-Induced Obesity. Nutrients 2023, 15, 68. [Google Scholar] [CrossRef]
- Mahmud, S.A.; Qureshi, M.A.; Sapkota, M.; Pellegrino, M.W. A pathogen branched-chain amino acid catabolic pathway subverts host survival by impairing energy metabolism and the mitochondrial UPR. PLoS Pathog. 2020, 16, e1008918. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; López-Maside, L.; Donapetry-García, C.; Fernández-Fernández, C.; Sixto-Leal, C. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 2017, 49, 1005–1028. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Cardoso, F.F.; Parys, C.; Cardoso, F.C.; Loor, J.J. Branched-Chain Amino Acid Supplementation Alters the Abundance of Mechanistic Target of Rapamycin and Insulin Signaling Proteins in Subcutaneous Adipose Explants from Lactating Holstein Cows. Animals 2021, 11, 2714. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Manning, B.D. MTORC1 Signaling and the Metabolic Control of Cell Growth. Curr. Opin. Cell Biol. 2017, 45, 72–82. [Google Scholar] [CrossRef]
- Hu, W.; Yang, P.; Fu, Z.; Wang, Y.; Zhou, Y.; Ye, Z.; Gong, Y.; Huang, A.; Sun, L.; Zhao, Y.; et al. High L-Valine Concentrations Associate with Increased Oxidative Stress and Newly-Diagnosed Type 2 Diabetes Mellitus: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. 2022, 15, 499–509. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, S.; Yan, W.; Xia, Y.; Chen, X.; Wang, W.; Zhang, J.; Gao, C.; Peng, C.; Yan, F.; et al. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine 2016, 13, 157–167. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Shin, A.C. Recent Progress on Branched-Chain Amino Acids in Obesity, Diabetes, and Beyond. Endocrinol. Metab. 2019, 34, 234–246. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Guénard, F.; Garneau, V.; Barbier, O.; Pérusse, L.; Vohl, M.-C. Associations between branched chain amino acid levels, obesity and cardiometabolic complications. Integr. Obes. Diabetes 2015, 1. [Google Scholar] [CrossRef]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.C.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A Branched-Chain Amino Acid Metabolite Drives Vascular Fatty Acid Transport and Causes Insulin Resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef]
- Moghei, M.; Tavajohi-Fini, P.; Beatty, B.; Adegoke, O.A.J. Ketoisocaproic acid, a metabolite of leucine, suppresses insulin-stimulated glucose transport in skeletal muscle cells in a BCAT2-dependent manner. Am. J. Physiol. Cell Physiol. 2016, 311, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Chen, P.J.; Xiao, W.H. The Effects of BCAAs on Insulin Resistance in Athletes. J. Nutr. Sci. Vitaminol. 2019, 65, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Liu, Y.; Ronnett, G.V.; Wu, A.; Cox, B.J.; Dai, F.F.; Röst, H.L.; Gunderson, E.P.; Wheeler, M.B. Amino acid and lipid metabolism in post-gestational diabetes and progression to type 2 diabetes: A metabolic profiling study. PLoS Med. 2020, 17, e1003112. [Google Scholar] [CrossRef] [PubMed]
- Harville, E.W.; Bazzano, L.; Qi, L.; He, J.; Dorans, K.; Perng, W.; Kelly, T. Branched-chain amino acids, history of gestational diabetes, and breastfeeding: The Bogalusa Heart Study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Huhtala, M.S.; Tertti, K.; Pellonperä, O.; Rönnemaa, T. Amino Acid Profile in Women with Gestational Diabetes Mellitus Treated with Metformin or Insulin. Diabetes Res. Clin. Pract. 2018, 146, 8–17. [Google Scholar] [CrossRef]
- Heath, H.; Luevano, J.; Johnson, C.M.; Phelan, S.; La Frano, M.R. Predictive Gestational Diabetes Biomarkers with Sustained Alterations Throughout Pregnancy. J. Endocr. Soc. 2022, 6, bvac134. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Lou, P.; Zhao, M.; Wang, Y.; Tang, M.; Gong, M.; Liao, G.; Yuan, Y.; Li, L. Elevated branched-chain α-keto acids exacerbate macrophage oxidative stress and chronic inflammatory damage in type 2 diabetes mellitus. Free Radic. Biol. Med. 2021, 175, 141–154. [Google Scholar] [CrossRef]
- Hamaya, R.; Mora, S.; Lawler, P.R.; Cook, N.R.; Ridker, P.M.; Buring, J.E.; Lee, I.-M.; Manson, J.E.; Tobias, D.K. Association of Plasma Branched-Chain Amino Acid with Biomarkers of Inflammation and Lipid Metabolism in Women. Circ. Genom. Precis. Med. 2021, 14, e003330. [Google Scholar] [CrossRef]
- Reddy, P.; Leong, J.; Jialal, I. Amino acid levels in nascent metabolic syndrome: A contributor to the pro-inflammatory burden. J. Diabetes Complicat. 2018, 32, 465–469. [Google Scholar] [CrossRef]
- Cosentino, R.G.; Churilla, J.R.; Josephson, S.; Molle-Rios, Z.; Hossain, J.; Prado, W.L.; Balagopal, P.B. Branched-chain Amino Acids and Relationship with Inflammation in Youth With Obesity: A Randomized Controlled Intervention Study. J. Clin. Endocrinol. Metab. 2021, 106, 3129–3139. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, R.J.O.; Rizo-Roca, D.; Chibalin, A.V.; Chorell, E.; Furrer, R.; Katayama, S.; Harada, J.; Karlsson, H.K.R.; Handschin, C.; Moritz, T.; et al. Branched-chain amino acid metabolism is regulated by ERRα in primary human myotubes and is further impaired by glucose loading in type 2 diabetes. Diabetologia 2021, 64, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Z.; Liu, L.; Han, T.; Yang, X.; Sun, C. Genetic predisposition to impaired metabolism of the branched chain amino acids, dietary intakes, and risk of type 2 diabetes. Genes Nutr. 2021, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhang, F. Amino Acids Metabolism in Retinopathy: From Clinical and Basic Research Perspective. Metabolites 2022, 12, 1244. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.; Son, J.L.; Rhee, S.Y.; Kim, D.Y.; Chon, S.; Lim, H.; Woo, J.T. Dietary Glutamic Acid and Aspartic Acid as Biomarkers for Predicting Diabetic Retinopathy. Sci. Rep. 2021, 11, 7244. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Tang, J.; Zhang, F.; Liu, L.; Zhou, J.; Chen, M.; Li, M.; Wu, X.; Nie, Y.; Duan, J. Global Trends and Performances in Diabetic Retinopathy Studies: A Bibliometric Analysis. Front. Public Health 2023, 11, 1128008. [Google Scholar] [CrossRef]
- Amoaku, W.M.; Ghanchi, F.; Bailey, C.; Banerjee, S.; Banerjee, S.; Downey, L.; Gale, R.; Hamilton, R.; Khunti, K.; Posner, E.; et al. Diabetic Retinopathy and Diabetic Macular Oedema Pathways and Management: UK Consensus Working Group. Eye 2020, 34, 1–51. [Google Scholar] [CrossRef]
- Gale, M.J.; Scruggs, B.A.; Flaxel, C.J. Diabetic Eye Disease: A Review of Screening and Management Recommendations. Clin. Exp. Ophthalmol. 2021, 49, 128–145. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial (DCCT): Results of Feasibility Study. The DCCT Research Group. Diabetes Care 1987, 10, 1–19. [CrossRef]
- King, P.; Peacock, I.; Donnelly, R. The UK Prospective Diabetes Study (UKPDS): Clinical and Therapeutic Implications for Type 2 Diabetes. Br. J. Clin. Pharmacol. 1999, 48, 643–648. [Google Scholar] [CrossRef]
- Zhan, L. Frontiers in Understanding the Pathological Mechanism of Diabetic Retinopathy. Med. Sci. Monit. 2023, 29, e939658. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M. The Polyol Pathway as a Mechanism for Diabetic Retinopathy: Attractive, Elusive, and Resilient. Exp. Diabesity Res. 2007, 2007, 61038. [Google Scholar] [CrossRef]
- Ahuja, S.; Saxena, S.; Akduman, L.; Meyer, C.H.; Kruzliak, P.; Khanna, V.K. Serum Vascular Endothelial Growth Factor Is a Biomolecular Biomarker of Severity of Diabetic Retinopathy. Int. J. Retina Vitr. 2019, 5, 29. [Google Scholar] [CrossRef]
- Ruberte, J.; Ayuso, E.; Navarro, M.; Carretero, A.; Nacher, V.; Haurigot, V.; George, M.; Llombart, C.; Casellas, A.; Costa, C.; et al. Increased Ocular Levels of IGF-1 in Transgenic Mice Lead to Diabetes-like Eye Disease. J. Clin. Investig. 2004, 113, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Boccuni, I.; Fairless, R. Retinal Glutamate Neurotransmission: From Physiology to Pathophysiological Mechanisms of Retinal Ganglion Cell Degeneration. Life 2022, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Milla-Navarro, S.; Diaz-Tahoces, A.; Ortuño-Lizarán, I.; Fernández, E.; Cuenca, N.; Germain, F.; de la Villa, P. Visual Disfunction Due to the Selective Effect of Glutamate Agonists on Retinal Cells. Int. J. Mol. Sci. 2021, 22, 6245. [Google Scholar] [CrossRef]
- Sweatt, A.J.; Garcia-Espinosa, M.A.; Wallin, R.; Hutson, S.M. Branched-Chain Amino Acids and Neurotransmitter Metabolism: Expression of Cytosolic Branched-Chain Aminotransferase (BCATC) in the Cerebellum and Hippocampus. J. Comp. Neurol. 2004, 477, 360–370. [Google Scholar] [CrossRef]
- Huemer, J.; Khalid, H.; Ferraz, D.; Faes, L.; Korot, E.; Jurkute, N.; Balaskas, K.; Egan, C.A.; Petzold, A.; Keane, P.A. Re-Evaluating Diabetic Papillopathy Using Optical Coherence Tomography and Inner Retinal Sublayer Analysis. Eye 2022, 36, 1476–1485. [Google Scholar] [CrossRef]
- Saad, G.; Ben Abdelkrim, A.; Beizig Maaroufi, A.; Njah Kacem, M.; Chaieb Chadli, M.; Ach, K. Clinical patterns of third nerve palsies in diabetic patients. Tunis Med. 2020, 98, 513–517. [Google Scholar]
- Zhou, C.; Zhang, Q.; Lu, L.; Wang, J.; Liu, D.; Liu, Z. Metabolomic Profiling of Amino Acids in Human Plasma Distinguishes Diabetic Kidney Disease From Type 2 Diabetes Mellitus. Front. Med. 2021, 8, 765873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, D.; He, Y.; Lou, K.; Zheng, D.; Han, W. Branched Chain Amino Acids Protects Rat Mesangial Cells from High Glucose by Modulating TGF-β1 and BMP-7. Diabetes Metab. Syndr. Obes. 2019, 12, 2433–2440, Erratum in: Diabetes Metab. Syndr. Obes. 2020, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Lichtenberg, S.; Lokhov, P.G. Potential Plasma Metabolite Biomarkers of Diabetic Nephropathy: Untargeted Metabolomics Study. J. Pers. Med. 2022, 12, 1889. [Google Scholar] [CrossRef]
- Fang, Q.; Liu, N.; Zheng, B.; Guo, F.; Zeng, X.; Huang, X.; Ouyang, D. Roles of Gut Microbial Metabolites in Diabetic Kidney Disease. Front. Endocrinol. 2021, 12, 636175. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, M.K.; Gnudi, L. Diabetic Nephropathy: An Overview. Methods Mol. Biol. 2020, 2067, 3–7. [Google Scholar] [CrossRef]
- Qi, W.; Chen, X.; Poronnik, P.; Pollock, C.A. Transforming growth factor-beta/connective tissue growth factor axis in the kidney. Int. J. Biochem. Cell Biol. 2008, 40, 9–13. [Google Scholar] [CrossRef]
- Hills, C.E.; Bland, R.; Bennett, J.; Ronco, P.M.; Squires, P.E. High glucose up-regulates ENaC and SGK1 expression in HCD-cells. Cell. Physiol. Biochem. 2006, 18, 337–346. [Google Scholar] [CrossRef]
- Feng, Y.; Jin, M.Y.; Liu, D.W.; Wei, L. Bone morphogenetic protein (BMP) 7 expression is regulated by the E3 ligase UBE4A in diabetic nephropathy. Arch. Physiol. Biochem. 2020, 126, 416–419. [Google Scholar] [CrossRef]
- McMahon, R.; Murphy, M.; Clarkson, M.; Taal, M.; Mackenzie, H.S.; Godson, C.; Martin, F.; Brady, H.R. IHG-2, a mesangial cell gene induced by high glucose, is human gremlin. Regulation by extracellular glucose concentration, cyclic mechanical strain, and transforming growth factor-beta1. J. Biol. Chem. 2000, 275, 9901–9904. [Google Scholar] [CrossRef]
- Asghari, G.; Farhadnejad, H.; Teymoori, F.; Mirmiran, P.; Tohidi, M.; Azizi, F. High dietary intake of branched-chain amino acids is associated with an increased risk of insulin resistance in adults. J. Diabetes 2018, 10, 357–364, Erratum in: J. Diabetes 2019, 11, 920. [Google Scholar] [CrossRef]
- Karusheva, Y.; Koessler, T.; Strassburger, K.; Markgraf, D.; Mastrototaro, L.; Jelenik, T.; Simon, M.C.; Pesta, D.; Zaharia, O.P.; Bódis, K.; et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2019, 110, 1098–1107. [Google Scholar] [CrossRef]
- Lim, L.-L.; Lau, E.S.; Lee, H.M.; Tam, C.H.; Lim, C.K.; Luk, A.; Chow, E.; Ma, R.C.; Chan, J.C.; Kong, A.P. 533-P: Association of Serum Branched-Chain Amino Acids with Kidney Function Decline in Type 2 Diabetes: The Hong Kong Diabetes Register. Diabetes 2019, 68 (Suppl. 1), 533-P. [Google Scholar] [CrossRef]
- Pillai, S.M.; Herzog, B.; Seebeck, P.; Pellegrini, G.; Roth, E.; Verrey, F. Differential Impact of Dietary Branched Chain and Aromatic Amino Acids on Chronic Kidney Disease Progression in Rats. Front. Physiol. 2019, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; González-Amor, M.; Rodrigues-Diez, R.R.; Esteban, V.; Ruiz-Ortega, M.; Salaices, M.; Mas, S.; Briones, A.M.; Egido, J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J. Cell. Mol. Med. 2018, 22, 4948–4962. [Google Scholar] [CrossRef] [PubMed]
- Mi, N.; Zhang, X.J.; Ding, Y.; Li, G.H.; Wang, W.D.; Xian, H.X.; Xu, J. Branched-chain amino acids attenuate early kidney injury in diabetic rats. Biochem. Biophys. Res. Commun. 2015, 466, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.H.; Gao, Z.X.; Liu, D.W.; Liu, Z.S.; Wu, P. Gut microbiota and its metabolites-molecular mechanisms and management strategies in diabetic kidney disease. Front. Immunol. 2023, 14, 1124704. [Google Scholar] [CrossRef]
- Winther, S.A.; Øllgaard, J.C.; Tofte, N.; Tarnow, L.; Wang, Z.; Ahluwalia, T.S.; Jorsal, A.; Theilade, S.; Parving, H.H.; Hansen, T.W.; et al. Utility of Plasma Concentration of Trimethylamine N-Oxide in Predicting Cardiovascular and Renal Complications in Individuals With Type 1 Diabetes. Diabetes Care 2019, 42, 1512–1520. [Google Scholar] [CrossRef]
- Kikuchi, K.; Saigusa, D.; Kanemitsu, Y.; Matsumoto, Y.; Thanai, P.; Suzuki, N.; Mise, K.; Yamaguchi, H.; Nakamura, T.; Asaji, K.; et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat. Commun. 2019, 10, 1835. [Google Scholar] [CrossRef]
- Salguero, M.V.; Al-Obaide, M.A.I.; Singh, R.; Siepmann, T.; Vasylyeva, T.L. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp. Ther. Med. 2019, 18, 3461–3469. [Google Scholar] [CrossRef]
- Fernandes, R.; Viana, S.D.; Nunes, S.; Reis, F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1876–1897. [Google Scholar] [CrossRef]
- Sriboonvorakul, N.; Pan-Ngum, W.; Poovorawan, K.; Muangnoicharoen, S.; Quinn, L.M.; Tan, B.K. Low Branched Chain Amino Acids and Tyrosine in Thai Patients with Type 2 Diabetes Mellitus Treated with Metformin and Metformin-Sulfonylurea Combination Therapies. J. Clin. Med. 2021, 10, 5424. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Drzewoski, J.; Hanefeld, M. The Current and Potential Therapeutic Use of Metformin-The Good Old Drug. Pharmaceuticals 2021, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Silver, B.; Ramaiya, K.; Andrew, S.B.; Fredrick, O.; Bajaj, S.; Kalra, S.; Charlotte, B.M.; Claudine, K.; Makhoba, A. EADSG Guidelines: Insulin Therapy in Diabetes. Diabetes Ther. 2018, 9, 449–492. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Jin, K.; Yue, M.; Chen, X.; Chen, J. Research Progress on the GIP/GLP-1 Receptor Coagonist Tirzepatide, a Rising Star in Type 2 Diabetes. J. Diabetes Res. 2023, 2023, 5891532. [Google Scholar] [CrossRef]
- Tee, K.B.; Ibrahim, L.; Hashim, N.M.; Saiman, M.Z.; Zakaria, Z.H.; Huri, H.Z. Pharmacokinetic–Pharmacometabolomic Approach in Early-Phase Clinical Trials: A Way Forward for Targeted Therapy in Type 2 Diabetes. Pharmaceutics 2022, 14, 1268. [Google Scholar] [CrossRef]
- Bao, Y.; Zhao, T.; Wang, X.; Qiu, Y.; Su, M.; Jia, W.; Jia, W. Metabonomic Variations in the Drug-Treated Type 2 Diabetes Mellitus Patients and Healthy Volunteers. J. Proteome Res. 2009, 8, 1623–1630. [Google Scholar] [CrossRef]
- Walford, G.A.; Davis, J.; Warner, A.S.; Ackerman, R.J.; Billings, L.K.; Chamarthi, B.; Fanelli, R.R.; Hernandez, A.M.; Huang, C.; Khan, S.Q.; et al. Branched Chain and Aromatic Amino Acids Change Acutely Following Two Medical Therapies for Type 2 Diabetes Mellitus. Metabolism 2013, 62, 1772–1778. [Google Scholar] [CrossRef]
- Sawicki, K.T.; Ning, H.; Allen, N.B.; Carnethon, M.R.; Wallia, A.; Otvos, J.D.; Ben-Sahra, I.; McNally, E.M.; Snell-Bergeon, J.K.; Wilkins, J.T. Longitudinal trajectories of branched chain amino acids through young adulthood and diabetes in later life. JCI Insight 2023, 8, e166956. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Bhatt, A.S.; Claggett, B.L.; Vaduganathan, M.; Kulac, I.J.; Lam, C.S.; Hernandez, A.F.; Martinez, F.A.; Inzucchi, S.E.; Shah, S.J.; et al. Effect of Dapagliflozin on Health Status in Patients with Preserved or Mildly Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2023, 81, 460–473. [Google Scholar] [CrossRef]
- Kupis, M.; Samelska, K.; Szaflik, J.; Skopiński, P. Novel therapies for diabetic retinopathy. Cent. Eur. J. Immunol. 2022, 47, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Wołos-Kłosowicz, K.; Matuszewski, W.; Rutkowska, J.; Krankowska, K.; Bandurska-Stankiewicz, E. Will GLP-1 Analogues and SGLT-2 Inhibitors Become New Game Changers for Diabetic Retinopathy? J. Clin. Med. 2022, 11, 6183. [Google Scholar] [CrossRef]
- Gong, Q.; Zhang, R.; Wei, F.; Fang, J.; Zhang, J.; Sun, J.; Sun, Q.; Wang, H. SGLT2 Inhibitor-Empagliflozin Treatment Ameliorates Diabetic Retinopathy Manifestations and Exerts Protective Effects Associated with Augmenting Branched Chain Amino Acids Catabolism and Transportation in Db/Db Mice. Biomed. Pharmacother. 2022, 152, 113222. [Google Scholar] [CrossRef] [PubMed]
- Sonnet, D.S.; NO’Leary, M.; AGutierrez, M.; MNguyen, S.; Mateen, S.; Hsu, Y.; PMitchell, K.; JLopez, A.; Vockley, J.; KKennedy, B.; et al. Metformin inhibits Branched Chain Amino Acid (BCAA) derived ketoacidosis and promotes metabolic homeostasis in MSUD. Sci. Rep. 2016, 6, 28775. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.E.; Lyon, E.S.; Vaughan, R.A. Effect of metformin on myotube BCAA catabolism. J. Cell. Biochem. 2020, 121, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Paterson, K.R.; Gyi, K.M.; McBride, D.; Cohen, H.N.; Shenkin, A.; Manderson, W.G.; MacCuish, A.C. Effect of Sulphonylurea Administration on Insulin Secretion and Amino Acid Metabolism in Non-insulin-dependent Diabetic Patients. Diabetic Med. 1985, 2, 38–40. [Google Scholar] [CrossRef]
- Iwasa, M.; Ishihara, T.; Mifuji-Moroka, R.; Fujita, N.; Kobayashi, Y.; Hasegawa, H.; Iwata, K.; Kaito, M.; Takei, Y. Elevation of branched-chain amino acid levels in diabetes and NAFL and changes with antidiabetic drug treatment. Obes. Res. Clin. Pract. 2015, 9, 293–297. [Google Scholar] [CrossRef]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef]
- Ellaway, A.; Dundas, R.; Olsen, J.R.; Shiels, P.G. Perceived neighbourhood problems over time and associations with adiposity. Int. J. Environ. Res. Public Health 2018, 15, 1854. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Macotela, Y.; Emanuelli, B.; Bång, A.M.; Espinoza, D.O.; Boucher, J.; Beebe, K.; Gall, W.; Kahn, C.R. Dietary leucine--an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS ONE 2011, 6, e21187. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326, Erratum in Cell Metab. 2009, 9, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Gumus Balikcioglu, P.; Jachthuber Trub, C.; Balikcioglu, M.; Ilkayeva, O.; White, P.J.; Muehlbauer, M.; Bain, J.R.; Armstrong, S.; Freemark, M. Branched-chain α-keto acids and glutamate/glutamine: Biomarkers of insulin resistance in childhood obesity. Endocrinol. Diabetes Metab. 2023, 6, e388. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.R.; Bihlmeyer, N.A.; Roche, K.; Catherine, C.; Jawahar, J.; Kwee, L.C.; Younge, N.E.; Silverman, J.; Ilkayeva, O.; Sarria, C.; et al. The Pediatric Obesity Microbiome and Metabolism Study (POMMS): Methods, Baseline Data, and Early Insights. Obesity 2021, 29, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Jachthuber Trub, C.; Balikcioglu, M.; Freemark, M.; Bain, J.; Muehlbauer, M.; Ilkayeva, O.; White, P.J.; Armstrong, S.; Østbye, T.; Grambow, S.; et al. Impact of lifestyle Intervention on branched-chain amino acid catabolism and insulin sensitivity in adolescents with obesity. Endocrinol. Diabetes Metab. 2021, 4, e00250. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Wu, Y.; Guo, Y.; Wang, X. Baseline Serum BCAAs are Related to the Improvement in Insulin Resistance in Obese People After a Weight Loss Intervention. Diabetes Metab. Syndr. Obes. 2023, 16, 179–186. [Google Scholar] [CrossRef]
- Du, C.; Liu, W.J.; Yang, J.; Zhao, S.S.; Liu, H.X. The Role of Branched-Chain Amino Acids and Branched-Chain α-Keto Acid Dehydrogenase Kinase in Metabolic Disorders. Front. Nutr. 2022, 9, 932670. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamashita, T.; Osone, T.; Hosooka, T.; Shinohara, M.; Kitahama, S.; Sasaki, K.; Sasaki, D.; Yoneshiro, T.; Suzuki, T.; et al. Bacteroides spp. promotes branched-chain amino acid catabolism in brown fat and inhibits obesity. iScience 2021, 24, 103342. [Google Scholar] [CrossRef]
- Tso, S.C.; Qi, X.; Gui, W.J.; Chuang, J.L.; Morlock, L.K.; Wallace, A.L.; Ahmed, K.; Laxman, S.; Campeau, P.M.; Lee, B.H.; et al. Structure-based design and mechanisms of allosteric inhibitors for mitochondrial branched-chain α-ketoacid dehydrogenase kinase. Proc. Natl. Acad. Sci. USA 2013, 110, 9728–9733. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, M.; He, X.; Wu, Q.; Li, D.L.; Zang, W.J. Pyridostigmine Protects Against Diabetic Cardiomyopathy by Regulating Vagal Activity, Gut Microbiota, and Branched-Chain Amino Acid Catabolism in Diabetic Mice. Front. Pharmacol. 2021, 12, 647481. [Google Scholar] [CrossRef]
- Torki, S.A.; Bahadori, E.; Aghakhaninejad, Z.; Mohseni, G.K.; Tajadod, S.; Rajabi Harsini, A.; Azaryan, F.; Saeedirad, Z.; Askarpour, S.A.; Mahmoudi, Z.; et al. Association between type 2 diabetes and branched chain amino acids (BCAA); a case-control study. J. Diabetes Metab. Disord. 2023. [Google Scholar] [CrossRef]
- Cai, D.; Hou, B.; Xie, S.L. Amino acid analysis as a method of discovering biomarkers for diagnosis of diabetes and its complications. Amino Acids 2023, 55, 563–578. [Google Scholar] [CrossRef]
- Ancel, P.; Martin, J.C.; Doukbi, E.; Houssays, M.; Gascon, P.; Righini, M.; Matonti, F.; Svilar, L.; Valmori, M.; Tardivel, C.; et al. Untargeted Multiomics Approach Coupling Lipidomics and Metabolomics Profiling Reveals New Insights in Diabetic Retinopathy. Int. J. Mol. Sci. 2023, 24, 12053. [Google Scholar] [CrossRef]
- Dutta, D.; Khandelwal, D. Lifestyle Interventions Reduce the Risk of Type 2 Diabetes through Decreasing Branched-Chain Amino Acids: Newer Insights. J. Clin. Endocrinol. Metab. 2023, 108, e27–e28. [Google Scholar] [CrossRef]

| Source | Trial Type | Molecule | Outcomes |
|---|---|---|---|
| Sriboonvorakul et al. [71] | A clinical trial on a cohort of T2DM patients that was compared to healthy controls following treatment with single (metformin) or multiple drug (metformin and sulfonylurea). | Metformin and Sulfonylurea |
In both of the treated T2DM groups, BCAAs were significantly lower than the healthy controls. Isoleucine was significantly lower in the single-treated T2DM group compared with the healthy controls. Valine was significantly lower in both treated T2DM groups compared with healthy controls. Leucine was significantly lower in both treated T2DM groups compared with healthy controls (p < 0.0001) |
| Gong et al. [83] | An experimental study on seven-week-old male diabetic db/db mice. | Empagliflozin | EMPA significantly inhibited oxidative stress and apoptosis and recovered tight junction in diabetic retinas. EMPA suppressed aberrant BCAA accumulation, which led to downregulation of inflammation and angiogenic factors, including TNF-α, IL-6, VCAM-1, and VEGF induced by diabetes. BCKAs were increased in diabetic retinas and decreased with EMPA application. BCKDK was enhanced and BCKDHA and BCKDHB were decreased in diabetic retinas |
| S Sonnet D et al. [84] | An experimental study on the iMSUD mouse model | Metformin | Metformin reduced levels of KIC in patient-derived fibroblasts by 20–50%; in the muscle by 69%, and in serum by 56% and restored levels of mitochondrial metabolites. Metformin decreased the expression of BCAT, which produces KIC in skeletal muscle. |
| Riviera et al. [85] | An experimental study on C2C12 mouse myoblasts (CRL-1772; ATCC, Manassas, VA). | Metformin | Metformin inhibited mitochondrial metabolism, promoting an activation of AMPK and subsequently PGC-1. Metformin reduced KLF15 (Kruppel-like factor 15) protein levels, leading to reduced expression/activation of BCAA catabolic enzymes. Metformin enhanced KLF15 mRNA expression, the implications of which were unknown. |
| Paterson et al. [86] | A clinical trial including nine non-insulin-dependent diabetic patients. | Gliclazide | Glycaemic control was improved, but fasting amino acid levels were not altered. Postprandial levels of BCAAs were significantly reduced: total BCAA (valine, leucine, and isoleucine) after 3 months of therapy (p < 0.01). |
| Iwasa M, et al. [87] | A clinical trial on 84 subjects with type 2 DM, NAFL, hypertension, and dyslipidemia. | Pioglitazone and Alogliptin | BCAA levels were negatively correlated with HDL cholesterol. BCAA levels were positively correlated with ALT, suggesting an association with fatty liver changes. There were no significant correlations with HbA1c, HOMA-IR, TG, hs-CRP, or adiponectin. Serum BCAA levels in diabetics were higher than in non-diabetics. Treatment with pioglitazone and alogliptin improved serum haemoglobin A1c and decreased BCAA levels. |
| NCT Number Trial | Status | Molecule Investigated | Trial Type | Primary Outcomes |
|---|---|---|---|---|
| NCT02351323 | Completed | glutamine and leucine | randomized, double-blind, placebo controlled, clinical trial | test the efficacy of 6 months of glutamine supplements in reducing biomarkers for IR and weight gain among 56 obese adolescents aged 12–19 years with a BMI ≥ 95th percentile and a family history of T2DM |
| NCT01211717 | Completed | isoleucine, leucine, and valine | randomized, interventional clinical trial | determine the effectiveness of three BCAAs (isoleucine, leucine, and valine) on treating delayed onset muscle soreness in T2DM |
| NCT02435277 NCT02151461 | Completed | leucine and metformin combinations | phase 2 trials randomized | change in fasting plasma glucose from baseline (day 1) to week 4 (day 28); change in HbA1c Levels |
| NCT01593605 | Completed | resveratrol/leucine and resveratrol/HMB | randomized controlled trial | their ability to control glucose levels in persons without diabetes but with impaired fasting glucose |
| NCT04461236 | Recruiting | isoleucine | randomized controlled clinical trial | change in whole-body protein metabolism in type 2 diabetic obese subjects; 24 h glucose levels in type 2 diabetic obese subjects |
| NCT04424537 | Withdrawn | MRBs made with BCAD2 powder (lacking BCAAs) | randomized controlled trial | change in weight and fasting blood glucose level change in insulin sensitivity |
| NCT03785951 | Unknown | wheat protein with leucine | double-blind, randomized, controlled, three-way, cross-over study | change in fasting and day-long glucose levels, and day-long insulin levels (using ELISA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, D.M.; Gosav, E.M.; Botoc, T.; Floria, M.; Tarniceriu, C.C.; Maranduca, M.A.; Haisan, A.; Cucu, A.I.; Rezus, C.; Costea, C.F. Depiction of Branched-Chain Amino Acids (BCAAs) in Diabetes with a Focus on Diabetic Microvascular Complications. J. Clin. Med. 2023, 12, 6053. https://doi.org/10.3390/jcm12186053
Tanase DM, Gosav EM, Botoc T, Floria M, Tarniceriu CC, Maranduca MA, Haisan A, Cucu AI, Rezus C, Costea CF. Depiction of Branched-Chain Amino Acids (BCAAs) in Diabetes with a Focus on Diabetic Microvascular Complications. Journal of Clinical Medicine. 2023; 12(18):6053. https://doi.org/10.3390/jcm12186053
Chicago/Turabian StyleTanase, Daniela Maria, Evelina Maria Gosav, Tina Botoc, Mariana Floria, Claudia Cristina Tarniceriu, Minela Aida Maranduca, Anca Haisan, Andrei Ionut Cucu, Ciprian Rezus, and Claudia Florida Costea. 2023. "Depiction of Branched-Chain Amino Acids (BCAAs) in Diabetes with a Focus on Diabetic Microvascular Complications" Journal of Clinical Medicine 12, no. 18: 6053. https://doi.org/10.3390/jcm12186053
APA StyleTanase, D. M., Gosav, E. M., Botoc, T., Floria, M., Tarniceriu, C. C., Maranduca, M. A., Haisan, A., Cucu, A. I., Rezus, C., & Costea, C. F. (2023). Depiction of Branched-Chain Amino Acids (BCAAs) in Diabetes with a Focus on Diabetic Microvascular Complications. Journal of Clinical Medicine, 12(18), 6053. https://doi.org/10.3390/jcm12186053