Minimal Detectable Change in Resting Blood Pressure and Cardiorespiratory Fitness: A Secondary Analysis of a Study on School-Based High-Intensity Interval Training Intervention
Abstract
:1. Introduction
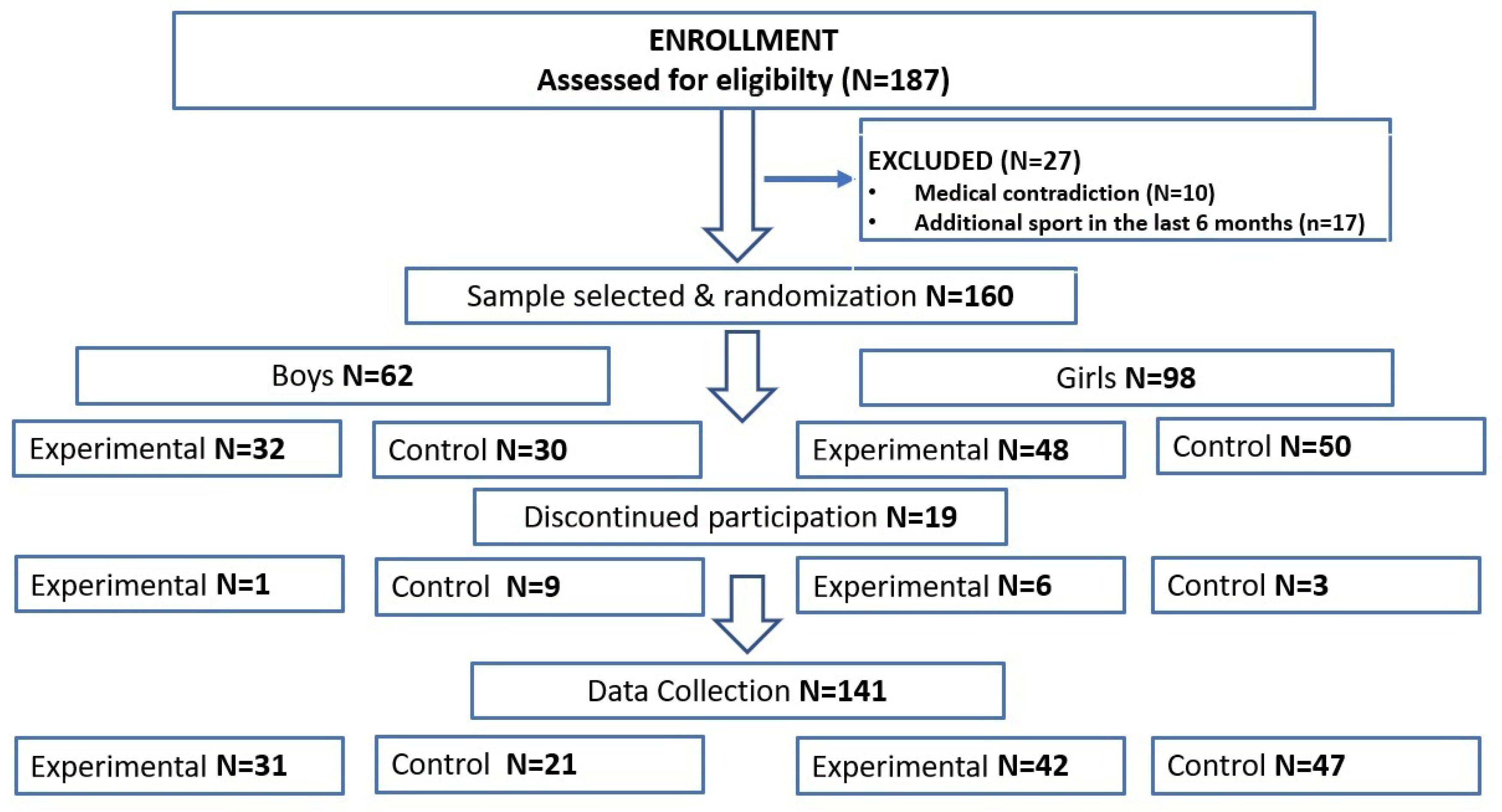
2. Materials and Methods
2.1. Participants
2.2. Procedures and HIIT Intervention
2.3. Measurements
2.3.1. Anthropometrical Measurements
2.3.2. Resting Blood Pressure Measurements
2.3.3. Fitness Index (FI) (Harvard Step Test)—Cardiorespiratory Fitness (CRF)
2.4. Minimal Detectable Change
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Högström, G.; Nordström, A.; Nordström, P. High aerobic fitness in late adolescence is associated with a reduced risk of myocardial infarction later in life: A nationwide cohort study in men. Eur. Heart J. 2014, 35, 3133–3140. [Google Scholar] [CrossRef]
- Ortega, F.B.; Ruiz, J.R.; Castillo, M.J.; Sjöström, M. Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. 2008, 32, 1–11. [Google Scholar] [CrossRef]
- Costigan, S.A.; Eather, N.; Plotnikoff, R.C.; Taaffe, D.R.; Lubans, D.R. High-intensity interval training for improving health-related fitness in adolescents: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1253–1261. [Google Scholar] [CrossRef]
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.; Ekelund, U. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257. [Google Scholar] [CrossRef]
- Dumith, S.C.; Gigante, D.P.; Domingues, M.R.; Kohl, H.W. Physical activity change during adolescence: A systematic review and a pooled analysis. Int. J. Epidemiol. 2012, 40, 685–698. [Google Scholar] [CrossRef]
- Buchan, D.S.; Ollis, S.; Thomas, N.E.; Buchanan, N.; Cooper, S.M.; Malina, R.M.; Baker, J.S. Physical activity interventions: Effects of duration and intensity. Scand. J. Med. Sci. 2011, 21, e341–e350. [Google Scholar] [CrossRef]
- Camacho-Cardenosa, A.; Brazo-Sayavera, J.; Camacho-Cardenosa, M.; Marcos-Serrano, M.; Timón, R.; Olcina, G. Effects of high intensity interval training on fat mass parameters in adolescents. Rev. Esp. Salud Publica 2016, 21, e1–e9. [Google Scholar]
- Costigan, S.A.; Ridgers, N.D.; Eather, N.; Plotnikoff, R.C.; Harris, N.; Lubans, D.R. Exploring the impact of high intensity interval training on adolescents’ objectively measured physical activity: Findings from a randomized controlled trial. J. Sports Sci. 2018, 36, 1087–1094. [Google Scholar] [CrossRef]
- Delgado-Floody, P.; Latorre-Roman, P.; Jerez-Mayorga, D.; Caamano-Navarrete, F.; Garcia-Pinillos, F. Feasibility of incorporating high-intensity interval training into physical education programs to improve body composition and cardiorespiratory capacity of overweight and obese children: A systematic review. J. Exerc. Sci. Fit. 2019, 17, 35–40. [Google Scholar] [CrossRef]
- Stratford, P.W.; Riddle, D.L. When minimal detectable change exceeds a diagnostic test-based threshold change value for an outcome measure: Resolving the conflict. Phys. Ther. 2012, 92, 1338–1347. [Google Scholar] [CrossRef]
- Beaton, D.E.; Boers, M.; Wells, G.A. Many faces of the minimal clinically important difference (MCID): A literature review and directions for future research. Curr. Opin. Rheumatol. 2002, 14, 109–114. [Google Scholar] [CrossRef]
- Turner, D.; Schünemann, H.J.; Griffith, L.E.; Beaton, D.E.; Griffiths, A.M.; Critch, J.N.; Guyatt, G.H. The minimal detectable change cannot reliably replace the minimal important difference. J. Clin. Epidemiol. 2010, 63, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.A.; Cook, C.E.; Baxter, G.D. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J. Orthop. Sports Phys. Ther. 2011, 41, 319–327. [Google Scholar] [CrossRef]
- Kovacs, F.M.; Abraira, V.; Royuela, A.; Corcoll, J.; Alegre, L.; Tomás, M.; Mir, M.A.; Cano, A.; Muriel, A.; Zamora, J.; et al. Minimum detectable and minimal clinically important changes for pain in patients with nonspecific neck pain. BMC Musculoskelet. Disord. 2008, 9, 43. [Google Scholar] [CrossRef]
- Andresen, E.M. Criteria for assessing the tools of disability outcomes research. Arch. Phys. Med. Rehabil. 2000, 81, 15–20. [Google Scholar] [CrossRef]
- Riddle, D.; Stratford, P. Is This Change Real? Interpreting Patient Outcomes in Physical Therapy; Davis Plus: Philadelphia, PA, USA, 2013. [Google Scholar]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status: Ascertaining the minimal clinically important difference. Control. Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, S.E.; Griffiths, P.L.; Clemes, S.A. Seasonal variation in physical activity, sedentary behaviour and sleep in a sample of UK adults. Ann. Hum. Biol. 2014, 41, 1–8. [Google Scholar] [CrossRef]
- Popowczak, M.; Rokita, A.; Domaradzki, J. Effects of Tabata training on health-related fitness components among secondary school students. Kinesiology 2022, 54, 221–229. [Google Scholar] [CrossRef]
- Domaradzki, J.; Koźlenia, D.; Popowczak, M. Prevalence of Positive Effects on Body Fat Percentage, Cardiovascular Parameters, and Cardiorespiratory Fitness after 10-Week High-Intensity Interval Training in Adolescents. Biology 2022, 11, 424. [Google Scholar] [CrossRef]
- Domaradzki, J.; Koźlenia, D.; Popowczak, M. The Prevalence of Responders and Non-Responders for Body Composition, Resting Blood Pressure, Musculoskeletal, and Cardiorespiratory Fitness after Ten Weeks of School-Based High-Intensity Interval Training in Adolescents. J. Clin. Med. 2023, 12, 4204. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.; Appadoo, S.; Bector, C.; Chandra, S. Measuring physical fitness and cardiovascular efficiency using harvard step test approach under fuzzy environment. ASAC 2008, 29, 129–158. [Google Scholar]
- Burnstein, B.D.; Steele, R.J.; Shrier, I. Reliability of fitness tests using methods and time periods common in sport and occupational management. J. Athl. Train. 2011, 46, 505–513. [Google Scholar] [CrossRef]
- Crosby, R.D.; Kolotkin, R.L.; Williams, G.R. Defining clinically meaningful change in health-related quality of life. J. Clin. Epidemiol. 2003, 56, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Haley, S.M.; Fragala-Pinkham, M.A. Interpreting change scores of tests and measures used in physical therapy. Phys. Ther. 2006, 86, 735–743. [Google Scholar] [CrossRef]
- Donoghue, D.; Stokes, E.K. How much change is true change? The minimum detectable change of the Berg Balance Scale in elderly people. J. Rehabil. Med. 2009, 41, 343–346. [Google Scholar] [CrossRef]
- Teixeira, R.V.; de Matos, D.; Da Silva, L.M.; Aidar, F.J.; De Araújo Tinôco Cabral, B.G. Behaviour of training loads and physical performance during a period of 6 weeks in high-intensity functional training practitioners. Hum. Mov. 2023, 24, 59–69. [Google Scholar] [CrossRef]
- Dominic, D.; Kishore, S. Effect of modified high intensity interval training on fat loss. Cent. Eur. J. Sport. Sci. Med. 2021, 35, 49–56. [Google Scholar] [CrossRef]
- Espinoza-Silva, M.; Latorre-Román, P.; Párraga-Montilla, J.; Caamaño-Navarrete, F.; Jerez-Mayorga, D.; Delgado-Floody, P. Response of obese schoolchildren to high-intensity interval training applied in the school context. Endocrinol. Diabetes Nutr. 2019, 66, 611–619. [Google Scholar] [CrossRef]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine response to high-intensity interval vs. resistance exercise: An individual approach. Front. Physiol. 2018, 9, 1735. [Google Scholar] [CrossRef]
- Engel, F.A.; Ackermann, A.; Chtourou, H.; Sperlich, B. High-intensity interval training performed by young athletes: A Systematic review and meta-analysis. Front. Physiol. 2018, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Racil, G.; Coquart, J.B.; Elmontassar, W.; Haddad, M.; Goebel, R.; Chaouachi, A.; Amri, M.; Chamari, K. Greater effects of high- compared with moderate-intensity interval training on cardio-metabolic variables, blood leptin concentration and ratings of perceived exertion in obese adolescent females. Biol. Sport 2016, 33, 145–152. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Cerrillo-Urbina, A.J.; Herrera-Valenzuela, T.; Cristi-Montero, C.; Saavedra, J.M.; Martínez-Vizcaíno, V. Is high-intensity interval training more effective on improving cardiometabolic risk and aerobic capacity than other forms of exercise in overweight and obese youth? A meta-analysis. Obes. Rev. 2016, 17, 531–540. [Google Scholar] [CrossRef]
- Moniz, S.C.; Islam, H.; Hazell, T.J. Mechanistic and methodological perspectives on the impact of intense interval training on post-exercise metabolism. Scand. J. Med. Sci. Sports 2020, 30, 638–651. [Google Scholar]
- Børsheim, E.; Bahr, R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med. 2003, 33, 1037–1060. [Google Scholar] [CrossRef]
- Farah, B.Q.; Ritti-Dias, R.M.; Balagopal, P.B.; Hill, J.O.; Prado, W.L. Does exercise intensity affect blood pressure and heart rate in obese adolescents? A 6-month multidisciplinary randomized intervention study. Pediatr. Obes. 2014, 9, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Corte de Araujo, A.C.; Roschel, H.; Picanço, A.R.; do Prado, D.M.; Villares, S.M.; de Sa Pinto, A.L.; Gualano, B. Similar health benefits of endurance and high-intensity interval training in obese children. PLoS ONE 2012, 7, e42747. [Google Scholar] [CrossRef]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef]
- Alvarez, C.; Ramírez-Campillo, R.; Ramírez-Vélez, R.; Izquierdo, M. Effects of 6-Weeks High-Intensity Interval Training in Schoolchildren with Insulin Resistance: Influence of Biological Maturation on Metabolic, Body Composition, Cardiovascular and Performance Non-responses. Front. Physiol. 2017, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Blair, S.N.; Church, T.S.; Earnest, C.P.; Hagberg, J.M.; Häkkinen, K.; Jenkins, N.T.; Karavirta, L.; Kraus, W.E.; Leon, A.S.; et al. Adverse metabolic response to regular exercise: Is it a rare or common occurrence? PLoS ONE 2012, 7, e37887. [Google Scholar] [CrossRef]
- Hopkins, W.G. How to Interpret Changes in an Athletic Performance Test. Available online: http://www.sportsci.org/jour/04/wghtests.htm (accessed on 20 July 2023).
- Jurić, P.; Dudley, D.A.; Petocz, P. Does incorporating high intensity interval training in physical education classes improve fitness outcomes of students? A cluster randomized controlled trial. Prev. Med. Rep. 2023, 32, 102127. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Lundby, C. Refuting the myth of non-response to exercise training: ‘non-responders’ do respond to higher dose of training: Trainability and exercise dose. J. Physiol. 2017, 595, 3377–3387. [Google Scholar] [CrossRef]
- Lan, C.; Liu, Y.; Wang, Y. Effects of different exercise programs on cardiorespiratory fitness and body composition in college students. J. Exerc. Sci. Fit. 2022, 20, 62–69. [Google Scholar] [CrossRef]
- Guo, L.; Chen, J.; Yuan, W. The effect of HIIT on body composition, cardiovascular fitness, psychological well-being, and executive function of overweight/obese female young adults. Front. Psychol. 2023, 13, 1095328. [Google Scholar] [CrossRef]
- França, C.; Marques, A.; Ihle, A.; Nuno, J.; Campos, P.; Gonçalves, F.; Martins, J.; Gouveia, É.R. Associations between muscular strength and vertical jumping performance in adolescent male football players. Hum. Mov. 2023, 24, 94–100. [Google Scholar] [CrossRef]
- Yong, F.; Heiss, G.; Couper, D.; Meyer, M.L.; Cheng, S.; Tanaka, H. Measurement Repeatability of Central and Peripheral Blood Pressures: The ARIC Study. Am. J. Hypertens. 2017, 30, 978–984. [Google Scholar] [CrossRef]
- Celler, G.B.; Butlin, M.; Argha, A.; Tan, I.; Yong, A.; Avolio, A. Are Korotkoff Sounds Reliable Markers for Accurate Estimation of Systolic and Diastolic Pressure Using Brachial Cuff Sphygmomanometry? IEEE Trans. Bio-Med. Eng. 2021, 68, 3593–3601. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Arel, J.; Brosseau, R.; Hicks, A.L.; Gagnon, D.H. Reliability and minimal detectable change of a new treadmill-based progressive workload incremental test to measure cardiorespiratory fitness in manual wheelchair users. J. Spinal Cord Med. 2017, 40, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Hol, A.T.; Eng, J.J.; Miller, W.C.; Sproule, S.; Krassioukov, A.V. Reliability and validity of the six-minute arm test for the evaluation of cardiovascular fitness in people with spinal cord injury. Arch. Phys. Med. Rehabil. 2007, 88, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Swift, D.L.; McGuire, D.K.; Ayers, C.R.; Neeland, I.J.; Blair, S.N.; Johannsen, N.; Earnest, C.P.; Berry, J.D.; Church, T.S. Metabolic effects of exercise training among fitness-nonresponsive patients with type 2 diabetes: The HART-D Study. Diabetes Care 2015, 38, 1494–1501. [Google Scholar] [CrossRef]
- Ross, R.; de Lannoy, L.; Stotz, P.J. Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response. Mayo Clin. Proc. 2015, 90, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
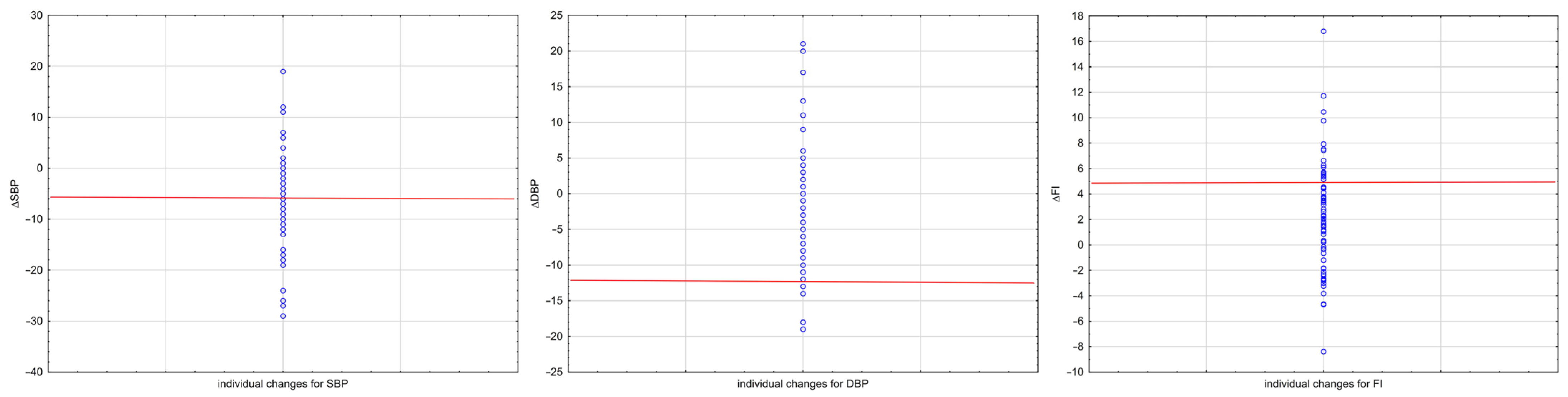
| Variable | EG | CG | t | p | Cohen’s d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 95%CI | Mean ± SD | 95%CI | ||||||||
| ΔSBP | −6.32 | −8.31 | −4.32 | 8.56 | 0.75 | −0.38 | 1.88 | 4.67 | −6.023 | <0.001 | −1.02 (−1.36, −0.66) |
| %Δ | −4.88 | −6.43 | −3.33 | 6.64 | 0.79 | −0.19 | 1.76 | 4.01 | −6.076 | <0.001 | −1.02 (−1.37, −0.67) |
| ΔDBP | −2.36 | −4.32 | −0.39 | 8.43 | −0.16 | −2.00 | 1.67 | 7.59 | −1.621 | 0.107 | −0.27 (−0.60, 0.06) |
| %Δ | −2.60 | −5.41 | 0.21 | 12.05 | 0.33 | −2.33 | 3.00 | 11.01 | −1.503 | 0.135 | −0.25 (−0.58, 0.08) |
| ΔFI | 2.36 | 1.40 | 3.32 | 4.13 | 0.35 | −0.42 | 1.12 | 3.19 | 3.223 | 0.002 | 0.54 (0.21, 0.88) |
| %Δ | 5.80 | 3.58 | 8.02 | 9.52 | 1.06 | −0.70 | 2.82 | 7.27 | 3.301 | 0.001 | 0.56 (0.22, 0.89) |
| Variable | Minimal Detectable Change | Baseline | ICC(3,1) | SEM | ||||
|---|---|---|---|---|---|---|---|---|
| MDC90 | MDC90% | Mean ± SD | 95%CI | |||||
| SBP | 7.82 | 6.68 | 117.03 | 9.06 | 114.83 | 119.22 | 0.86 (0.78–0.91) | 3.37 |
| DBP | 12.45 | 17.27 | 72.13 | 7.58 | 70.30 | 73.97 | 0.50 (0.30–0.66) | 5.37 |
| FI | 5.39 | 12.15 | 44.35 | 3.77 | 43.44 | 45.26 | 0.62 (0.45–0.75) | 2.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domaradzki, J. Minimal Detectable Change in Resting Blood Pressure and Cardiorespiratory Fitness: A Secondary Analysis of a Study on School-Based High-Intensity Interval Training Intervention. J. Clin. Med. 2023, 12, 6146. https://doi.org/10.3390/jcm12196146
Domaradzki J. Minimal Detectable Change in Resting Blood Pressure and Cardiorespiratory Fitness: A Secondary Analysis of a Study on School-Based High-Intensity Interval Training Intervention. Journal of Clinical Medicine. 2023; 12(19):6146. https://doi.org/10.3390/jcm12196146
Chicago/Turabian StyleDomaradzki, Jarosław. 2023. "Minimal Detectable Change in Resting Blood Pressure and Cardiorespiratory Fitness: A Secondary Analysis of a Study on School-Based High-Intensity Interval Training Intervention" Journal of Clinical Medicine 12, no. 19: 6146. https://doi.org/10.3390/jcm12196146
APA StyleDomaradzki, J. (2023). Minimal Detectable Change in Resting Blood Pressure and Cardiorespiratory Fitness: A Secondary Analysis of a Study on School-Based High-Intensity Interval Training Intervention. Journal of Clinical Medicine, 12(19), 6146. https://doi.org/10.3390/jcm12196146









