Reduced Reliability of Procalcitonin (PCT) as a Biomarker of Bacterial Superinfection: Concerns about PCT-Driven Antibiotic Stewardship in Critically Ill COVID-19 Patients—Results from a Retrospective Observational Study in Intensive Care Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study, Population, Settings, Data Collection, and Outcomes
2.2. Diagnosis of SARS-CoV-2 Infection and COVID-19-Related Pneumonia, and COVID-19 Treatments
2.3. Clinical Evaluation, PCT Dosage, and Microbiological Analysis
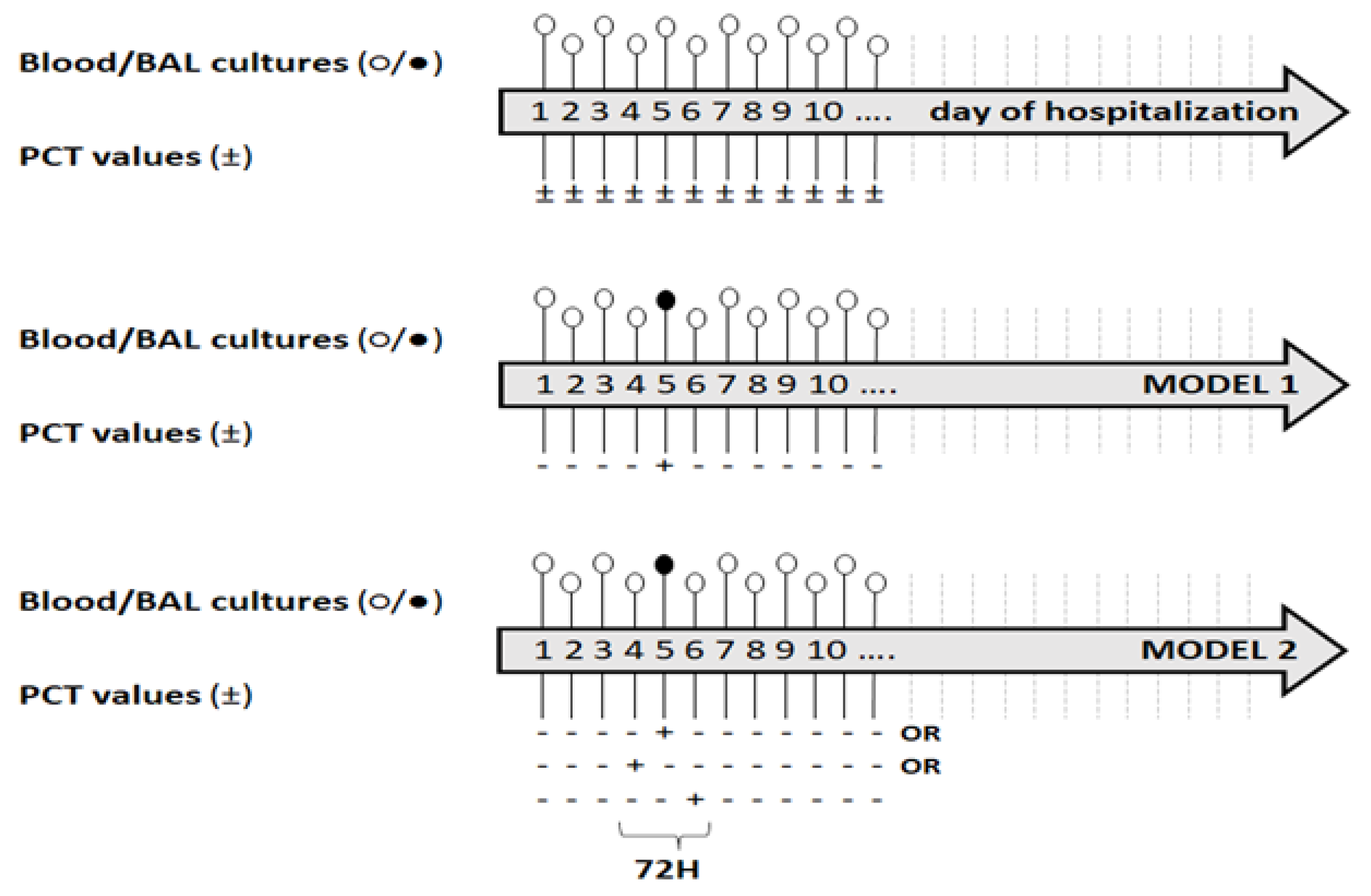
2.4. Definition
2.5. Statistical Analysis
2.6. Ethics Committee Approval
3. Results
3.1. Predictors of VAP/BSI
3.2. Diagnostic Accuracy of PCT and PCT-72 h
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Falcone, M.; Suardi, L.R.; Tiseo, G.; Galfo, V.; Occhineri, S.; Verdenelli, S.; Ceccarelli, G.; Poli, M.; Merli, M.; Bavaro, D.; et al. Superinfections caused by carbapenem-resistant Enterobacterales in hospitalized patients with COVID-19, a multicentre observational study from Italy (CREVID Study). JAC Antimicrob. Resist. 2022, 4, dlac064. [Google Scholar] [CrossRef] [PubMed]
- Polly, M.; de Almeida, B.L.; Lennon, R.P.; Cortês, M.F.; Costa, S.F.; Guimarães, T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am. J. Infect. Control 2022, 50, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Alshrefy, A.J.; Alwohaibi, R.N.; Alhazzaa, S.A.; Almaimoni, R.A.; AlMusailet, L.I.; Al Qahtani, S.Y.; Alshahrani, M.S. Incidence of Bacterial and Fungal Secondary Infections in COVID-19 Patients Admitted to the ICU. Int. J. Gen. Med. 2022, 15, 7475–7485. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Beishuizen, A.; Broyles, M.; Ferrer, R.; Gavazzi, G.; Gluck, E.H.; González Del Castillo, J.; Jensen, J.U.; Kanizsai, P.L.; Kwa, A.L.H.; et al. Procalcitonin (PCT)-guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clin. Chem. Lab. Med. 2019, 57, 1308–1318. [Google Scholar] [CrossRef]
- Meisner, M. Update on procalcitonin measurements. Ann. Lab. Med. 2014, 34, 263–273. [Google Scholar] [CrossRef]
- Linscheid, P.; Seboek, D.; Nylen, E.S.; Langer, I.; Schlatter, M.; Becker, K.L.; Keller, U.; Müller, B. In vitro and in vivo calcitonin I gene expression in parenchymal cells: A novel product of human adipose tissue. Endocrinology 2003, 144, 5578–5584. [Google Scholar] [CrossRef] [PubMed]
- Linscheid, P.; Seboek, D.; Zulewski, H.; Keller, U.; Müller, B. Autocrine/paracrine role of inflammation mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology 2005, 146, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.N. Role of procalcitonin in the management of infected patients in the intensive care unit. Infect. Dis. Clin. N. Am. 2017, 31, 435–453. [Google Scholar] [CrossRef]
- Bergin, S.P.; Tsalik, E.L. Procalcitonin: The right answer but to which question? Clin. Infect. Dis. 2017, 65, 191–193. [Google Scholar] [CrossRef][Green Version]
- Gautam, S.; Cohen, A.J.; Stahl, Y.; Valda Toro, P.; Young, G.M.; Datta, R.; Yan, X.; Ristic, N.T.; Bermejo, S.D.; Sharma, L.; et al. Severe respiratory viral infection induces procalcitonin in the absence of bacterial pneumonia. Thorax 2020, 75, 974–981. [Google Scholar] [CrossRef]
- Linscheid, P.; Seboek, D.; Schaer, D.J.; Zulewski, H.; Keller, U.; Müller, B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit. Care Med. 2004, 32, 1715–1721. [Google Scholar] [CrossRef]
- Whang, K.T.; Vath, S.D.; Becker, K.L.; Snider, R.H.; Nylen, E.S.; Muller, B.; Li, Q.; Tamarkin, L.; White, J.C. Procalcitonin and proinflammatory cytokine in interactions in sepsis. Shock 2000, 14, 73–78. [Google Scholar] [CrossRef]
- Nijsten, M.W.; Olinga, P.; The, T.H.; de Vries, E.G.; Koops, H.S.; Groothuis, G.M.; Limburg, P.C.; ten Duis, H.J.; Moshage, H.; Hoekstra, H.J.; et al. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit. Care Med. 2000, 28, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Preas, H.L., 2nd; Nylen, E.S.; Snider, R.H.; Becker, K.L.; White, J.C.; Agosti, J.M.; Suffredini, A.F. Effects of anti-inflammatory agents on serum levels of calcitonin precursors during human experimental endotoxemia. J. Infect. Dis. 2001, 184, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Clinical Utility of Procalcitonin on Antibiotic Stewardship: A Narrative Review. Infect. Chemother. 2022, 54, 610–620. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Clinical Management of COVID-19, Interim Guidance. World Health Organization 2020. Available online: https://apps.who.int/iris/handle/10665/332196 (accessed on 27 December 2022).
- Mussini, C.; Falcone, M.; Nozza, S.; Sagnelli, C.; Parrella, R.; Meschiari, M.; Petrosillo, N.; Mastroianni, C.; Cascio, A.; Iaria, C.; et al. Italian Society of Infectious and Tropical Diseases. Therapeutic strategies for severe COVID-19, A position paper from the Italian Society of Infectious and Tropical Diseases (SIMIT). Clin. Microbiol. Infect. 2021, 27, 389–395. [Google Scholar] [CrossRef]
- Meisner, M.; Schmidt, J.; Hüttner, H.; Tschaikowsky, K. The natural elimination rate of procalcitonin in patients with normal and impaired renal function. Intensive Care Med. 2000, 26 (Suppl. 2), S212–S216. [Google Scholar] [CrossRef]
- Eucast: EUCAST. Available online: https://www.eucast.org (accessed on 24 December 2022).
- Plachouras, D.; Lepape, A.; Suetens, C. ECDC definitions and methods for the surveillance of healthcare-associated infections in intensive care units. Intensive Care Med. 2018, 44, 2216–2218. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Staquet, M.; Rozencweig, M.; Lee, Y.J.; Muggia, F.M. Methodology for the assessment of new dichotomous diagnostic tests. J. Chronic Dis. 1981, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Church, D.L. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin. Microbiol. Rev. 2014, 27, 647–664. [Google Scholar] [CrossRef]
- Genders, T.S.; Spronk, S.; Stijnen, T.; Steyerberg, E.W.; Lesaffre, E.; Hunink, M.G. Methods for calculating sensitivity and specificity of clustered data: A tutorial. Radiology 2012, 265, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Omoush, S.A.; Alzyoud, J.A.M. The Prevalence and Impact of Coinfection and Superinfection on the Severity and Outcome of COVID-19 Infection: An Updated Literature Review. Pathogens 2022, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- De Marignan, D.; Vacheron, C.H.; Ader, F.; Lecocq, M.; Richard, J.C.; Frobert, E.; Casalegno, J.S.; Couray-Targe, S.; Argaud, L.; Rimmele, T.; et al. A retrospective comparison of COVID-19 and seasonal influenza mortality and outcomes in the ICUs of a French university hospital. Eur. J. Anaesthesiol. 2022, 39, 427–435. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Invest. 2020, 50, e13319. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, F.; Ceccarelli, G.; Migliara, G.; Baccolini, V.; Russo, A.; Marzuillo, C.; Ceparano, M.; Giordano, G.; Tozzi, P.; Galardo, G.; et al. High Incidence of Candidemia in Critically Ill COVID-19 Patients Supported by Veno-Venous Extracorporeal Membrane Oxygenation: A Retrospective Study. J. Fungi 2023, 9, 119. [Google Scholar] [CrossRef]
- Russo, A.; Venditti, M.; Ceccarelli, G.; Mastroianni, C.M.; d’Ettorre, G. Procalcitonin in daily clinical practice: An evergreen tool also during a pandemic. Intern. Emerg. Med. 2021, 16, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, L.; Lin, L.; Liu, X. Predictive values of procalcitonin for coinfections in patients with COVID-19, a systematic review and meta-analysis. Virol. J. 2023, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Pink, I.; Raupach, D.; Fuge, J.; Vonberg, R.P.; Hoeper, M.M.; Welte, T.; Rademacher, J. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection 2021, 49, 935–943. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Chang, M.; Dietz, D.; Shoucri, S.; Laracy, J.; Sobieszczyk, M.E.; Uhlemann, A.C.; Zucker, J.; Kubin, C.J. Limited Utility of Procalcitonin in Identifying Community-Associated Bacterial Infections in Patients Presenting with Coronavirus Disease 2019. Antimicrob. Agents Chemother. 2021, 65, e02167-20. [Google Scholar] [CrossRef]
- Vanhomwegen, C.; Veliziotis, I.; Malinverni, S.; Konopnicki, D.; Dechamps, P.; Claus, M.; Roman, A.; Cotton, F.; Dauby, N. Procalcitonin accurately predicts mortality but not bacterial infection in COVID-19 patients admitted to intensive care unit. Ir. J. Med. Sci. 2021, 190, 1649–1652. [Google Scholar] [CrossRef]
- Heer, R.S.; Mandal, A.K.; Kho, J.; Szawarski, P.; Csabi, P.; Grenshaw, D.; Walker, I.A.; Missouris, C.G. Elevated procalcitonin concentrations in severe Covid-19 may not reflect bacterial co-infection. Ann. Clin. Biochem. 2021, 58, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Dolci, A.; Robbiano, C.; Aloisio, E.; Chibireva, M.; Serafini, L.; Falvella, F.S.; Pasqualetti, S.; Panteghini, M. Searching for a role of procalcitonin determination in COVID-19, a study on a selected cohort of hospitalized patients. Clin. Chem. Lab. Med. 2020, 59, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Adams, C.; Brunetti, L.; Bargoud, C.; Teichman, A.L.; Choron, R.L. Evaluation of Procalcitonin’s Utility to Predict Concomitant Bacterial Pneumonia in Critically Ill COVID-19 Patients. J. Intensive Care Med. 2022, 37, 1486–1492. [Google Scholar] [CrossRef]
- Lugito, N.P.H. Is procalcitonin a part of human immunological response to SARS-CoV-2 infection or “just” a marker of bacterial coinfection? Curr. Res. Transl. Med. 2021, 69, 103289. [Google Scholar] [CrossRef]
- Martinez, F.O.; Combes, T.W.; Orsenigo, F.; Siamon Gordon, S. Monocyte activation in systemic Covid-19 infection: Assay and rationale. EBioMedicine 2020, 59, 102964. [Google Scholar] [CrossRef]
- Daubin, C.; Fournel, F.; Thiollière, F.; Daviaud, F.; Ramakers, M.; Polito, A.; Flocard, B.; Valette, X.; Du Cheyron, D.; Terzi, N.; et al. Ability of procalcitonin to distinguish between bacterial and nonbacterial infection in severe acute exacerbation of chronic obstructive pulmonary syndrome in the ICU. Ann. Intensive Care 2021, 11, 39. [Google Scholar] [CrossRef]
- Cohen, A.J.; Glick, L.R.; Lee, S.; Kunitomo, Y.; Tsang, D.A.; Pitafi, S.; Valda Toro, P.; Ristic, N.R.; Zhang, E.; Carey, G.B.; et al. Nonutility of procalcitonin for diagnosing bacterial pneumonia in patients with severe COVID-19. Eur. Clin. Respir. J. 2023, 10, 2174640. [Google Scholar] [CrossRef]
- Hughes, S.; Mughal, N.; Moore, L.S.P. Procalcitonin to Guide Antibacterial Prescribing in Patients Hospitalised with COVID-19. Antibiotics 2021, 10, 1119. [Google Scholar] [CrossRef] [PubMed]
- Atallah, N.J.; Warren, H.M.; Roberts, M.B.; Elshaboury, R.H.; Bidell, M.R.; Gandhi, R.G.; Adamsick, M.; Ibrahim, M.K.; Sood, R.; Bou Zein Eddine, S.; et al. Baseline procalcitonin as a predictor of bacterial infection and clinical outcomes in COVID-19, A case-control study. PLoS ONE 2022, 17, e0262342. [Google Scholar] [CrossRef] [PubMed]
| COVID-19 Patients n (%) | ||
|---|---|---|
| Patients | 184 | |
| Gender | ||
| Female | 48 (26.1) | |
| Male | 136 (73.9) | |
| Age, years | ||
| Median (IQR) | 66 (55.5, 73) | |
| SAPS II (n = 162) | ||
| Median (IQR) | 35 (27, 43) | |
| Comorbidities (yes) | 146 (79.4) | |
| Diabetes mellitus (yes) | 40 (21.7) | |
| Obesity (yes) | 22 (12.0) | |
| Hypertension (yes) | 86 (46.7) | |
| Cardiopathy (yes) | 35 (19.0) | |
| Renal failure (yes) | 12 (6.5) | |
| COPD (yes) | 26 (14.1) | |
| Hepatopathy (yes) | 4 (2.2) | |
| Neurological disorders (yes) | 28 (15.2) | |
| Other disorders (yes) | 63 (34.3) | |
| Outcome | ||
| Discharge | 115 (62.5) | |
| Death | 69 (37.5) | |
| Model 1 a (n = 162, Observations = 1702) | Model 2 b (n = 162, Observations = 1810) | |||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
| Positive PCT (yes) | 1.13 | 0.51–2.52 | 0.764 | -- | -- | -- |
| Positive PCT-72 h (yes) | -- | -- | -- | 1.32 | 0.66–2.64 | 0.427 |
| Age in years | 1.02 | 1.00–1.05 | 0.099 | 1.02 | 1.00–1.05 | 0.099 |
| Gender (male) | 1.00 | 0.57–1.05 | 0.993 | 0.98 | 0.58–1.67 | 0.939 |
| SAPS II | 0.98 | 0.95–1.01 | 0.202 | 0.98 | 0.95–1.01 | 0.129 |
| Comorbidity (yes) | 1.13 | 0.55–2.39 | 0.623 | 1.25 | 0.62–2.51 | 0.536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarelli, G.; Alessandri, F.; Migliara, G.; Baccolini, V.; Giordano, G.; Galardo, G.; Marzuillo, C.; De Vito, C.; Russo, A.; Ciccozzi, M.; et al. Reduced Reliability of Procalcitonin (PCT) as a Biomarker of Bacterial Superinfection: Concerns about PCT-Driven Antibiotic Stewardship in Critically Ill COVID-19 Patients—Results from a Retrospective Observational Study in Intensive Care Units. J. Clin. Med. 2023, 12, 6171. https://doi.org/10.3390/jcm12196171
Ceccarelli G, Alessandri F, Migliara G, Baccolini V, Giordano G, Galardo G, Marzuillo C, De Vito C, Russo A, Ciccozzi M, et al. Reduced Reliability of Procalcitonin (PCT) as a Biomarker of Bacterial Superinfection: Concerns about PCT-Driven Antibiotic Stewardship in Critically Ill COVID-19 Patients—Results from a Retrospective Observational Study in Intensive Care Units. Journal of Clinical Medicine. 2023; 12(19):6171. https://doi.org/10.3390/jcm12196171
Chicago/Turabian StyleCeccarelli, Giancarlo, Francesco Alessandri, Giuseppe Migliara, Valentina Baccolini, Giovanni Giordano, Gioacchino Galardo, Carolina Marzuillo, Corrado De Vito, Alessandro Russo, Massimo Ciccozzi, and et al. 2023. "Reduced Reliability of Procalcitonin (PCT) as a Biomarker of Bacterial Superinfection: Concerns about PCT-Driven Antibiotic Stewardship in Critically Ill COVID-19 Patients—Results from a Retrospective Observational Study in Intensive Care Units" Journal of Clinical Medicine 12, no. 19: 6171. https://doi.org/10.3390/jcm12196171
APA StyleCeccarelli, G., Alessandri, F., Migliara, G., Baccolini, V., Giordano, G., Galardo, G., Marzuillo, C., De Vito, C., Russo, A., Ciccozzi, M., Villari, P., Venditti, M., Mastroianni, C. M., Pugliese, F., & d’Ettorre, G. (2023). Reduced Reliability of Procalcitonin (PCT) as a Biomarker of Bacterial Superinfection: Concerns about PCT-Driven Antibiotic Stewardship in Critically Ill COVID-19 Patients—Results from a Retrospective Observational Study in Intensive Care Units. Journal of Clinical Medicine, 12(19), 6171. https://doi.org/10.3390/jcm12196171











