Subfertility as Overlapping of Nutritional, Endocrine, Immune, and Cardiometabolic Dysregulations—A Study Focused on Biochemical Endophenotypes of Subfertile Couples
Abstract
:1. Introduction
2. Materials and Methods
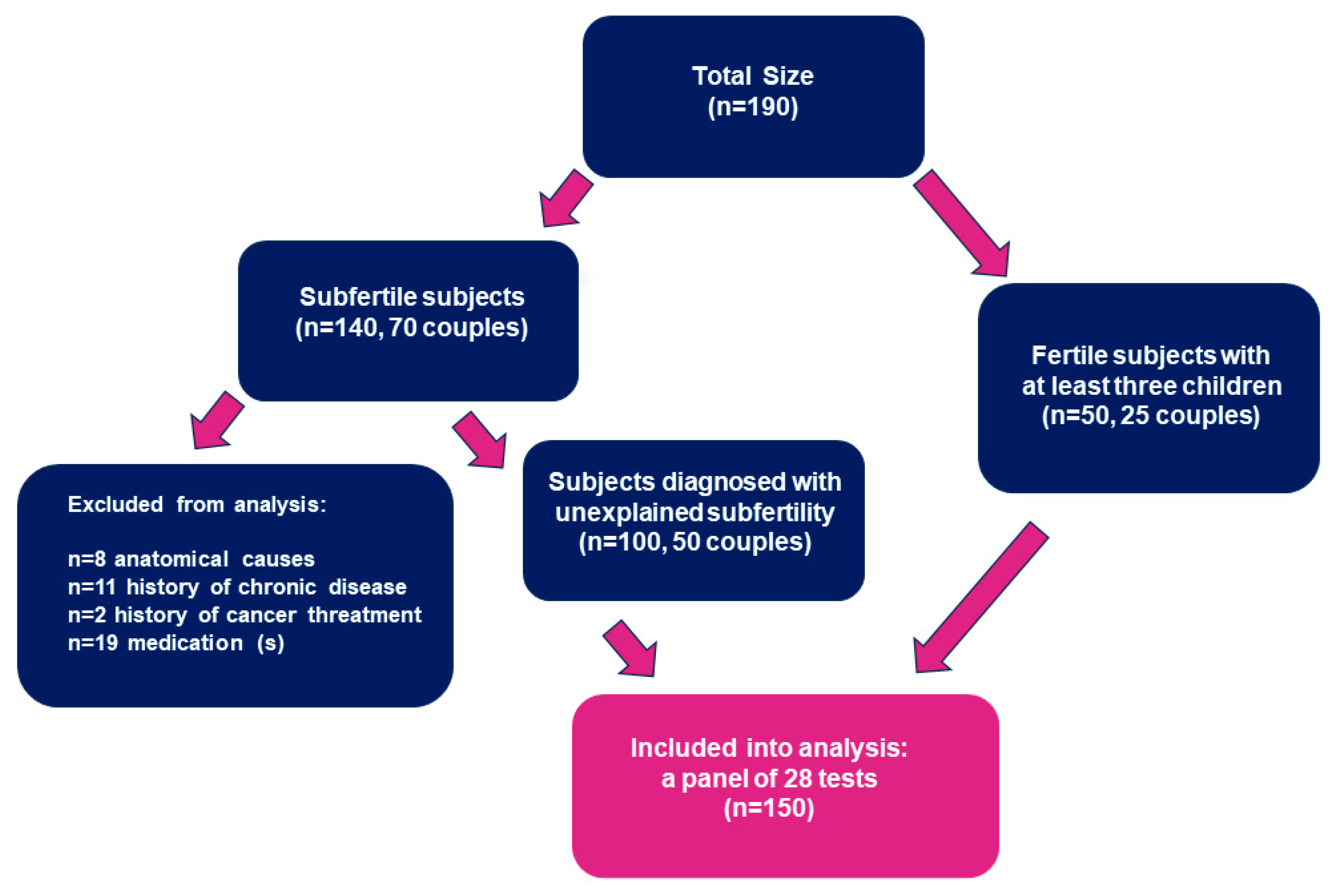
2.1. Patients and Samples
2.2. Methods
2.3. Laboratory Criteria
2.3.1. Thyroid Function Tests
2.3.2. Lipid, Liver, and Carbohydrate Metabolism
2.3.3. Total IgE and Allergen-Specific IgE Sensitization
2.4. Statistical Analysis
3. Results
3.1. Thyroid Function and Thyroid Autoimmunity
3.1.1. Thyroid Function Tests: Thyroid-Stimulating Hormone (TSH) and Free Thyroxine (fT4)
3.1.2. Autoimmune Thyroid Disorder (AITD): Anti-Thyroid Peroxidase Autoantibodies (Anti-TPO) and Anti-Thyroglobulin Autoantibodies (Anti-TG)
3.1.3. Biochemical Characteristics of TPO-Positive Subfertile Participants
3.2. Lipid Profile
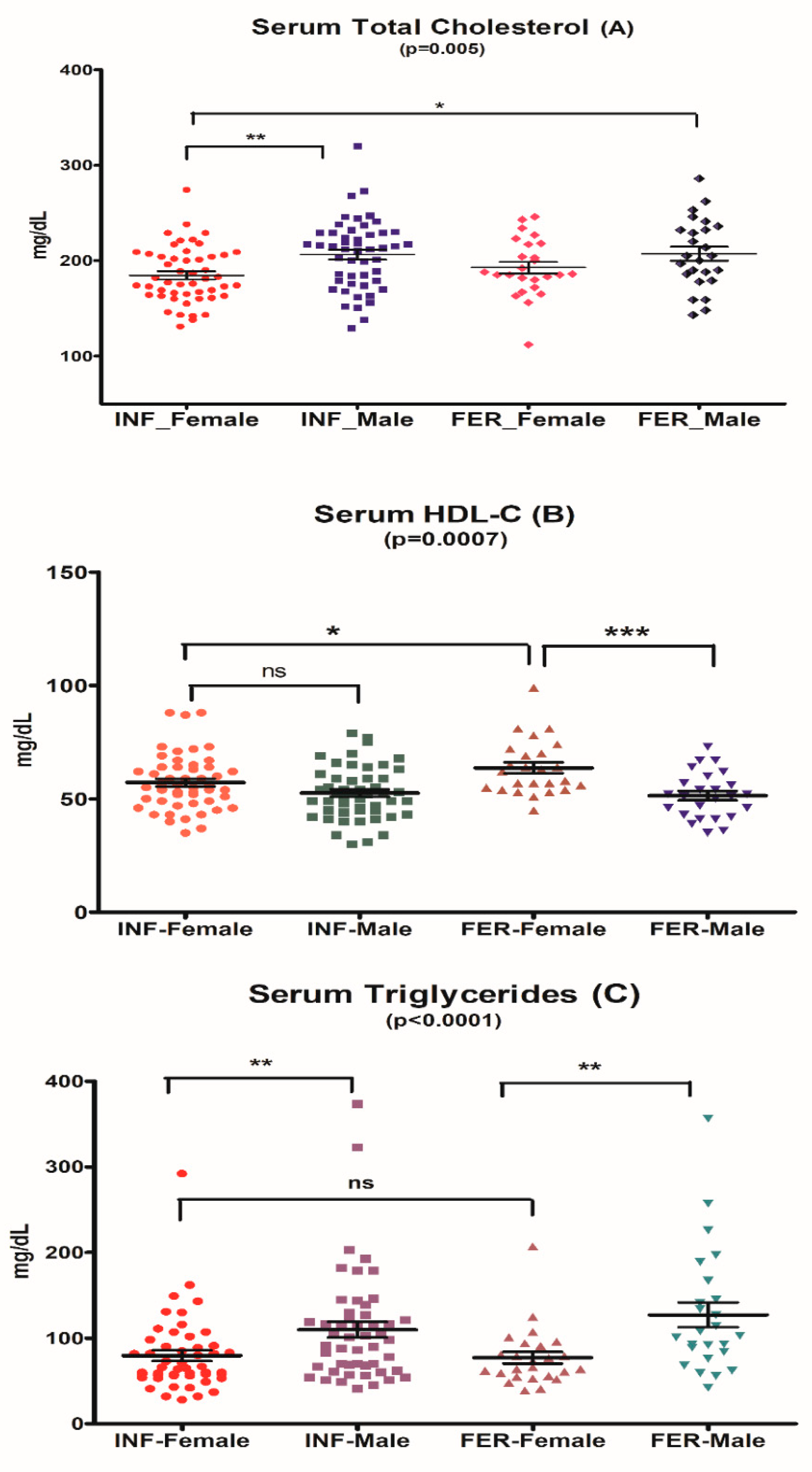
3.2.1. Serum Total Cholesterol
3.2.2. Serum LDL-Cholesterol
3.2.3. Serum Triglycerides
3.2.4. Serum HDL-Cholesterol
3.2.5. Spearman’s Correlations for Lipid Profile Indices
3.3. Glucose and Insulin Levels and HOMA-IR
3.4. Total IgE and Allergen-Specific IgE Sensitization
sIgE Sensitization
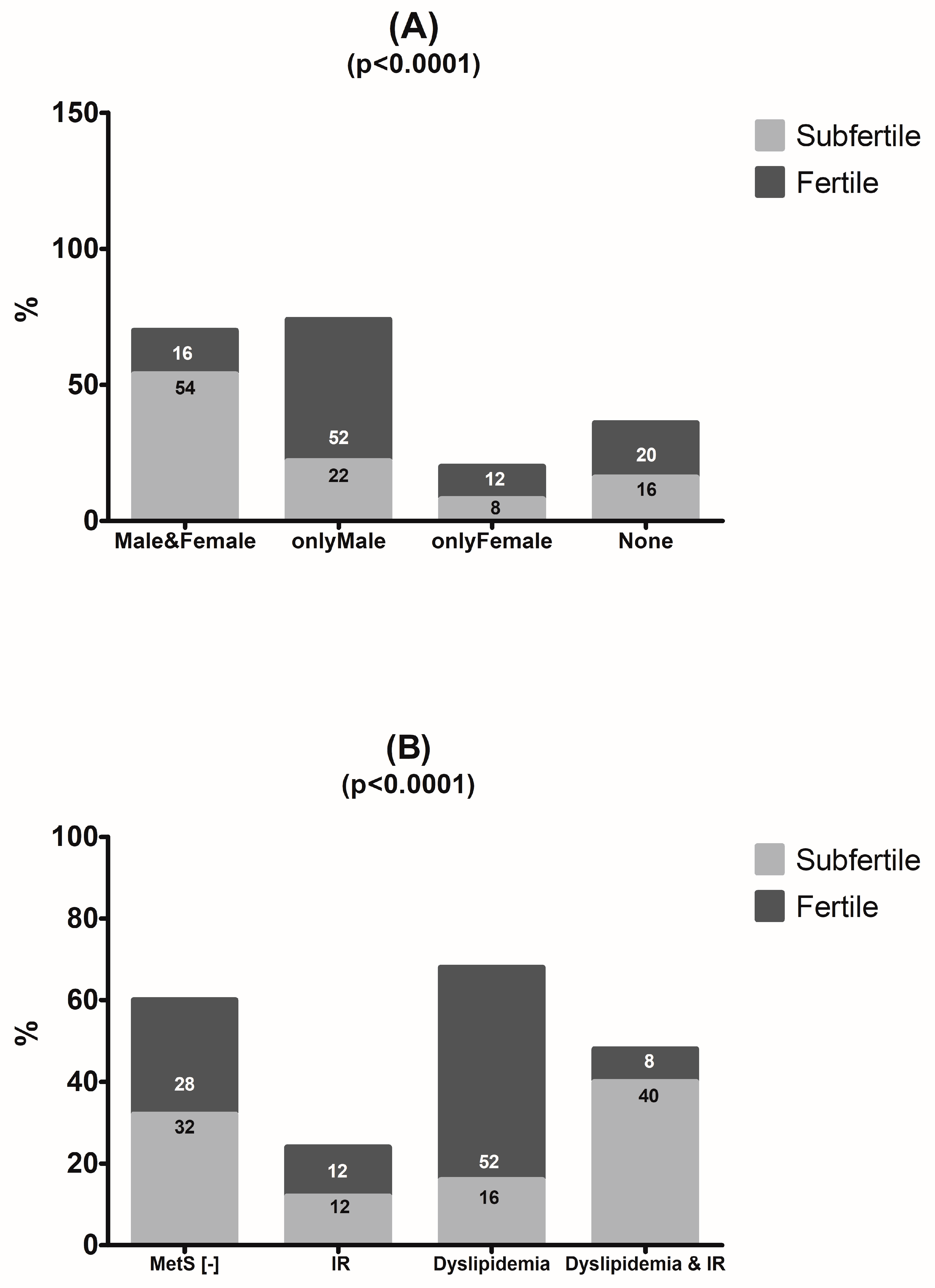
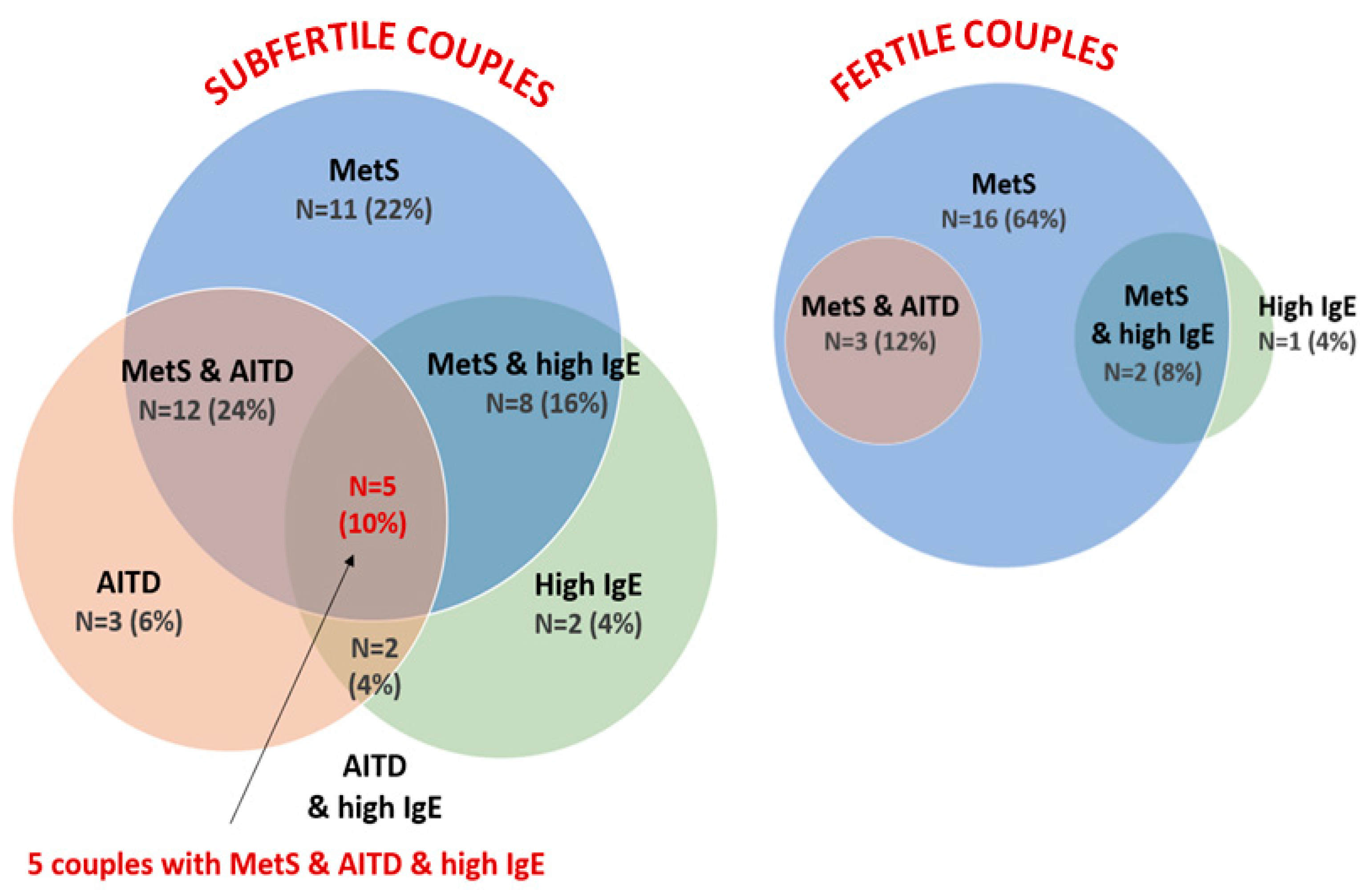
3.5. Laboratory Test Result Comparison between Couples
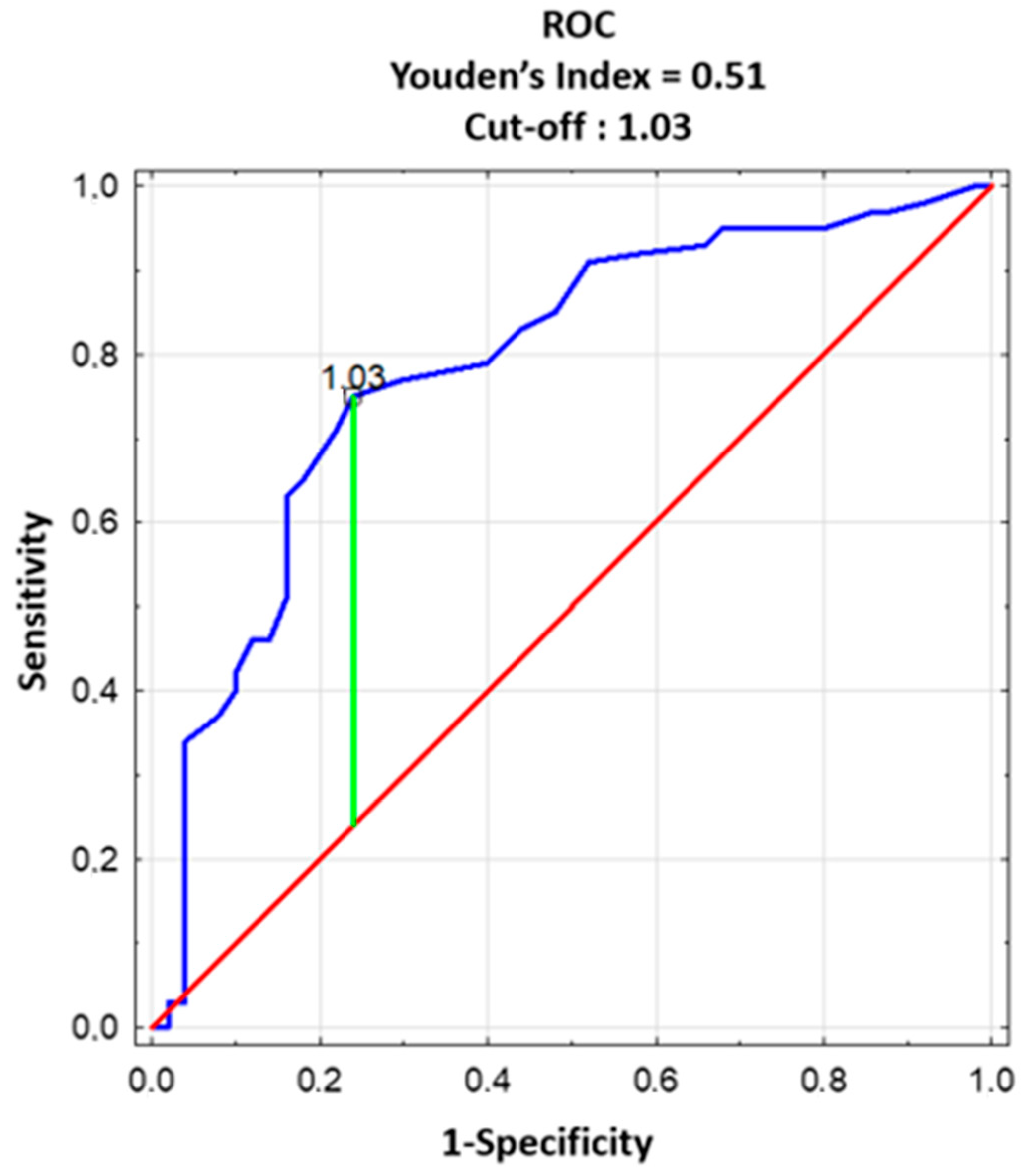
3.6. Laboratory Predictors of Individual and Couple Subfertility
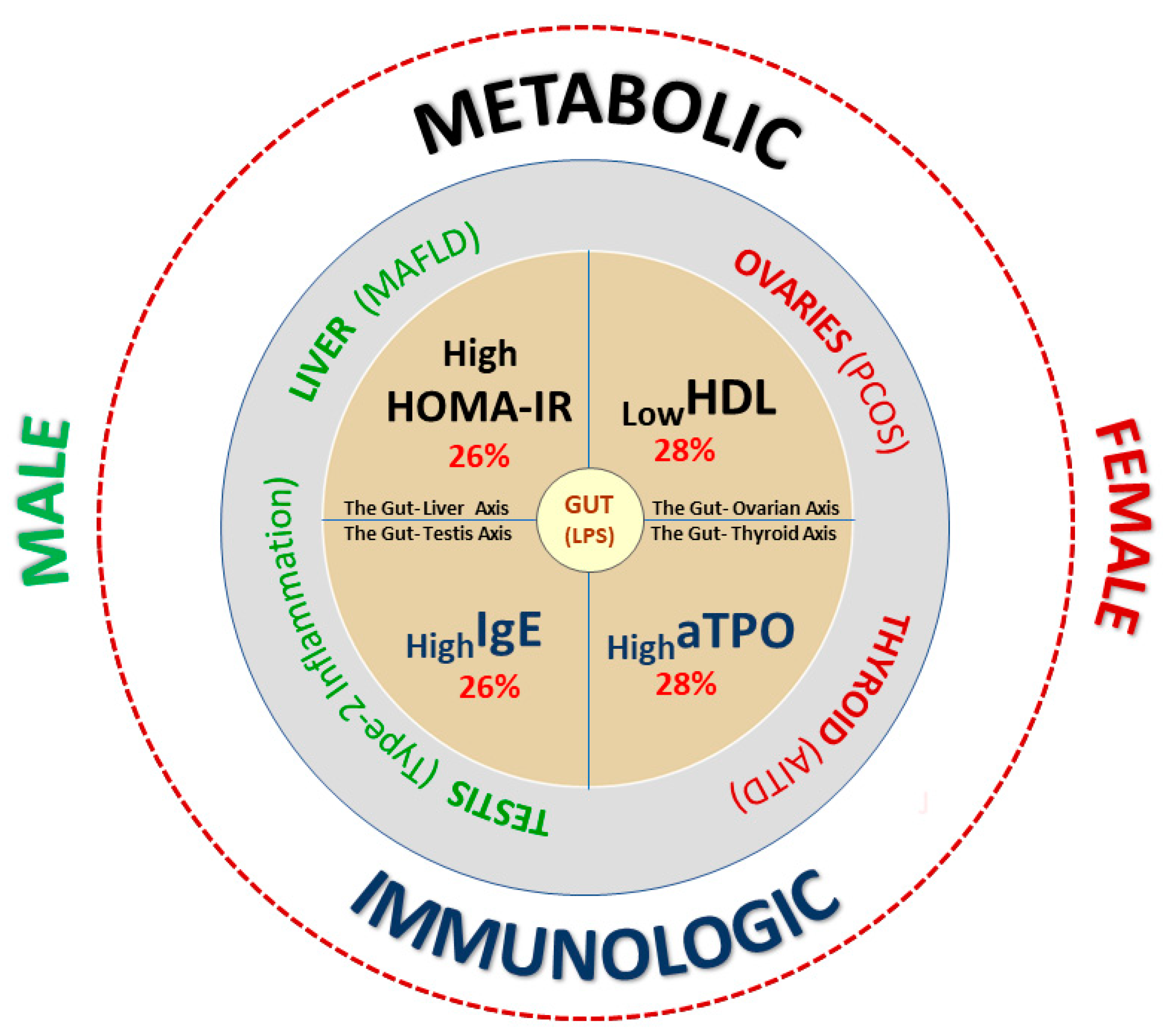
4. Discussion
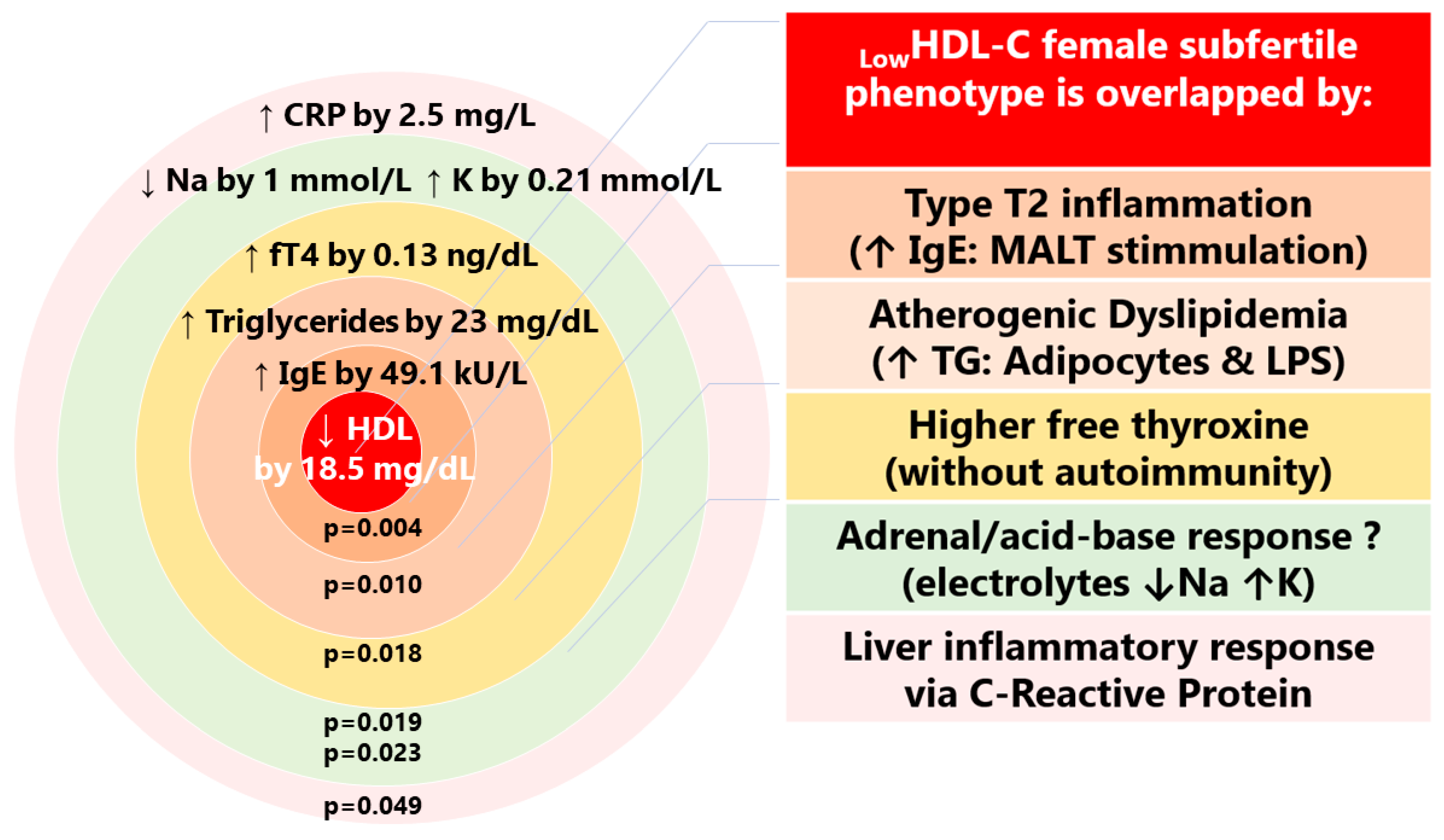
4.1. Cardio-Metabolic Female Phenotype of Subfertility (LowHDL-Cholesterol)
4.2. Hepato-Metabolic Male Phenotype of Subfertility (HighHOMA-IR)
4.3. Autoimmune Female Phenotype of Subfertility (HighAnti-Thyroid Peroxidase)
4.4. Type-2 Immune Response Phenotype of Subfertility (highIgE) in Men
4.5. Metabolic Predictors of Couple Subfertility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gnoth, C.; Godehardt, E.; Frank-Herrmann, P.; Friol, K.; Tigges, J.; Freundl, G. Definition and prevalence of subfertility and infertility. Hum. Reprod. 2005, 20, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2020, 113, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Siemienowicz, K.J.; Furmanska, K.; Filis, P.; Talia, C.; Thomas, J.; Fowler, P.A.; Rae, M.T.; Duncan, W.C. Pubertal FGF21 deficit is central in the metabolic pathophysiology of an ovine model of polycystic ovary syndrome. Mol. Cell Endocrinol. 2021, 525, 111196. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Leridon, H. Studies of fertility and fecundity: Comparative approaches from demography and epidemiology. Comptes Rendus Biol. 2007, 330, 339–346. [Google Scholar] [CrossRef]
- Pathak, U.I.; Gabrielsen, J.S.; Lipshultz, L.I. Cutting-Edge Evaluation of Male Infertility. Urol. Clin. N. Am. 2020, 47, 129–138. [Google Scholar] [CrossRef]
- Farquhar, C.M.; Bhattacharya, S.; Repping, S.; Mastenbroek, S.; Kamath, M.S.; Marjoribanks, J.; Boivin, J. Female subfertility. Nat. Rev. Dis. Primers 2019, 5, 7. [Google Scholar]
- Siristatidis, C.; Bhattacharya, S. Unexplained infertility: Does it really exist? Does it matter? Hum. Reprod. 2007, 22, 2084–2087. [Google Scholar] [CrossRef]
- Miyamoto, T.; Minase, G.; Shin, T.; Ueda, H.; Okada, H.; Sengoku, K. Human male infertility and its genetic causes. Reprod. Med. Biol. 2017, 16, 81–88. [Google Scholar] [CrossRef]
- Sadeghi, M.R. Unexplained infertility, the controversial matter in management of infertile couples. J. Reprod. Infertil. 2015, 16, 1–2. [Google Scholar]
- Tamrakar, S.R.; Bastakoti, R. Determinants of Infertility in Couples. J. Nepal. Health. Res. Counc. 2019, 17, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Brazdova, A.; Senechal, H.; Peltre, G.; Poncet, P. Immune Aspects of Female Infertility. Int. J. Fertil. Steril. 2016, 10, 1–10. [Google Scholar] [PubMed]
- Salvio, G.; Ciarloni, A.; Cutini, M.; Delli Muti, N.; Finocchi, F.; Perrone, M.; Rossi, S.; Balercia, G. Metabolic Syndrome and Male Fertility: Beyond Heart Consequences of a Complex Cardiometabolic Endocrinopathy. Int. J. Mol. Sci. 2022, 23, 5497. [Google Scholar] [CrossRef] [PubMed]
- Maffei, S.; Forini, F.; Canale, P.; Nicolini, G.; Guiducci, L. Gut Microbiota and Sex Hormones: Crosstalking Players in Cardiometabolic and Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 7154. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: A guideline. Fertil. Steril. 2015, 104, 545–553. [Google Scholar] [CrossRef]
- Eldar-Geva, T.; Shoham, M.; Rosler, A.; Margalioth, E.J.; Livne, K.; Meirow, D. Subclinical hypothyroidism in infertile women: The importance of continuous monitoring and the role of the thyrotropin-releasing hormone stimulation test. Gynecol. Endocrinol. 2007, 23, 332–337. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, W.; Liu, Y.; Wang, X.; Guan, Y.; Jia, L. Analysis of basal serum TSH, FT3, and FT4 levels based on age, sampling time in women with infertility. BMC Womens Health 2021, 21, 317. [Google Scholar] [CrossRef]
- Wei, H.J.; Young, R.; Kuo, I.L.; Liaw, C.M.; Chiang, H.S.; Yeh, C.Y. Prevalence of insulin resistance and determination of risk factors for glucose intolerance in polycystic ovary syndrome: A cross-sectional study of Chinese infertility patients. Fertil. Steril. 2009, 91, 1864–1868. [Google Scholar] [CrossRef]
- Fabozzi, G.; Verdone, G.; Allori, M.; Cimadomo, D.; Tatone, C.; Stuppia, L.; Franzago, M.; Ubaldi, N.; Vaiarelli, A.; Ubaldi, F.M.; et al. Personalized Nutrition in the Management of Female Infertility: New Insights on Chronic Low-Grade Inflammation. Nutrients 2022, 14, 1918. [Google Scholar] [CrossRef]
- Martinot, E.; Thirouard, L.; Holota, H.; Monrose, M.; Garcia, M.; Beaudoin, C.; Volle, D.H. Intestinal microbiota defines the GUT-TESTIS axis. Gut 2022, 71, 844–845. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Feng, Y.; Hao, Y.; Zhong, R.; Jiang, Y.; Tang, X.; Lu, D.; Fang, H.; Agarwal, M.; Chen, L.; et al. Gut-Testis Axis: Microbiota Prime Metabolome to Increase Sperm Quality in Young Type 2 Diabetes. Microbiol. Spectr. 2022, 10, e0142322. [Google Scholar] [CrossRef] [PubMed]
- Fabozzi, G.; Rebuzzini, P.; Cimadomo, D.; Allori, M.; Franzago, M.; Stuppia, L.; Garagna, S.; Ubaldi, F.M.; Zuccotti, M.; Rienzi, L. Endocrine-Disrupting Chemicals, Gut Microbiota, and Human (In)Fertility-It Is Time to Consider the Triad. Cells 2022, 11, 3335. [Google Scholar] [CrossRef]
- Fatourechi, V. Subclinical hypothyroidism: An update for primary care physicians. Mayo. Clin. Proc. 2009, 84, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.; Buse, J.; Ferrannini, E.; Stern, M. The metabolic syndrome: Time for a critical appraisal: Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes. Care 2005, 28, 2289–2304. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, R.; Kavitha, S. Atherogenic indices in stroke patients: A retrospective study. Iran. J. Neurol. 2017, 16, 78–82. [Google Scholar] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart. J. 2020, 41, 111–188. [Google Scholar]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Song, P.; Xu, L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug. Discov. Ther. 2015, 9, 380–385. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, J.; Sim, D.W.; Lee, S.C. Comparison of Singleplex Specific IgE Detection Immunoassays: ImmunoCAP Phadia 250 and Immulite 2000 3gAllergy. Ann. Lab. Med. 2018, 38, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Popescu, F.D.; Vieru, M. Precision medicine allergy immunoassay methods for assessing immunoglobulin E sensitization to aeroallergen molecules. World J. Methodol. 2018, 8, 17–36. [Google Scholar] [CrossRef]
- Wild, R.A.; Rizzo, M.; Clifton, S.; Carmina, E. Lipid levels in polycystic ovary syndrome: Systematic review and meta-analysis. Fertil. Steril. 2011, 95, 1073–1079.e11. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Choi, Y.M. Dyslipidemia in women with polycystic ovary syndrome. Obs. Gynecol. Sci. 2013, 56, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.C. A guide to understanding polycystic ovary syndrome (PCOS). J. Fam. Plann. Reprod. Health Care. 2014, 40, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Y.; Fan, R.; Wang, M.; Ren, C.; Jiang, A.; Yang, T. Role of inflammatory factors in the etiology and treatment of recurrent implantation failure. Reprod. Biol. 2022, 22, 100698. [Google Scholar] [CrossRef]
- Endo, Y.; Fujita, M.; Ikewaki, K. HDL Functions-Current Status and Future Perspectives. Biomolecules 2023, 13, 105. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart. J. 2022, 44, 1394–1407. [Google Scholar] [CrossRef]
- Arias, A.; Quiroz, A.; Santander, N.; Morselli, E.; Busso, D. Implications of High-Density Cholesterol Metabolism for Oocyte Biology and Female Fertility. Front. Cell Dev. Biol. 2022, 10, 941539. [Google Scholar] [CrossRef]
- Nunez Calonge, R.; Guijarro, J.A.; Andres, C.; Cortes, S.; Saladino, M.; Caballero, P.; Kireev, R. Relationships between lipids levels in blood plasma, follicular fluid and seminal plasma with ovarian response and sperm concentration regardless of age and body mass index. Rev. Int. Androl. 2022, 20, 178–188. [Google Scholar] [CrossRef]
- Field, F.J.; Watt, K.; Mathur, S.N. TNF-alpha decreases ABCA1 expression and attenuates HDL cholesterol efflux in the human intestinal cell line Caco-2. J. Lipid. Res. 2010, 51, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Figarska, S.M.; Gustafsson, S.; Sundstrom, J.; Arnlov, J.; Malarstig, A.; Elmstahl, S.; Fall, T.; Lind, L.; Ingelsson, E. Associations of Circulating Protein Levels with Lipid Fractions in the General Population. Arter. Thromb. Vasc. Biol. 2018, 38, 2505–2518. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H. Similarities in Pathogenetic Mechanisms Underlying the Bidirectional Relationship between Endometriosis and Pelvic Inflammatory Disease. Diagnostics 2023, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.; Wang, J.; Leng, Y.; Li, L.; Wang, H. Role of Obesity in Female Reproduction. Int. J. Med. Sci. 2023, 20, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Li, X. Effects of intestinal flora on polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1151723. [Google Scholar] [CrossRef] [PubMed]
- Kanney, N.; Patki, A.; Chandler-Laney, P.; Garvey, W.T.; Hidalgo, B.A. Epigenetic Age Acceleration in Mothers and Offspring 4-10 Years after a Pregnancy Complicated by Gestational Diabetes and Obesity. Metabolites 2022, 12, 1226. [Google Scholar] [CrossRef]
- Llarena, N.; Hine, C. Reproductive Longevity and Aging: Geroscience Approaches to Maintain Long-Term Ovarian Fitness. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1551–1560. [Google Scholar] [CrossRef]
- Li Piani, L.; Vigano, P.; Somigliana, E. Epigenetic clocks and female fertility timeline: A new approach to an old issue? Front. Cell Dev. Biol. 2023, 11, 1121231. [Google Scholar] [CrossRef]
- Grieger, J.A.; Grzeskowiak, L.E.; Smithers, L.G.; Bianco-Miotto, T.; Leemaqz, S.Y.; Andraweera, P.; Poston, L.; McCowan, L.M.; Kenny, L.C.; Myers, J.; et al. Metabolic syndrome and time to pregnancy: A retrospective study of nulliparous women. BJOG 2019, 126, 852–862. [Google Scholar] [CrossRef]
- Harris, H.W.; Gosnell, J.E.; Kumwenda, Z.L. The lipemia of sepsis: Triglyceride-rich lipoproteins as agents of innate immunity. J. Endotoxin. Res. 2000, 6, 421–430. [Google Scholar]
- Macpherson, M.E.; Skarpengland, T.; Hov, J.R.; Ranheim, T.; Vestad, B.; Dahl, T.B.; Fraz, M.S.A.; Michelsen, A.E.; Holven, K.B.; Fevang, B.; et al. Increased Plasma Levels of Triglyceride-Enriched Lipoproteins Associate with Systemic Inflammation, Lipopolysaccharides, and Gut Dysbiosis in Common Variable Immunodeficiency. J. Clin. Immunol. 2023, 43, 1229–1240. [Google Scholar] [CrossRef]
- Magata, F.; Tsukamura, H.; Matsuda, F. The impact of inflammatory stress on hypothalamic kisspeptin neurons: Mechanisms underlying inflammation-associated infertility in humans and domestic animals. Peptides 2023, 162, 170958. [Google Scholar]
- Biernacka-Bartnik, A.; Kocelak, P.; Owczarek, A.J.; Choreza, P.S.; Markuszewski, L.; Madej, P.; Puzianowska-Kuznicka, M.; Chudek, J.; Olszanecka-Glinianowicz, M. The cut-off value for HOMA-IR discriminating the insulin resistance based on the SHBG level in women with polycystic ovary syndrome. Front. Med. 2023, 10, 1100547. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Way, S.S.; Chen, K. Immunology of the Uterine and Vaginal Mucosae. Trends Immunol. 2018, 39, 302–314. [Google Scholar] [CrossRef]
- Marusic, M.; Paic, M.; Knobloch, M.; Liberati Prso, A.M. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6613827. [Google Scholar]
- An, R.; Ma, S.; Zhang, N.; Lin, H.; Xiang, T.; Chen, M.; Tan, H. AST-to-ALT ratio in the first trimester and the risk of gestational diabetes mellitus. Front. Endocrinol. 2022, 13, 1017448. [Google Scholar] [CrossRef]
- Belladelli, F.; Boeri, L.; Pozzi, E.; Fallara, G.; Corsini, C.; Candela, L.; Cazzaniga, W.; Cignoli, D.; Pagliardini, L.; D’Arma, A.; et al. Triglycerides/Glucose Index Is Associated with Sperm Parameters and Sperm DNA Fragmentation in Primary Infertile Men: A Cross-Sectional Study. Metabolites 2022, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Dutta, S.; Karkada, I.R.; Chinni, S.V. Endocrinopathies and Male Infertility. Life 2021, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.; El-Faissal, Y.; Kamel, A.; Kamal, O.; Aboulserour, G.; Aboulghar, M.; Fahmy, I. Increased insulin resistance in men with unexplained infertility. Reprod. Biomed. Online 2017, 35, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, D.E.; Niskanen, L.; Punnonen, K.; Nyyssonen, K.; Tuomainen, T.P.; Salonen, R.; Rauramaa, R.; Salonen, J.T. Sex hormones, inflammation and the metabolic syndrome: A population-based study. Eur. J. Endocrinol. 2003, 149, 601–608. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Y.N.; Zhang, J.; Liu, J. Elevated serum levels of diamine oxidase, D-lactate and lipopolysaccharides are associated with metabolic-associated fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2023, 35, 94–101. [Google Scholar] [CrossRef]
- Holota, H.; De Haze, A.; Martinot, E.; Monrose, M.; Saru, J.P.; Caira, F.; Beaudoin, C.; Volle, D.H. Identification of the Role of TGR5 in the Regulation of Leydig Cell Homeostasis. Int. J. Mol. Sci. 2022, 23, 15398. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Tan, A.; Knox, E.; Kilby, M.D.; Franklyn, J.; Coomarasamy, A. Association between thyroid autoantibodies and miscarriage and preterm birth: Meta-analysis of evidence. BMJ 2011, 342, d2616. [Google Scholar] [CrossRef] [PubMed]
- Hubalewska-Dydejczyk, A.; Gietka-Czernel, M.; Trofimiuk-Muldner, M.; Zgliczynski, W.; Ruchala, M.; Lewinski, A.; Bednarczuk, T.; Syrenicz, A.; Kos-Kudla, B.; Jarzab, B.; et al. Thyroid diseases and fertility disorders—Guidelines of the Polish Society of Endocrinology [Choroby tarczycy a zaburzenia plodnosci—rekomendacje Polskiego Towarzystwa Endokrynologicznego]. Endokrynol. Pol. 2022, 73, 645–679. [Google Scholar] [CrossRef]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar]
- Lu, D.; Song, Z.; Gao, Y.; Zhang, J.; Guo, X. Patients with Autoimmune Thyroid Diseases Have Higher Prevalence of Positive Antiphospholipid Antibodies: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2022, 2022, 8271951. [Google Scholar] [CrossRef]
- Tanska, K.; Gietka-Czernel, M.; Glinicki, P.; Kozakowski, J. Thyroid autoimmunity and its negative impact on female fertility and maternal pregnancy outcomes. Front. Endocrinol. 2022, 13, 1049665. [Google Scholar] [CrossRef]
- van den Boogaard, E.; Vissenberg, R.; Land, J.A.; van Wely, M.; van der Post, J.A.; Goddijn, M.; Bisschop, P.H. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: A systematic review. Hum. Reprod. Update 2011, 17, 605–619. [Google Scholar] [CrossRef]
- Jensen, R.C.; Glintborg, D.; Timmermann, C.A.G.; Nielsen, F.; Boye, H.; Madsen, J.B.; Bilenberg, N.; Grandjean, P.; Jensen, T.K.; Andersen, M.S. Higher free thyroxine associated with PFAS exposure in first trimester. The Odense Child Cohort. Environ. Res. 2022, 212 Pt D, 113492. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, Y.; Zhai, L.; Bai, Y.; Jia, L. Urinary bisphenol A and S are associated with diminished ovarian reserve in women from an infertility clinic in Northern China. Ecotoxicol. Environ. Saf. 2023, 256, 114867. [Google Scholar] [CrossRef]
- Gong, B.; Wang, C.; Meng, F.; Wang, H.; Song, B.; Yang, Y.; Shan, Z. Association Between Gut Microbiota and Autoimmune Thyroid Disease: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 774362. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Gutaj, N.; Gruszczynski, D.; Zawalna, N.; Nijakowski, K.; Muller, I.; Karpinski, T.; Salvi, M.; Ruchala, M. Microbiota Alterations in Patients with Autoimmune Thyroid Diseases: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13450. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lu, G.; Gao, D.; Lv, Z.; Li, D. The relationships between the gut microbiota and its metabolites with thyroid diseases. Front. Endocrinol. 2022, 13, 943408. [Google Scholar] [CrossRef] [PubMed]
- Bargiel, P.; Szczuko, M.; Stachowska, L.; Prowans, P.; Czapla, N.; Markowska, M.; Petriczko, J.; Kledzik, J.; Jedrzejczyk-Kledzik, A.; Palma, J.; et al. Microbiome Metabolites and Thyroid Dysfunction. J. Clin. Med. 2021, 10, 3609. [Google Scholar] [CrossRef]
- Zhao, F.; Feng, J.; Li, J.; Zhao, L.; Liu, Y.; Chen, H.; Jin, Y.; Zhu, B.; Wei, Y. Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid 2018, 28, 175–186. [Google Scholar] [CrossRef]
- Stanbery, A.G.; Shuchi, S.; Jakob von, M.; Tait Wojno, E.D.; Ziegler, S.F. TSLP, IL-33, and IL-25: Not just for allergy and helminth infection. J. Allergy. Clin. Immunol. 2022, 150, 1302–1313. [Google Scholar] [CrossRef]
- Haniuda, K.; Kitamura, D. Multi-faceted regulation of IgE production and humoral memory formation. Allergol. Int. 2021, 70, 163–168. [Google Scholar] [CrossRef]
- Ekladios, E.M.; Girgis, S.M.; Salem, D.; Fahmy, I.M.; Mostafa, T.; Khalil, G.R. Immunoglobulin E in serum and semen of infertile men. Andrologia 1988, 20, 485–491. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Fang, Y.; Shen, Q.; Zhao, K.; Liu, C.; Zhang, H. Microbiology and immune mechanisms associated with male infertility. Front. Immunol. 2023, 14, 1139450. [Google Scholar] [CrossRef]
- Harrison, R.F.; Unwin, A. Atopy in couples with unexplained infertility. Br. J. Obs. Gynaecol. 1989, 96, 192–195. [Google Scholar] [CrossRef]
- Hanzlikova, J.; Ulcova-Gallova, Z.; Malkusova, I.; Sefrna, F.; Panzner, P. TH1-TH2 response and the atopy risk in patients with reproduction failure. Am. J. Reprod. Immunol. 2009, 61, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Williamson, H.O.; Baker, E.R.; Fudenberg, H.H. Immunoglobulin E levels and antisperm antibody titers in infertile couples. Am. J. Obs. Gynecol. 1981, 140, 923–930. [Google Scholar] [CrossRef]
- Pham, L.; Baiocchi, L.; Kennedy, L.; Sato, K.; Meadows, V.; Meng, F.; Huang, C.K.; Kundu, D.; Zhou, T.; Chen, L.; et al. The interplay between mast cells, pineal gland, and circadian rhythm: Links between histamine, melatonin, and inflammatory mediators. J. Pineal. Res. 2021, 70, e12699. [Google Scholar] [CrossRef]
- Houle, E.; Li, Y.; Schroder, M.; McRitchie, S.L.; Rahil, T.; Sites, C.K.; Jenkins Sumner, S.; Pilsner, J.R. Exploring the internal exposome of seminal plasma with semen quality and live birth: A Pilot Study. Syst. Biol. Reprod. Med. 2023, 69, 296–309. [Google Scholar] [CrossRef]
- Alcaniz, L.; Vega, A.; Chacon, P.; El Bekay, R.; Ventura, I.; Aroca, R.; Blanca, M.; Bergstralh, D.T.; Monteseirin, J. Histamine production by human neutrophils. FASEB J. 2013, 27, 2902–2910. [Google Scholar] [CrossRef] [PubMed]
- Matalliotakis, I.; Fragouli, Y.; Kyriakou, D.; Goumenou, A.; Matalliotakis, G.; Koumantakis, E. Enhancement of soluble CD23 levels in the seminal plasma of infertile men with idiopathic testicular lesion. Arch. Androl. 1999, 43, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Smith-Norowitz, T.A.; Bluth, M.H.; Drew, H.; Norowitz, K.B.; Chice, S.; Shah, V.N.; Nowakowski, M.; Josephson, A.S.; Durkin, H.G.; Joks, R. Effect of minocycline and doxycycline on IgE responses. Ann. Allergy Asthma. Immunol. 2002, 89, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, W.; Shang, H.; Wei, H.; Deng, C. The Interplay between Androgen and Gut Microbiota: Is There a Microbiota-Gut-Testis Axis. Reprod. Sci. 2022, 29, 1674–1684. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995, 269 Pt 1, G467–G475. [Google Scholar] [CrossRef]
- Charles-Messance, H.; Mitchelson, K.A.J.; De Marco Castro, E.; Sheedy, F.J.; Roche, H.M. Regulating metabolic inflammation by nutritional modulation. J. Allergy. Clin. Immunol. 2020, 146, 706–720. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; Kwiatkowski, M.; Govorukhina, N.; Bischoff, R.; Melgert, B.N. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front. Immunol. 2021, 12, 746151. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.X.; Deng, X.R.; Zhang, C.H.; Yuan, H.J. Gut microbiota and metabolic syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef] [PubMed]
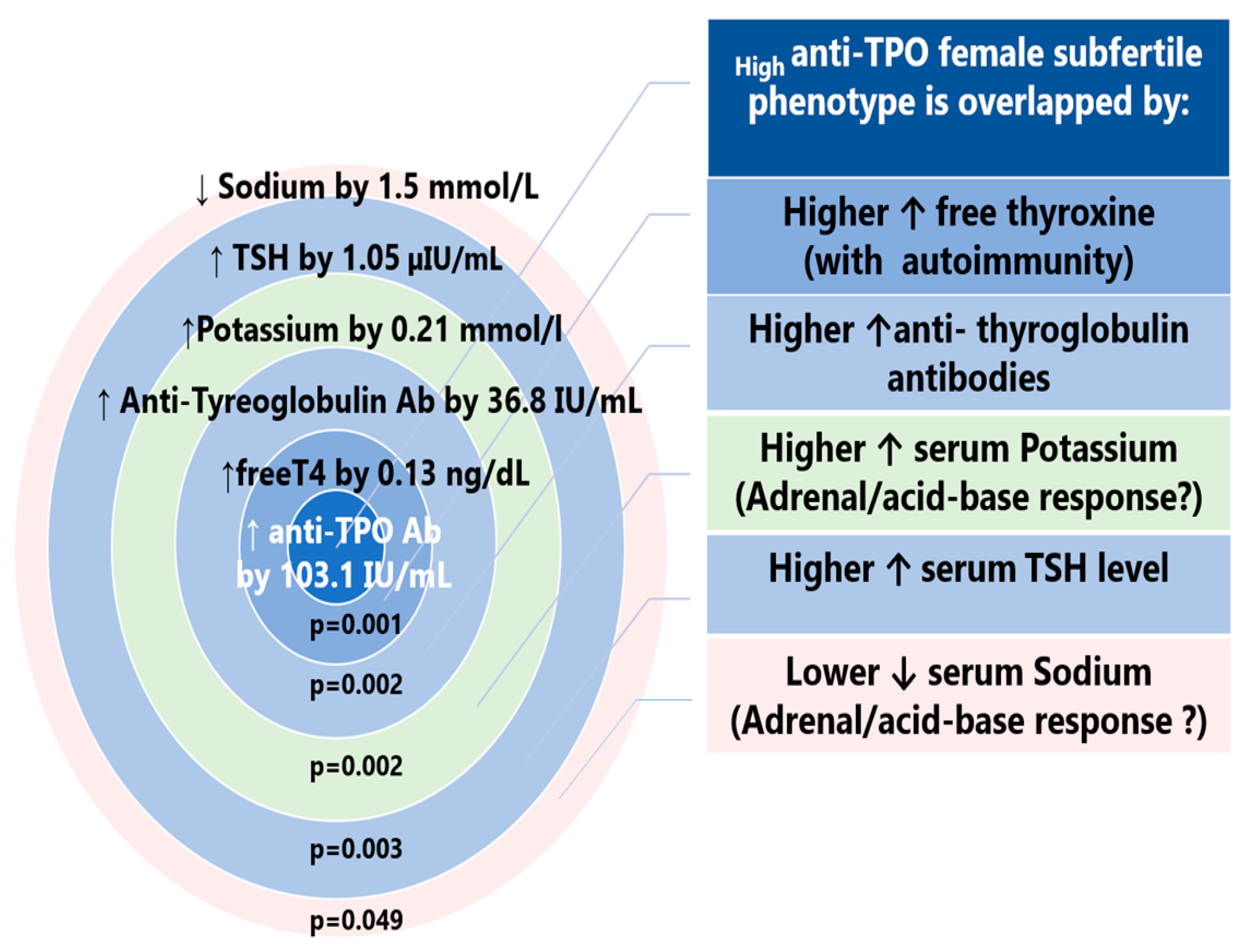
| Parameter | TPO-Positive Subfertile Female Group (N = 14) | TPO-Negative Fertile Female Group (N = 23) | p-Value |
|---|---|---|---|
| anti-TPO Mdn (IQR) min–max | 103.2 (22.5–233.5) 5.8–1000 | 0.150 (0.03–0.47) 0.0–1.3 | <0.0001 |
| anti-TG Mdn (IQR) min–max | 38.4 (4.2–291.2) 0.7–563.6 | 1.6 (0.9–4.9) 0.56–49.8 | 0.002 |
| TSH µIU/mL Mdn (IQR) 95% CI | 2.34 (1.62–3.05) 1.79–2.91 | 1.29 (0.85–1.69) 1.12–1.73 | 0.003 |
| fT4 ng/dL Mdn (IQR) | 1.08 (1.02–1.23) | 0.95 (0.89–1.02) | 0.001 |
| Insulin µU/mL Mdn (IQR) | 8.3 (5.5–11.2) | 5.2 (4.0–8.8) | 0.060 |
| HOMA-IR Mdn (IQR) | 1.80 (1.08–2.61) | 1.07 (0.84–1.80) | 0.094 |
| Sodium mmol/L Mdn (IQR) | 139 (138–140) | 140 (139–141) | 0.049 |
| Potassium mmol/L 95% CI | 4.50 4.46–4.71 | 4.30 4.20–4.43 | 0.002 |
| Parameter | LowHDL-C Subfertile Group (N = 14 Women) | OptimalHDL-C Fertile Group (N = 24 Women) | p-Value |
|---|---|---|---|
| HDL-C mg/dL Mdn (IQR) | 44.00 (40.75–47.25) | 62.50 (55.25–71.50) | <0.0001 |
| Total Cholesterol /HDL-C Ratio | 3.82 (3.46–4.70) | 3.07 (2.62–3.42) | 0.0002 |
| Triglycerides /HDL Ratio | 2.00 (1.73–2.84) | 1.12 (0.79–1.37) | <0.0001 |
| Triglycerides mg/dL | 88 (79–116) | 65 (55–89) | 0.010 |
| TSH µIU/mL Mdn (IQR) | 1.33 (0.98–2.33) | 1.29 (0.83–1.67) | 0.661 |
| Anti-TPO autoantibodies | 0.36 (0.00–0.56) | 0.19 (0.05–0.71) | 0.880 |
| Free T4 ng/dL Mdn (IQR) | 1.08 (0.97–1.17) | 0.95 (0.90–1.02) | 0.018 |
| Insulin µU/mL Mdn (IQR) | 8.00 (5.25–11.15) | 5.25 (4.05 –9.00) | 0.063 |
| HOMA-IR Mdn (IQR) | 1.80 (1.07–2.52) | 1.11 (0.86–2.00) | 0.099 |
| ALT | 13.5 (8.0–20.0) | 9.0 (7.0–12.8) | 0.092 |
| ALT > 19 IU/mL | 5/14 (35.7%) | 1/24 (4.2%) | 0.019 |
| AST/ALT | 1.25 (1.00–1.84) | 1.71 (1.5–1.96) | 0.056 |
| Sodium mmol/L Mdn (IQR) | 139 (138–140) | 140.0 (139–142) | 0.019 |
| Potassium mmol/L Mdn (IQR) | 4.49 (4.28–4.85) | 4.28 (4.13–4.44) | 0.023 |
| Total IgE | 67.9 (22.2–99.7) | 18.8 (11.7–27.4) | 0.004 |
| CRP | 3.65 (1.33–9.03) | 1.20 (0.70–2.30) | 0.049 |
| Fe | 65.5 (46.5–82.5) | 85.5 (47.3–106.5) | 0.126 |
| UIBC | 239 (193–304) | 212 (164–285) | 0.169 |
| IgA | 1.8 (1.5–2.2) | 1.9 (1.6–2.3) | 0.628 |
| CRP vs. | Subjects Diagnosed with Subfertility | Fertile Subjects | ||
|---|---|---|---|---|
| RS | p-Value | RS | p-Value | |
| Glucose | 0.17 | 0.092 | 0.20 | 0.155 |
| Insulin | 0.39 | <0.0001 | 0.25 | 0.082 |
| HOMA-IR | 0.40 | <0.0001 | 0.29 | 0.041 |
| Triglycerides | 0.45 | <0.0001 | 0.08 | 0.588 |
| Cholesterol | 0.16 | 0.116 | −0.14 | 0.351 |
| Ch/HDL-C | 0.40 | <0.0001 | 0.20 | 0.144 |
| nonHDL-cholesterol | 0.29 | 0.003 | 0.01 | 0.945 |
| LDL-cholesterol | 0.22 | 0.028 | −0.01 | 0.979 |
| HDL-cholesterol | −0.29 | 0.003 | −0.25 | 0.078 |
| Parameter | HighHOMA-IR Subfertile Group (N = 13) | NormalHOMA-IR Fertile Group (N = 14) | p-Value |
|---|---|---|---|
| HDL-C mg/dL Mdn (IQR) | 49 (45–54) | 53 (47–65) | 0.189 |
| Total cholesterol/HDL-C Ratio | 4.4 (4.2–5.5) | 4.5 (3.6–4.8) | 0.235 |
| Triglycerides /HDL-C Ratio | 2.7 (1.3–3.8) | 1.7 (1.1–3.3) | 0.216 |
| Triglycerides mg/Dl | 145 (70–186) | 93 (67–148) | 0.297 |
| Total cholesterol mg/dL | 227 (217–253) | 231 (204–248) | 0.560 |
| LDL cholesterol mg/dL | 162 151–175 | 147 132–164 | 0.090 |
| TSH µIU/mL Mdn (IQR) | 1.50 (0.91–1.79) | 1.28 (0.92–1.64) | 0.790 |
| Free T4 ng/dL Mdn (IQR) | 1.03 (0.98–1.09) | 1.00 (0.94–1.11) | 0.771 |
| Glucose | 91 (87–100) | 87 (81–96) | 0.080 |
| Insulin µU/mL Mdn (IQR) | 11.3 (9.6–15.9) | 5.6 (4.9 –7.2) | <0.0001 |
| HOMA-IR Mdn (IQR) | 2.44 (2.08–3.82) | 1.27 (1.08–1.46) | <0.0001 |
| ALT IU/mL | 32 (25–40) | 15 (10–21) | 0.0009 |
| ALT > 30 IU/mL | 8/13 (62%) | 1/14 (7%) | <0.0001 |
| AST/ALT | 0.8 (0.7–1.1) | 1.6 (1.0–1.9) | 0.008 |
| AST IU/mL (95% CI) | 28 22–32 22.8–33.8 | 22 18–23 18.5–22.8 | 0.007 |
| SHBG | 30.0 (21.2–44.5) | 43.7 (31.3–56.7) | 0.049 |
| Sodium mmol/L Mdn (IQR) | 140 (139–141) | 140 (139–142) | 0.584 |
| Potassium mmol/L Mdn (IQR) | 4.49 (4.39–4.73) | 4.46 (4.23–4.67) | 0.680 |
| Total IgE | 29 (9–116) | 47 (28–54) | 0.827 |
| CRP | 1.2 (0.5–2.1) | 1.3 (0.4–2.5) | 0.865 |
| Parameter | HighIgE Subfertile Group (N = 13) | normalSerum IgE Fertile Group (N = 23) | p-Value |
|---|---|---|---|
| Total IgE kU/L | 182 (136–271) | 35 (9–54) | <0.0001 |
| sIgE pollen sensitization N (%) | 4/13 (30%) | 1/23 (4%) | 0.047 |
| Non-atopic highIgE N (%) | 9/13 (70%) | 1/23 (4%) | <0.0001 |
| Total cholesterol mg/dL | 227.0 (193.0–233.5) | 205.0 (179.0–236.0) | 0.621 |
| HDL-C mg/dL Mdn (IQR) | 52.0 (40.5–62.5) | 51.0 (42.0–60.0) | 0.961 |
| Triglyceride mg/dL Mdn (IQR) | 116.0 (64.0–134.5) | 103.0 (76.0–145.0) | 0.987 |
| TSH µIU/mL Mdn (IQR) | 1.35 (0.87–1.56) | 1.21 (0.79–1.81) | 1.00 |
| Anti-TPO autoantibodies | 0.31 (0.19–0.69) | 0.14 (0.05–0.30) | 0.0097 |
| Free T4 ng/dL Mdn (IQR) | 1.05 (1.03–1.9) | 1.00 (0.93–1.04) | 0.007 |
| Sodium mmol/L Mdn (IQR) | 140 (139–141) | 141.0 (139–142) | 0.086 |
| Potassium mmol/L Mdn (IQR) | 4.48 (4.38–4.71) | 4.43 (4.18–4.66) | 0.347 |
| A | ||||
| Predictor | Odds Ratio | Std Error | p-Value | 95% Confidence Interval |
| HOMA-IR > 1.9 vs. <1.9 (HighHOMA-IR) | 6.07 | 4.9 | 0.025 | 1.25; 29.50 |
| Total IgE > 100 vs. <100 kU/L (HighIgE) | 6.37 | 5.5 | 0.034 | 1.15; 35.11 |
| Prediabetes: Yes vs. No | 2.7 | 3.3 | 0.407 | 0.25; 28.59 |
| HDL-C < 40 vs. >40 mg/dL | 0.56 | 0.57 | 0.571 | 0.078; 4.08 |
| Anti-TPO antibodies: Yes, No | 5.70 | 6.9 | 0.152 | 0.53; 62.17 |
| Anti-TG antibodies: Yes, No | 0.55 | 0.51 | 0.523 | 0.089; 3.4 |
| Cons | 1.37 | 0.063 | 0.700 | 0.056; 3.36 |
| B | ||||
| Predictor | Odds Ratio | Std Error | p-value | 95% Confidence Interval |
| HDL-C < 50 mg/dL (LowHDL) | 10.92 | 11.95 | 0.029 | 1.27; 93.27 |
| Anti-TPO positivity (highaTPO) | 5.48 | 4.70 | 0.047 | 1.02; 29.40 |
| Anti-TG positivity | 1.12 | 0.65 | 0.840 | 0.36; 3.47 |
| Hyperinsulinemia > 12 µU/mL | 1.92 | 2.34 | 0.594 | 0.18; 21.03 |
| Total IgE > 100 kU/L | 4.87 | 5.54 | 0.164 | 0.52; 45.25 |
| Cons | 0.87 | 0.33 | 0.727 | 0.41; 1.86 |
| C | ||||
| Predictor | Odds Ratio | Std Error | p-value | 95% Confidence Interval |
| Total IgE > 100 kU/L | 6.50 | 5.56 | 0.029 | 1.22; 34.74 |
| No. of laboratory components of MetS * | 1.68 | 0.43 | 0.046 | 1.01; 2.78 |
| Anti-TPO positivity | 4.22 | 3.67 | 0.098 | 0.76; 23.25 |
| Single sign of lipid profile dysregulation ** | 0.23 | 0.17 | 0.046 | 0.05; 0.98 |
| Cons | 1.14 | 0.52 | 0.779 | 0.46; 2.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewski, T.; Wasilewska, J.; Łukaszewicz-Zając, M.; Mroczko, B. Subfertility as Overlapping of Nutritional, Endocrine, Immune, and Cardiometabolic Dysregulations—A Study Focused on Biochemical Endophenotypes of Subfertile Couples. J. Clin. Med. 2023, 12, 6094. https://doi.org/10.3390/jcm12186094
Wasilewski T, Wasilewska J, Łukaszewicz-Zając M, Mroczko B. Subfertility as Overlapping of Nutritional, Endocrine, Immune, and Cardiometabolic Dysregulations—A Study Focused on Biochemical Endophenotypes of Subfertile Couples. Journal of Clinical Medicine. 2023; 12(18):6094. https://doi.org/10.3390/jcm12186094
Chicago/Turabian StyleWasilewski, Tadeusz, Jolanta Wasilewska, Marta Łukaszewicz-Zając, and Barbara Mroczko. 2023. "Subfertility as Overlapping of Nutritional, Endocrine, Immune, and Cardiometabolic Dysregulations—A Study Focused on Biochemical Endophenotypes of Subfertile Couples" Journal of Clinical Medicine 12, no. 18: 6094. https://doi.org/10.3390/jcm12186094
APA StyleWasilewski, T., Wasilewska, J., Łukaszewicz-Zając, M., & Mroczko, B. (2023). Subfertility as Overlapping of Nutritional, Endocrine, Immune, and Cardiometabolic Dysregulations—A Study Focused on Biochemical Endophenotypes of Subfertile Couples. Journal of Clinical Medicine, 12(18), 6094. https://doi.org/10.3390/jcm12186094







