Exploring CAR-T Cell Therapy Side Effects: Mechanisms and Management Strategies
Abstract
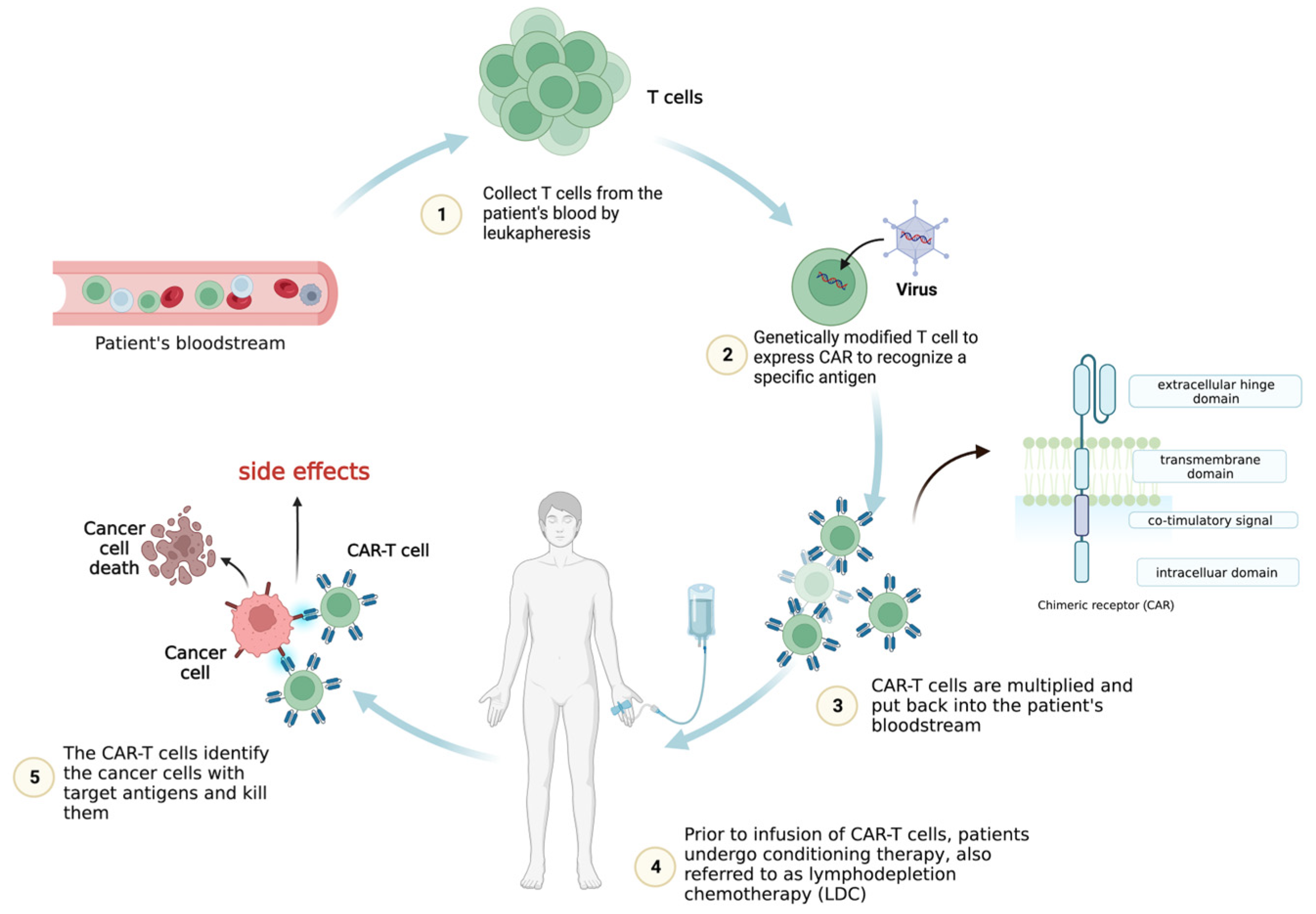
:1. Introduction
2. Mechanism and the Clinical Manifestations of Side Effects
2.1. Cytokine Release Syndrome (CRS)
2.2. Immune-Effector-Cell-Associated Neurotoxicity Syndrome (ICANS)
2.3. Tumor Lysis Syndrome (TLS)
2.4. On-Target, Off-Tumor Toxicity (OTOT)
2.5. Additional Factors Associated with Toxicity
3. Institutional Management Strategies for CAR-T Cell Toxicity
3.1. Prediction and Prevention of Side Effects
3.2. Treatment and Supportive Care
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| SCV | single chain of variable fragment |
| LDC | lymphodepletion chemotherapy |
| CRS | cytokine release syndrome |
| ICANS | immune-effector-cell-associated neurotoxicity syndrome |
| IFN-γ | interferon-gamma |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-alpha |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| APCs | antigen-presenting cells |
| DCs | dendritic cells |
| CD40L | ligand for CD40 |
| DAMPs | danger-associated molecular patterns |
| PT | prothrombin time |
| PPT | partial thromboplastin time |
| HLH | hemophagocytic lymph histiocytosis |
| ASTCT | American Society for Transplantation and Cellular Therapy |
| BBB | blood–brain barrier |
| CNS | central nervous system |
| TLS | tumor lysis syndrome |
| BCMA | B cell maturation antigen |
| CLS | capillary leak syndrome |
| OTOT | on-target, off-tumor toxicity |
| TAAs | tumor-associated antigens |
| TSAs | tumor-specific antigens |
| CBC | complete blood count |
| CRP | C-reactive protein |
| LDH | lactate dehydrogenase |
| ECG | electrocardiogram |
| sICAM | soluble intercellular adhesion molecule |
| sVCAM | soluble vascular cell adhesion molecule |
| FOLR1 | folate receptor 1 |
| TAG72 | tumor-associated glycoprotein 72 |
| IL-6R | interleukin-6 receptor |
| i.v. | intravenous injection |
| ICP | intracranial pressure |
References
- Jayaraman, J.; Mellody, M.P.; Hou, A.J.; Desai, R.P.; Fung, A.W.; Pham, A.H.T.; Chen, Y.Y.; Zhao, W. CAR-T Design: Elements and Their Synergistic Function. EBioMedicine 2020, 58, 102931. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Maus, M.V.; Porter, D.L. Chimeric Antigen Receptor T Cell Therapy: 25years in the Making. Blood Rev. 2016, 30, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Fesnak, A.D.; June, C.H.; Levine, B.L. Engineered T Cells: The Promise and Challenges of Cancer Immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy Bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Guo, Y.; Wang, Y.; Wu, Z.; Bo, J.; Zhang, B.; Zhu, J.; Han, W. Clinical Development of CAR T Cell Therapy in China: 2020 Update. Cell Mol. Immunol. 2021, 18, 792–804. [Google Scholar] [CrossRef] [PubMed]
- El-Khazragy, N.; Ghozy, S.; Emad, P.; Mourad, M.; Razza, D.; Farouk, Y.K.; Mohamed, N.A.; Ahmed, M.K.; Youssef, T.; Bahnasawy, Y.M.; et al. Chimeric Antigen Receptor T Cells Immunotherapy: Challenges and Opportunities in Hematological Malignancies. Immunotherapy 2020, 12, 1341–1357. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Huang, S.; Chen, S.; Wang, Y.; Sun, Q.; Xu, X.; Li, Y. Mechanisms of Cytokine Release Syndrome and Neurotoxicity of CAR T-Cell Therapy and Associated Prevention and Management Strategies. J. Exp. Clin. Cancer Res. 2021, 40, 367. [Google Scholar] [CrossRef] [PubMed]
- Brentjens, R.; Yeh, R.; Bernal, Y.; Riviere, I.; Sadelain, M. Treatment of Chronic Lymphocytic Leukemia with Genetically Targeted Autologous T Cells: Case Report of an Unforeseen Adverse Event in a Phase I Clinical Trial. Mol. Ther. 2010, 18, 666–668. [Google Scholar] [CrossRef]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric Antigen Receptor–Modified T Cells for Acute Lymphoid Leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced with a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Bullock, T.N.J. CD40 Stimulation as a Molecular Adjuvant for Cancer Vaccines and Other Immunotherapies. Cell Mol. Immunol. 2022, 19, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Cheng, X.; Truong, B.; Sun, L.; Yang, X.; Wang, H. Molecular Basis and Therapeutic Implications of CD40/CD40L Immune Checkpoint. Pharmacol. Ther. 2021, 219, 107709. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Hofmann, T.J.; Gershenson, Z.; Levine, B.L.; Grupp, S.A.; Teachey, D.T.; Barrett, D.M. Monocyte Lineage-Derived IL-6 Does Not Affect Chimeric Antigen Receptor T-Cell Function. Cytotherapy 2017, 19, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Interleukin-6: Designing Specific Therapeutics for a Complex Cytokine. Available online: https://pubmed.ncbi.nlm.nih.gov/29725131/ (accessed on 6 April 2023).
- Teachey, D.T.; Lacey, S.F.; Shaw, P.A.; Melenhorst, J.J.; Maude, S.L.; Frey, N.; Pequignot, E.; Gonzalez, V.E.; Chen, F.; Finklestein, J.; et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-Cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016, 6, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, L.; Zhang, H.; Chen, S.; Xiao, Y. CAR-T Cell Therapy in Hematological Malignancies: Current Opportunities and Challenges. Front. Immunol. 2022, 13, 927153. [Google Scholar] [CrossRef] [PubMed]
- Granzyme a from Cytotoxic Lymphocytes Cleaves GSDMB to Trigger Pyroptosis in Target Cells. Available online: https://pubmed.ncbi.nlm.nih.gov/32299851/ (accessed on 6 April 2023).
- Gasdermin E-Mediated Target Cell Pyroptosis by CAR T Cells Triggers Cytokine Release Syndrome. Available online: https://pubmed.ncbi.nlm.nih.gov/31953257/ (accessed on 6 April 2023).
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-Sensing Receptors in Sterile Inflammation and Inflammatory Diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current Concepts in the Diagnosis and Management of Cytokine Release Syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of Chimeric Antigen Receptor T Cells: Recognition and Management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Recent Advances in CAR T-Cell Toxicity: Mechanisms, Manifestations and Management. Blood Rev. 2019, 34, 45–55. [Google Scholar] [CrossRef]
- Freyer, C.W.; Porter, D.L. Cytokine Release Syndrome and Neurotoxicity Following CAR T-Cell Therapy for Hematologic Malignancies. J. Allergy Clin. Immunol. 2020, 146, 940–948. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Rivière, I.; Park, J.H.; Davila, M.L.; Wang, X.; Stefanski, J.; Taylor, C.; Yeh, R.; Bartido, S.; Borquez-Ojeda, O.; et al. Safety and Persistence of Adoptively Transferred Autologous CD19-Targeted T Cells in Patients with Relapsed or Chemotherapy Refractory B-Cell Leukemias. Blood 2011, 118, 4817–4828. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T Cells Expressing CD19 Chimeric Antigen Receptors for Acute Lymphoblastic Leukaemia in Children and Young Adults: A Phase 1 Dose-Escalation Trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Hwang, W.-T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric Antigen Receptor T Cells Persist and Induce Sustained Remissions in Relapsed Refractory Chronic Lymphocytic Leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Han, Q.; Liu, Y.; Dai, H.; Guo, Y.; Bo, J.; Fan, H.; Zhang, Y.; Zhang, Y.; et al. Effective Response and Delayed Toxicities of Refractory Advanced Diffuse Large B-Cell Lymphoma Treated by CD20-Directed Chimeric Antigen Receptor-Modified T Cells. Clin. Immunol. 2014, 155, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR–T Cells of Defined CD4+:CD8+ Composition in Adult B Cell ALL Patients. J. Clin. Investig. 2016, 126, 2123–2138. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.; Avigdor, A.; Bielorai, B.; Meir, A.; Besser, M.J.; Schachter, J.; Shimoni, A.; Nagler, A.; Toren, A.; Jacoby, E. Early and Late Hematologic Toxicity Following CD19 CAR-T Cells. Bone Marrow Transplant. 2019, 54, 1643–1650. [Google Scholar] [CrossRef]
- Brudno, J.N.; Somerville, R.P.T.; Shi, V.; Rose, J.J.; Halverson, D.C.; Fowler, D.H.; Gea-Banacloche, J.C.; Pavletic, S.Z.; Hickstein, D.D.; Lu, T.L.; et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J. Clin. Oncol. 2016, 34, 1112–1121. [Google Scholar] [CrossRef]
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T Cells Expressing an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Multiple Myeloma. Blood 2016, 128, 1688–1700. [Google Scholar] [CrossRef]
- Jensen, M.C.; Popplewell, L.; Cooper, L.J.; DiGiusto, D.; Kalos, M.; Ostberg, J.R.; Forman, S.J. Antitransgene Rejection Responses Contribute to Attenuated Persistence of Adoptively Transferred CD20/CD19-Specific Chimeric Antigen Receptor Redirected T Cells in Humans. Biol. Blood Marrow Transplant. 2010, 16, 1245–1256. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Dudley, M.E.; Feldman, S.A.; Wilson, W.H.; Spaner, D.E.; Maric, I.; Stetler-Stevenson, M.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. B-Cell Depletion and Remissions of Malignancy along with Cytokine-Associated Toxicity in a Clinical Trial of Anti-CD19 Chimeric-Antigen-Receptor-Transduced T Cells. Blood 2012, 119, 2709–2720. [Google Scholar] [CrossRef] [PubMed]
- Canna, S.W.; Marsh, R.A. Pediatric Hemophagocytic Lymphohistiocytosis. Blood 2020, 135, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Cardiotoxicity from Chimeric Antigen Receptor-T Cell Therapy for Advanced Malignancies. Available online: https://pubmed.ncbi.nlm.nih.gov/35257157/ (accessed on 3 April 2023).
- Camilli, M.; Maggio, L.; Tinti, L.; Lamendola, P.; Lanza, G.A.; Crea, F.; Lombardo, A. Chimeric Antigen Receptor-T Cell Therapy-Related Cardiotoxicity in Adults and Children Cancer Patients: A Clinical Appraisal. Front. Cardiovasc. Med. 2023, 10, 1090103. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Wang, K.; Wei, C.; Feng, D.; Liu, Y.; He, Q.; Xu, X.; Wang, C.; Zhao, S.; Lv, L.; et al. The BCMA-Targeted Fourth-Generation CAR-T Cells Secreting IL-7 and CCL19 for Therapy of Refractory/Recurrent Multiple Myeloma. Front. Immunol. 2021, 12, 609421. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Gust, J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Giavridis, T.; van der Stegen, S.J.C.; Eyquem, J.; Hamieh, M.; Piersigilli, A.; Sadelain, M. CAR T Cell-Induced Cytokine Release Syndrome Is Mediated by Macrophages and Abated by IL-1 Blockade. Nat. Med. 2018, 24, 731–738. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-Derived IL-1 and IL-6 Are Differentially Required for Cytokine-Release Syndrome and Neurotoxicity Due to CAR T Cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Predominant Cerebral Cytokine Release Syndrome in CD19-Directed Chimeric Antigen Receptor-Modified T Cell Therapy. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4986179/ (accessed on 6 April 2023).
- Parker, K.R.; Migliorini, D.; Perkey, E.; Yost, K.E.; Bhaduri, A.; Bagga, P.; Haris, M.; Wilson, N.E.; Liu, F.; Gabunia, K.; et al. Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies. Cell 2020, 183, 126–142.e17. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric Antigen Receptor T-Cell Therapy—Assessment and Management of Toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Hunter, B.D.; Jacobson, C.A. CAR T-Cell Associated Neurotoxicity: Mechanisms, Clinicopathologic Correlates, and Future Directions. J. Natl. Cancer Inst. 2019, 111, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.S.; Gauthier, J. Taming the Beast: CRS and ICANS after CAR T-Cell Therapy for ALL. Bone Marrow Transplant. 2021, 56, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Barbar, T.; Jaffer Sathick, I. Tumor Lysis Syndrome. Adv. Chronic Kidney Dis. 2021, 28, 438–446.e1. [Google Scholar] [CrossRef]
- Maus, M.V.; Haas, A.R.; Beatty, G.L.; Albelda, S.M.; Levine, B.L.; Liu, X.; Zhao, Y.; Kalos, M.; June, C.H. T Cells Expressing Chimeric Antigen Receptors Can Cause Anaphylaxis in Humans. Cancer Immunol. Res. 2013, 1, 26–31. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Dudley, M.E.; Carpenter, R.O.; Kassim, S.H.; Rose, J.J.; Telford, W.G.; Hakim, F.T.; Halverson, D.C.; Fowler, D.H.; Hardy, N.M.; et al. Donor-Derived CD19-Targeted T Cells Cause Regression of Malignancy Persisting after Allogeneic Hematopoietic Stem Cell Transplantation. Blood 2013, 122, 4129–4139. [Google Scholar] [CrossRef] [PubMed]
- Thistlethwaite, F.C.; Gilham, D.E.; Guest, R.D.; Rothwell, D.G.; Pillai, M.; Burt, D.J.; Byatte, A.J.; Kirillova, N.; Valle, J.W.; Sharma, S.K.; et al. The Clinical Efficacy of First-Generation Carcinoembryonic Antigen (CEACAM5)-Specific CAR T Cells Is Limited by Poor Persistence and Transient Pre-Conditioning-Dependent Respiratory Toxicity. Cancer Immunol. Immunother. 2017, 66, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Flugel, C.L.; Majzner, R.G.; Krenciute, G.; Dotti, G.; Riddell, S.R.; Wagner, D.L.; Abou-el-Enein, M. Overcoming On-Target, off-Tumour Toxicity of CAR T Cell Therapy for Solid Tumours. Nat. Rev. Clin. Oncol. 2022, 20, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.T.; Maude, S.L.; Porter, D.L.; Frey, N.; Wood, P.; Han, X.; Waldron, E.; Chakraborty, A.; Awasthi, R.; Levine, B.L.; et al. Cellular Kinetics of CTL019 in Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia and Chronic Lymphocytic Leukemia. Blood 2017, 130, 2317–2325. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Davila, M.L.; Riviere, I.; Park, J.; Wang, X.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Olszewska, M.; et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2013, 5, 177ra38. [Google Scholar] [CrossRef]
- Finney, O.C.; Brakke, H.M.; Rawlings-Rhea, S.; Hicks, R.; Doolittle, D.; Lopez, M.; Futrell, R.B.; Orentas, R.J.; Li, D.; Gardner, R.A.; et al. CD19 CAR T Cell Product and Disease Attributes Predict Leukemia Remission Durability. J. Clin. Investig. 2019, 129, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2014, 6, 224ra25. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, Y.; Du, J.; Dong, Y.; Pang, H.; Ma, L.; Si, S.; Zhang, Z.; He, M.; Yue, Y.; et al. Reducing Hinge Flexibility of CAR-T Cells Prolongs Survival In Vivo With Low Cytokines Release. Front. Immunol. 2021, 12, 724211. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Huang, X.F.; Xiang, X.; Liu, Y.; Kang, X.; Song, Y.; Guo, X.; Liu, H.; Ding, N.; Zhang, T.; et al. A Safe and Potent Anti-CD19 CAR T Cell Therapy. Nat. Med. 2019, 25, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Alabanza, L.; Pegues, M.; Geldres, C.; Shi, V.; Wiltzius, J.J.W.; Sievers, S.A.; Yang, S.; Kochenderfer, J.N. Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains. Mol. Ther. 2017, 25, 2452–2465. [Google Scholar] [CrossRef]
- Pietrobon, V.; Todd, L.A.; Goswami, A.; Stefanson, O.; Yang, Z.; Marincola, F. Improving CAR T-Cell Persistence. Int. J. Mol. Sci. 2021, 22, 10828. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef]
- Thompson, J.A. New NCCN Guidelines: Recognition and Management of Immunotherapy-Related Toxicity. J. Natl. Compr. Cancer Netw. 2018, 16, 594–596. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Chen, D.H.; Guha, A.; Mackenzie, S.; Walker, J.M.; Roddie, C. CAR T Cell Therapy–Related Cardiovascular Outcomes and Management. JACC Cardio Oncol. 2020, 2, 97–109. [Google Scholar] [CrossRef]
- Hay, K.A.; Hanafi, L.-A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; López, J.A.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and Biomarkers of Severe Cytokine Release Syndrome after CD19 Chimeric Antigen Receptor-Modified T-Cell Therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef]
- Hong, F.; Shi, M.; Cao, J.; Wang, Y.; Gong, Y.; Gao, H.; Li, Z.; Zheng, J.; Zeng, L.; He, A.; et al. Predictive Role of Endothelial Cell Activation in Cytokine Release Syndrome after Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukaemia. J. Cell Mol. Med. 2021, 25, 11063–11074. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Pyo, S.; Kang, C.H.; Lee, C.O.; Lee, H.K.; Choi, S.U.; Park, C.H. Folate Receptor 1 (FOLR1) Targeted Chimeric Antigen Receptor (CAR) T Cells for the Treatment of Gastric Cancer. PLoS ONE 2018, 13, e0198347. [Google Scholar] [CrossRef] [PubMed]
- Murad, J.P.; Kozlowska, A.K.; Lee, H.J.; Ramamurthy, M.; Chang, W.-C.; Yazaki, P.; Colcher, D.; Shively, J.; Cristea, M.; Forman, S.J.; et al. Effective Targeting of TAG72+ Peritoneal Ovarian Tumors via Regional Delivery of CAR-Engineered T Cells. Front. Immunol. 2018, 9, 2268. [Google Scholar] [CrossRef] [PubMed]
- Kosti, P.; Maher, J.; Arnold, J.N. Perspectives on Chimeric Antigen Receptor T-Cell Immunotherapy for Solid Tumors. Front. Immunol. 2018, 9, 1104. [Google Scholar] [CrossRef] [PubMed]
- Saafan, H.; Alahdab, A.; Michelet, R.; Gohlke, L.; Ziemann, J.; Holdenrieder, S.; McLaughlin, K.-M.; Wass, M.N.; Cinatl, J.; Michaelis, M.; et al. Constitutive Cell Proliferation Regulating Inhibitor of Protein Phosphatase 2A (CIP2A) Mediates Drug Resistance to Erlotinib in an EGFR Activating Mutated NSCLC Cell Line. Cells 2021, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Grada, Z.; Hegde, M.; Byrd, T.; Shaffer, D.R.; Ghazi, A.; Brawley, V.S.; Corder, A.; Schönfeld, K.; Koch, J.; Dotti, G.; et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol. Ther.-Nucleic Acids 2013, 2, e105. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, J.; Huang, B.; Zhang, Z.; Wang, S.; Tang, F.; Teng, M.; Li, Y. Special Chimeric Antigen Receptor (CAR) Modifications of T Cells: A Review. Front. Oncol. 2022, 12, 832765. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.H.; Watchmaker, P.B.; Simic, M.S.; Gilbert, R.D.; Li, A.W.; Krasnow, N.A.; Downey, K.M.; Yu, W.; Carrera, D.A.; Celli, A.; et al. SynNotch-CAR T Cells Overcome Challenges of Specificity, Heterogeneity, and Persistence in Treating Glioblastoma. Sci. Transl. Med. 2021, 13, eabe7378. [Google Scholar] [CrossRef]
- Moghimi, B.; Muthugounder, S.; Jambon, S.; Tibbetts, R.; Hung, L.; Bassiri, H.; Hogarty, M.D.; Barrett, D.M.; Shimada, H.; Asgharzadeh, S. Preclinical Assessment of the Efficacy and Specificity of GD2-B7H3 SynNotch CAR-T in Metastatic Neuroblastoma. Nat. Commun. 2021, 12, 511. [Google Scholar] [CrossRef]
- Stern, L.A.; Gholamin, S.; Moraga, I.; Yang, X.; Saravanakumar, S.; Cohen, J.R.; Starr, R.; Aguilar, B.; Salvary, V.; Hibbard, J.C.; et al. Engineered IL13 Variants Direct Specificity of IL13Rα2-Targeted CAR T Cell Therapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2112006119. [Google Scholar] [CrossRef]
- Vitanza, N.A.; Wilson, A.L.; Huang, W.; Seidel, K.; Brown, C.; Gustafson, J.A.; Yokoyama, J.K.; Johnson, A.J.; Baxter, B.A.; Koning, R.W.; et al. Intraventricular B7-H3 CAR T Cells for Diffuse Intrinsic Pontine Glioma: Preliminary First-in-Human Bioactivity and Safety. Cancer Discov. 2023, 13, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Johnson, A.J.; Wilson, A.L.; Brown, C.; Yokoyama, J.K.; Künkele, A.; Chang, C.A.; Rawlings-Rhea, S.; Huang, W.; Seidel, K.; et al. Locoregional Infusion of HER2-Specific CAR T Cells in Children and Young Adults with Recurrent or Refractory CNS Tumors: An Interim Analysis. Nat. Med. 2021, 27, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.K.; Delaidelli, A.; Joseph, S.K.; Bielamowicz, K.; Fousek, K.; Holgado, B.L.; Manno, A.; Srikanthan, D.; Gad, A.Z.; Van Ommeren, R.; et al. Locoregional Delivery of CAR T Cells to the Cerebrospinal Fluid for Treatment of Metastatic Medulloblastoma and Ependymoma. Nat. Med. 2020, 26, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Tchou, J.; Zhao, Y.; Levine, B.L.; Zhang, P.J.; Davis, M.M.; Melenhorst, J.J.; Kulikovskaya, I.; Brennan, A.L.; Liu, X.; Lacey, S.F.; et al. Safety and Efficacy of Intratumoral Injections of Chimeric Antigen Receptor (CAR) T Cells in Metastatic Breast Cancer. Cancer Immunol. Res. 2017, 5, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, P.S.; Cherkassky, L.; Villena-Vargas, J.; Colovos, C.; Servais, E.; Plotkin, J.; Jones, D.R.; Sadelain, M. Regional Delivery of Mesothelin-Targeted CAR T Cell Therapy Generates Potent and Long-Lasting CD4-Dependent Tumor Immunity. Sci. Transl. Med. 2014, 6, 261ra151. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Burga, R.A.; McCormack, E.; Wang, L.J.; Mooring, W.; Point, G.R.; Khare, P.D.; Thorn, M.; Ma, Q.; Stainken, B.F.; et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-Cell Therapy for CEA+ Liver Metastases. Clin. Cancer Res. 2015, 21, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- The Inducible Caspase-9 Suicide Gene System as a “Safety Switch” to Limit on-Target, off-Tumor Toxicities of Chimeric Antigen Receptor T Cells. Available online: https://pubmed.ncbi.nlm.nih.gov/25389405/ (accessed on 4 April 2023).
- Dong, R.; Jiang, S.; Chen, Y.; Ma, Y.; Sun, L.; Xing, C.; Zhang, S.; Yu, K. Prognostic Significance of Cytokine Release Syndrome in B Cell Hematological Malignancies Patients After Chimeric Antigen Receptor T Cell Therapy. J. Interferon Cytokine Res. 2021, 41, 469–476. [Google Scholar] [CrossRef]
- Hayden, P.J. Management of Adults and Children Receiving CAR T-Cell Therapy: 2021 Best Practice Recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann. Oncol. 2022, 33, 259–275. [Google Scholar]
- Neelapu, S.S. Managing the Toxicities of CAR T-cell Therapy. Hematol. Oncol. 2019, 37, 48–52. [Google Scholar] [CrossRef]
- Earlier Steroid Use with Axicabtagene Ciloleucel (Axi-Cel) in Patients with Relapsed/Refractory Large B Cell Lymphoma (R/R LBCL)—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1083879119315320 (accessed on 4 April 2023).
- Le, R.Q.; Li, L.; Yuan, W.; Shord, S.S.; Nie, L.; Habtemariam, B.A.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist 2018, 23, 943–947. [Google Scholar] [CrossRef]
- Markham, A.; Patel, T. Siltuximab: First Global Approval. Drugs 2014, 74, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5996391/ (accessed on 1 April 2023).
- Sterner, R.M.; Sakemura, R.; Cox, M.J.; Yang, N.; Khadka, R.H.; Forsman, C.L.; Hansen, M.J.; Jin, F.; Ayasoufi, K.; Hefazi, M.; et al. GM-CSF Inhibition Reduces Cytokine Release Syndrome and Neuroinflammation but Enhances CAR-T Cell Function in Xenografts. Blood 2019, 133, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.J.; Kuhlmann, C.; Sterner, R.M.; Sakemura, R.; Sinha, S.; Hefazi, M.; Ahmed, O.; Durrant, C.; Chappell, D.; Roman, C.M.; et al. Improved Anti-Tumor Response of Chimeric Antigen Receptor T Cell (CART) Therapy after GM-CSF Inhibition Is Mechanistically Supported by a Novel Direct Interaction of GM-CSF with Activated Carts. Biol. Blood Marrow Transplant. 2020, 26, S60–S61. [Google Scholar] [CrossRef]
- Strati, P.; Jallouk, A.; Deng, Q.; Li, X.; Feng, L.; Sun, R.; Adkins, S.; Johncy, S.; Cain, T.; Steiner, R.E.; et al. A Phase I Study of Prophylactic Anakinra to Mitigate ICANS in Patients with Large B-Cell Lymphoma. Blood Adv. 2023. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Nath, K.; Devlin, S.M.; Sauter, C.S.; Palomba, M.L.; Shah, G.; Dahi, P.; Lin, R.J.; Scordo, M.; Perales, M.-A.; et al. CD19 CAR T-Cell Therapy and Prophylactic Anakinra in Relapsed or Refractory Lymphoma: Phase 2 Trial Interim Results. Nat. Med. 2023, 29, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Wehrli, M.; Gallagher, K.; Chen, Y.-B.; Leick, M.B.; McAfee, S.L.; El-Jawahri, A.R.; DeFilipp, Z.; Horick, N.; O’Donnell, P.; Spitzer, T.; et al. Single-Center Experience Using Anakinra for Steroid-Refractory Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS). J. Immunother. Cancer 2022, 10, e003847. [Google Scholar] [CrossRef] [PubMed]
- Nenna, A.; Carpenito, M.; Chello, C.; Nappi, P.; Annibali, O.; Vincenzi, B.; Grigioni, F.; Chello, M.; Nappi, F. Cardiotoxicity of Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: Pathophysiology, Clinical Implications, and Echocardiographic Assessment. Int. J. Mol. Sci. 2022, 23, 8242. [Google Scholar] [CrossRef]



| Side Effects | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| CRS [84,85] | Fever or organ toxicity |
| |||
| Hypotension | Grading and supportive care |
| |||
| If hypotension persists after two fluid boluses and anti-IL-6 therapy, start vasopressors, and consider transfer to an intensive care unit | Monitoring in the intensive care unit | ||||
| In patients at high risk or hypotension persists after 1–2 doses of anti-IL-6 therapy, dexamethasone can be used at 10 mg i.v. every 6 h for 1–3 days | Dexamethasone at 10 mg i.v. every 6 h for 1–3 days If refractory, increase to 20 mg i.v. every 6 h | Methylprednisolone i.v. 1000 mg/day for 3 days 250 mg × 2/day for 2 days 125 mg × 2/day for 2 days 60 mg × 2/day for 2 days | |||
| Hypoxia | Grading and supportive care | Tocilizumab or siltuximab ± corticosteroids and supportive care | |||
| Supplemental oxygen | Supplemental oxygen, including high-flow oxygen delivery and non-invasive positive-pressure ventilation | Mechanical ventilation | |||
| ICANS [45,85] | Supportive care and neurological work-up:
| ||||
| Transferring the patient to an intensive care unit if grade ≥ 2 CRS | Intensive care unit transfer | Intensive care unit monitoring; mechanical ventilation | |||
| Dexamethasone at 10–20 mg i.v. every 6 h or its equivalent of methylprednisolone for 1–3 days. | Lower ICP with hyperventilation, hyperosmolar therapy with mannitol/hypertonic saline, and/or neurosurgery consultation for a ventriculoperitoneal shunt in patients with cerebral edema | ||||
250 mg × 2/day for 2 days 125 mg × 2/day for 2 days 60 mg × 2/day for 2 days | |||||
| Classification of Examination | Specific Examination and Tests |
|---|---|
| Regular laboratory examination | Blood routine; blood biochemistry; coagulation function test; arterial blood gas analysis; infection-related test; cytokines (IL-1, IL-2, IL-6, TNF-α, IFN-γ, etc.) |
| Regular imaging examination | Chest and abdomen enhanced CT; cerebral enhanced MRI; abdominal ultrasound; echocardiogram |
| Proliferation of CAR-T cells in vivo | Quantitative PCR detection of peripheral blood CAR gene; flow cytometry |
| Examination of organ function | Electrocardiogram; echocardiogram; systemic, superficial lymph node ultrasound; lung function test; CARTOX-10 scoring; electroencephalogram; cerebrospinal fluid pressure; abdominal ultrasound; (regular laboratory examination is helpful to assess organ function) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qin, D.; Shou, A.C.; Liu, Y.; Wang, Y.; Zhou, L. Exploring CAR-T Cell Therapy Side Effects: Mechanisms and Management Strategies. J. Clin. Med. 2023, 12, 6124. https://doi.org/10.3390/jcm12196124
Zhang Y, Qin D, Shou AC, Liu Y, Wang Y, Zhou L. Exploring CAR-T Cell Therapy Side Effects: Mechanisms and Management Strategies. Journal of Clinical Medicine. 2023; 12(19):6124. https://doi.org/10.3390/jcm12196124
Chicago/Turabian StyleZhang, Yugu, Diyuan Qin, Arthur Churchill Shou, Yanbin Liu, Yongsheng Wang, and Lingyun Zhou. 2023. "Exploring CAR-T Cell Therapy Side Effects: Mechanisms and Management Strategies" Journal of Clinical Medicine 12, no. 19: 6124. https://doi.org/10.3390/jcm12196124
APA StyleZhang, Y., Qin, D., Shou, A. C., Liu, Y., Wang, Y., & Zhou, L. (2023). Exploring CAR-T Cell Therapy Side Effects: Mechanisms and Management Strategies. Journal of Clinical Medicine, 12(19), 6124. https://doi.org/10.3390/jcm12196124






