Normative Reference Values of the Tibial Nerve in Healthy Individuals Using Ultrasonography: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Literature Search and Study Selection
2.2. Risk of Bias Assessment
2.3. Meta-Analysis
3. Results
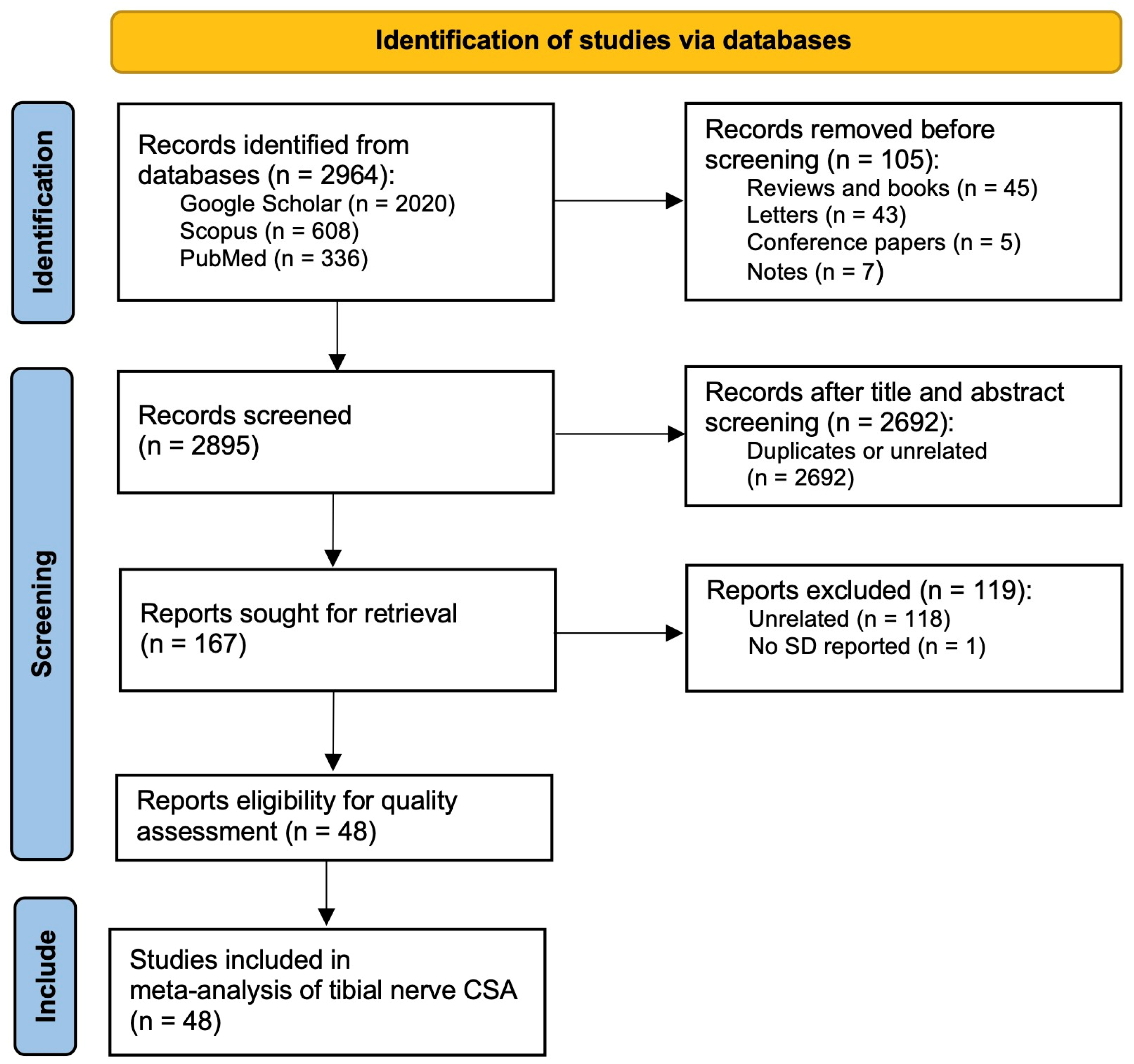
3.1. Systematic Review
3.2. Risk of Bias Assessment
3.3. Demography of the Subjects
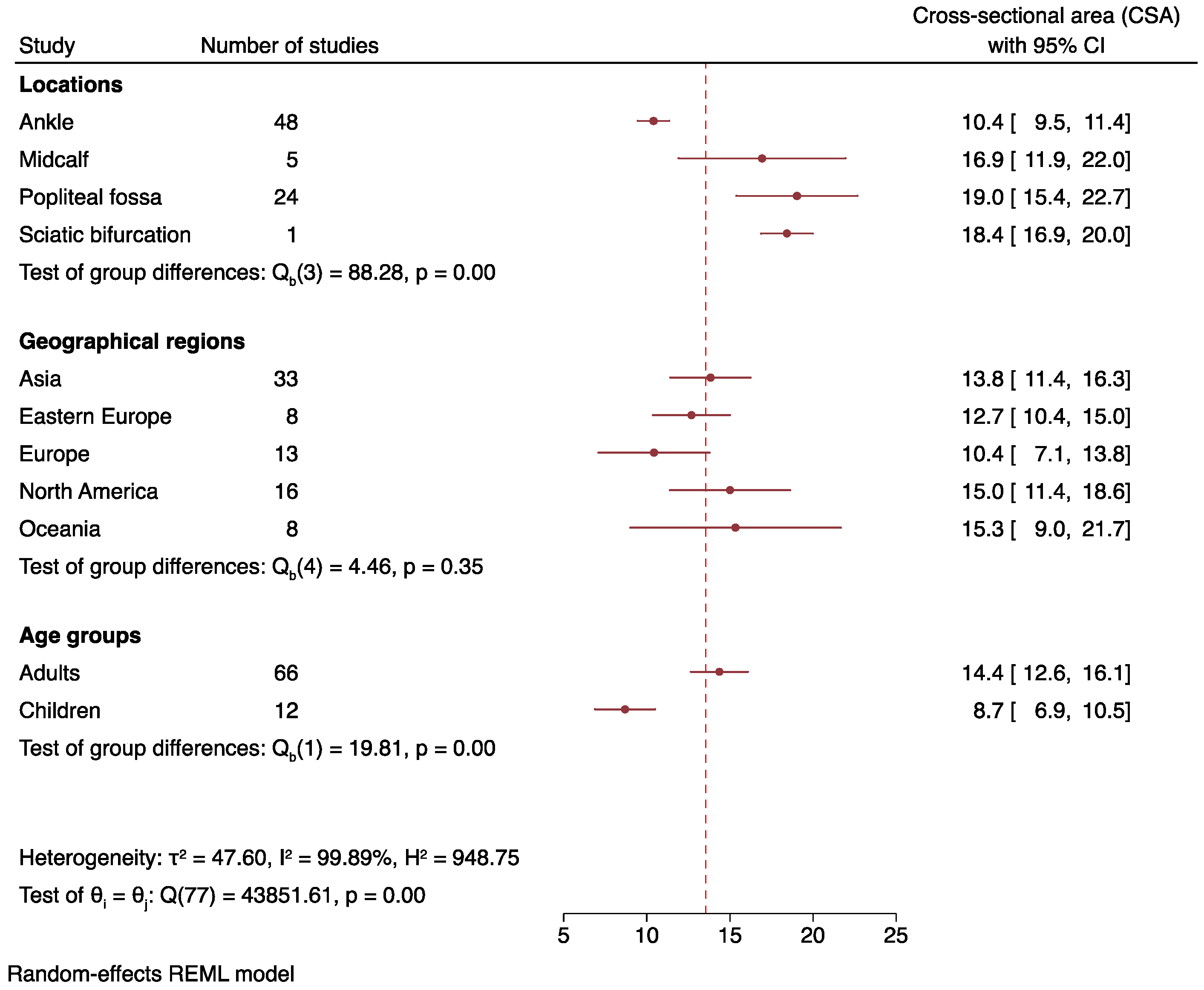
3.4. Overall Tibial Nerve Cross-Sectional Area
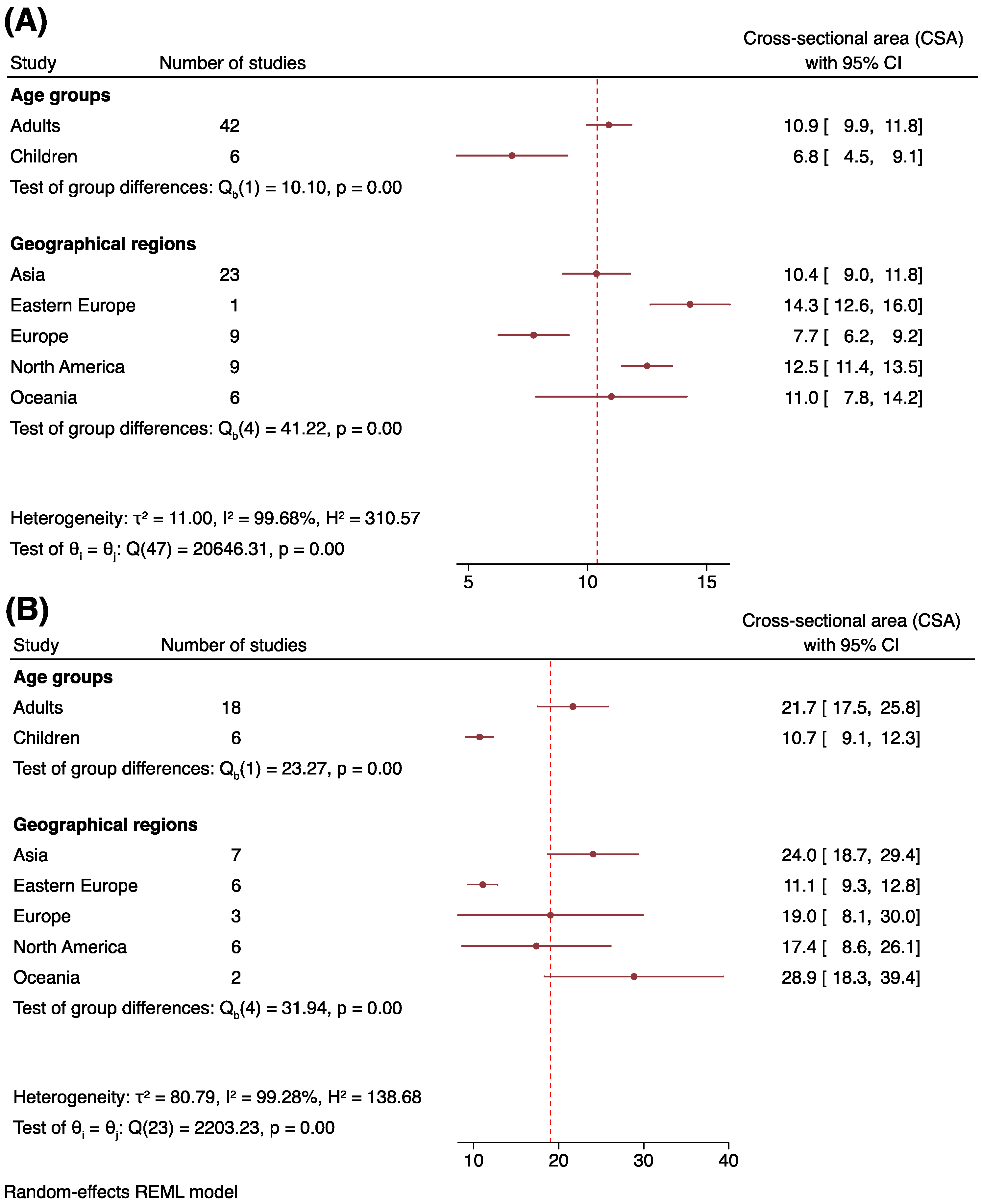
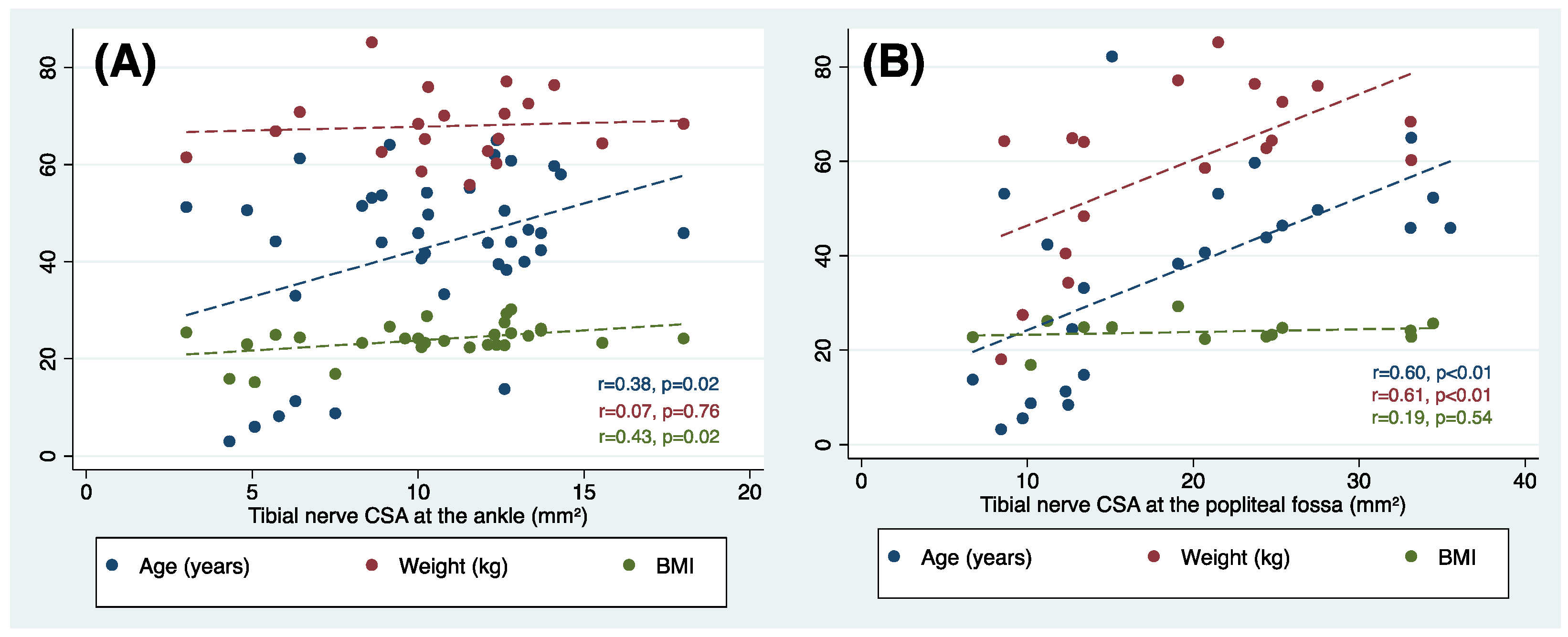
3.5. Tibial Nerve Cross-Sectional Area at the Popliteal Fossa and Ankle
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawande, A.D.; Warrier, S.S.; Joshi, M.S. Role of ultrasound in evaluation of peripheral nerves. Indian J. Radiol. Imaging 2014, 24, 254–258. [Google Scholar] [CrossRef]
- Telleman, J.A.; Grimm, A.; Goedee, S.; Visser, L.H.; Zaidman, C.M. Nerve ultrasound in polyneuropathies. Muscle Nerve 2018, 57, 716–728. [Google Scholar] [CrossRef]
- Telleman, J.A.; Herraets, I.J.T.; Goedee, H.S.; van Asseldonk, J.T.; Visser, L.H. Ultrasound scanning in the diagnosis of peripheral neuropathies. Pract. Neurol. 2021, 21, 186. [Google Scholar] [CrossRef]
- Ishibashi, F.; Taniguchi, M.; Kojima, R.; Kawasaki, A.; Kosaka, A.; Uetake, H. Elasticity of the tibial nerve assessed by sonoelastography was reduced before the development of neuropathy and further deterioration associated with the severity of neuropathy in patients with type 2 diabetes. J. Diabetes Investig. 2016, 7, 404–412. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.H.; Yang, S.N.; Yoon, J.S. Sonographic features of peripheral nerves at multiple sites in patients with diabetic polyneuropathy. J. Diabetes Complicat. 2016, 30, 518–523. [Google Scholar] [CrossRef]
- Kelle, B.; Evran, M.; Ballı, T.; Yavuz, F. Diabetic peripheral neuropathy: Correlation between nerve cross-sectional area on ultrasound and clinical features. J. Back Musculoskelet. Rehabil. 2016, 29, 717–722. [Google Scholar] [CrossRef]
- Kerasnoudis, A.; Pitarokoili, K.; Behrendt, V.; Gold, R.; Yoon, M.S. Cross sectional area reference values for sonography of peripheral nerves and brachial plexus. Clin. Neurophysiol. 2013, 124, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Riazi, S.; Bril, V.; Perkins, B.A.; Abbas, S.; Chan, V.W.; Ngo, M.; Lovblom, L.E.; El-Beheiry, H.; Brull, R. Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy?: A cross-sectional study. Diabetes Care 2012, 35, 2575–2579. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, K.; Kaur, S. High resolution ultrasonography of the tibial nerve in diabetic peripheral neuropathy. J. Ultrason. 2017, 17, 246–252. [Google Scholar] [CrossRef]
- Singh, K.P.; Kaur, S.; Arora, V. Reference values for the cross sectional area of normal tibial nerve on high-resolution ultrasonography. J. Ultrason. 2022, 22, e144–e152. [Google Scholar] [CrossRef]
- Watanabe, T.; Ito, H.; Sekine, A.; Katano, Y.; Nishimura, T.; Kato, Y.; Takeda, J.; Seishima, M.; Matsuoka, T. Sonographic evaluation of the peripheral nerve in diabetic patients: The relationship between nerve conduction studies, echo intensity, and cross-sectional area. J. Ultrasound Med. 2010, 29, 697–708. [Google Scholar] [CrossRef]
- Fisse, A.L.; Katsanos, A.H.; Gold, R.; Krogias, C.; Pitarokoili, K. Cross-sectional area reference values for peripheral nerve ultrasound in adults: A systematic review and meta-analysis-Part II: Lower extremity nerves. Eur. J. Neurol. 2021, 28, 2313–2318. [Google Scholar] [CrossRef]
- Druzhinin, D.; Naumova, E.; Nikitin, S. Nerve ultrasound normal values in children and young adults. Muscle Nerve 2019, 60, 757–761. [Google Scholar] [CrossRef]
- Cartwright, M.S.; Mayans, D.R.; Gillson, N.A.; Griffin, L.P.; Walker, F.O. Nerve cross-sectional area in extremes of age. Muscle Nerve 2013, 47, 890–893. [Google Scholar] [CrossRef]
- Seok, H.Y.; Jang, J.H.; Won, S.J.; Yoon, J.S.; Park, K.S.; Kim, B.J. Cross-sectional area reference values of nerves in the lower extremities using ultrasonography. Muscle Nerve 2014, 50, 564–570. [Google Scholar] [CrossRef]
- Cartwright, M.S.; Passmore, L.V.; Yoon, J.S.; Brown, M.E.; Caress, J.B.; Walker, F.O. Cross-sectional area reference values for nerve ultrasonography. Muscle Nerve 2008, 37, 566–571. [Google Scholar] [CrossRef]
- Qrimli, M.; Ebadi, H.; Breiner, A.; Siddiqui, H.; Alabdali, M.; Abraham, A.; Lovblom, L.E.; Perkins, B.A.; Bril, V. Reference values for ultrasonograpy of peripheral nerves. Muscle Nerve 2016, 53, 538–544. [Google Scholar] [CrossRef]
- Abdelnaby, R.; Elsayed, M.; Mohamed, K.A.; Dardeer, K.T.; Sonbol, Y.T.; Elgenidy, A.; Barakat, M.H.; NasrEldin, Y.K.; Maier, A. Sonographic reference values of vagus nerve: A systematic review and meta-analysis. Clin. Neurophysiol. Pract. 2022, 39, 59–71. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Yammine, K. Evidence-based anatomy. Clin. Anat. 2014, 27, 847–852. [Google Scholar] [CrossRef]
- D’Antoni, A.V.; Tubbs, R.S.; Patti, A.C.; Higgins, Q.M.; Tiburzi, H.; Battaglia, F. The Critical Appraisal Tool for Anatomical Meta-analysis (CATAM): A framework for critically appraising anatomical meta-analyses. Clin. Anat. 2022, 35, 323–331. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Bae, D.W.; An, J.Y. Cross-sectional area reference values for high-resolution ultrasonography of the lower extremity nerves in healthy Korean adults. Medicine 2022, 101, e29842. [Google Scholar] [CrossRef]
- Bedewi, M.A.; Abodonya, A.; Kotb, M.; Kamal, S.; Mahmoud, G.; Aldossari, K.; Alqabbani, A.; Swify, S. Estimation of ultrasound reference values for the lower limb peripheral nerves in adults: A cross-sectional study. Medicine 2018, 97, e0179. [Google Scholar] [CrossRef]
- Bedewi, M.A.; Elsifey, A.A.; Alfaifi, T.; Kotb, M.A.; Abdelgawad, M.S.; Bediwy, A.M.; Swify, S.M.; Awad, E.M. Shear wave elastography of the tibial nerve in healthy subjects. Medicine 2021, 100, e23999. [Google Scholar] [CrossRef]
- Boehm, J.; Scheidl, E.; Bereczki, D.; Schelle, T.; Arányi, Z. High-resolution ultrasonography of peripheral nerves: Measurements on 14 nerve segments in 56 healthy subjects and reliability assessments. Ultraschall. Med. 2014, 35, 459–467. [Google Scholar] [CrossRef]
- Borire, A.A.; Issar, T.; Kwai, N.C.; Visser, L.H.; Simon, N.G.; Poynten, A.M.; Kiernan, M.C.; Krishnan, A.V. Correlation between markers of peripheral nerve function and structure in type 1 diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3028. [Google Scholar] [CrossRef]
- Boyd, B.S.; Dilley, A. Altered tibial nerve biomechanics in patients with diabetes mellitus. Muscle Nerve 2014, 50, 216–223. [Google Scholar] [CrossRef]
- Breiner, A.; Qrimli, M.; Ebadi, H.; Alabdali, M.; Lovblom, L.E.; Abraham, A.; Albulahi, H.; Perkins, B.A.; Bril, V. Peripheral nerve high-resolution ultrasound in diabetes. Muscle Nerve 2017, 55, 171–178. [Google Scholar] [CrossRef]
- Chen, R.; Wang, X.-L.; Xue, W.-L.; Sun, J.-W.; Dong, X.-Y.; Jiang, Z.-P.; Wu, H.; Ma, R.; Zhou, X.-L. Application value of conventional ultrasound and real-time shear wave elastography in patients with type 2 diabetic polyneuropathy. Eur. J. Neurol. 2020, 126, 108965. [Google Scholar] [CrossRef]
- Dikici, A.S.; Ustabasioglu, F.E.; Delil, S.; Nalbantoglu, M.; Korkmaz, B.; Bakan, S.; Kula, O.; Uzun, N.; Mihmanli, I.; Kantarci, F. Evaluation of the tibial nerve with shear-wave elastography: A potential sonographic method for the diagnosis of diabetic peripheral neuropathy. Radiology 2017, 282, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Elfattah Hassan Gadalla, A.A.; Nada, H.R.; Kaddah, R.O.; Khalil, A.S.; Saleh, M.M. Quantitative shear wave elastography assessment of tibial nerve in diagnosis of diabetic peripheral neuropathy. Egypt. J. Radiol. Nucl. Med. 2022, 53, 131. [Google Scholar] [CrossRef]
- Fantino, O.; Bouysset, M.; Pialat, J.B. Can the axial cross-sectional area of the tibial nerve be used to diagnose tarsal tunnel syndrome? An ultrasonography study. Orthop. Traumatol. Surg. Res. 2021, 107, 102630. [Google Scholar] [CrossRef]
- Garg, N.; Park, S.B.; Howells, J.; Noto, Y.I.; Vucic, S.; Yiannikas, C.; Tomlinson, S.E.; Huynh, W.; Simon, N.G.; Mathey, E.K.; et al. Anti-MAG neuropathy: Role of IgM antibodies, the paranodal junction and juxtaparanodal potassium channels. Clin. Neurophysiol. 2018, 129, 2162–2169. [Google Scholar] [CrossRef]
- Goyal, K.; Aggarwal, P.; Gupta, M. Ultrasound evaluation of peripheral nerves of the lower limb in diabetic peripheral neuropathy. Eur. J. Neurol. 2021, 145, 110058. [Google Scholar] [CrossRef]
- Grimm, A.; Axer, H.; Heiling, B.; Winter, N. Nerve ultrasound normal values—Readjustment of the ultrasound pattern sum score UPSS. Clin. Neurophysiol. 2018, 129, 1403–1409. [Google Scholar] [CrossRef]
- He, Y.; Xiang, X.; Zhu, B.-H.; Qiu, L. Shear wave elastography evaluation of the median and tibial nerve in diabetic peripheral neuropathy. Quant. Imaging Med. Surg. 2019, 9, 273. [Google Scholar] [CrossRef]
- Hobbelink, S.M.R.; Brockley, C.R.; Kennedy, R.A.; Carroll, K.; de Valle, K.; Rao, P.; Davis, M.R.; Laing, N.G.; Voermans, N.C.; Ryan, M.M.; et al. Dejerine-Sottas disease in childhood-Genetic and sonographic heterogeneity. Brain Behav. 2018, 8, e00919. [Google Scholar] [CrossRef]
- Hooper, D.R.; Lawson, W.; Smith, L.; Baker, S.K. Sonographic features in hereditary neuropathy with liability to pressure palsies. Muscle Nerve 2011, 44, 862–867. [Google Scholar] [CrossRef]
- Ibrahim, H.R. Diagnostic value of shear wave ultrasound elastography of tibial nerve in patients with diabetic peripheral neuropathy. Egypt. J. Radiol. Nucl. Med. 2022, 53, 102. [Google Scholar] [CrossRef]
- Issar, T.; Walker, S.; Arnold, R.; Poynten, A.M.; Endre, Z.H.; Krishnan, A.V. Peripheral nerve morphology and intraneural blood flow in chronic kidney disease with and without diabetes. Muscle Nerve 2022, 65, 603–607. [Google Scholar] [CrossRef]
- Ito, T.; Kijima, M.; Watanabe, T.; Sakuta, M.; Nishiyama, K. Ultrasonography of the tibial nerve in vasculitic neuropathy. Muscle Nerve 2007, 35, 379–382. [Google Scholar] [CrossRef]
- Jain, S.; Visser, L.H.; Praveen, T.L.; Rao, P.N.; Surekha, T.; Ellanti, R.; Abhishek, T.L.; Nath, I. High-resolution sonography: A new technique to detect nerve damage in leprosy. PLOS Negl. Trop. Dis. 2009, 3, e498. [Google Scholar] [CrossRef]
- Jang, J.H.; Cho, C.S.; Yang, K.S.; Seok, H.Y.; Kim, B.J. Pattern analysis of nerve enlargement using ultrasonography in chronic inflammatory demyelinating polyneuropathy. Clin. Neurophysiol. 2014, 125, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Kronlage, M.; Pitarokoili, K.; Schwarz, D.; Godel, T.; Heiland, S.; Yoon, M.S.; Bendszus, M.; Bäumer, P. Diffusion tensor imaging in chronic inflammatory demyelinating polyneuropathy: Diagnostic accuracy and correlation with electrophysiology. Investig. Radiol. 2017, 52, 701–707. [Google Scholar] [CrossRef]
- Lothet, E.H.; Bishop, T.J.; Walker, F.O.; Cartwright, M.S. Ultrasound-derived nerve cross-sectional area in extremes of height and weight. J. Neuroimaging 2019, 29, 406–409. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, L.; Ding, Q.; Liu, J.; Zhang, Z.; Cui, L.; Liu, M. Reference values for lower limb nerve ultrasound and its diagnostic sensitivity. J. Clin. Neurosci. 2021, 86, 276–283. [Google Scholar] [CrossRef]
- Noto, Y.I.; Garg, N.; Li, T.; Timmins, H.C.; Park, S.B.; Shibuya, K.; Shahrizaila, N.; Huynh, W.; Matamala, J.M.; Dharmadasa, T.; et al. Comparison of cross-sectional areas and distal-proximal nerve ratios in amyotrophic lateral sclerosis. Muscle Nerve 2018, 58, 777–783. [Google Scholar] [CrossRef]
- Pitarokoili, K.; Kerasnoudis, A.; Behrendt, V.; Labedi, A.; Ayzenberg, I.; Gold, R.; Yoon, M.S. Facing the diagnostic challenge: Nerve ultrasound in diabetic patients with neuropathic symptoms. Muscle Nerve 2016, 54, 18–24. [Google Scholar] [CrossRef]
- Razali, S.N.O.; Arumugam, T.; Yuki, N.; Rozalli, F.I.; Goh, K.J.; Shahrizaila, N. Serial peripheral nerve ultrasound in Guillain-Barré syndrome. Clin. Neurophysiol. 2016, 127, 1652–1656. [Google Scholar] [CrossRef]
- Schubert, C.; Grimm, A.S.; Stahl, J.H.; Küpper, H.; Kegele, J.; Wittlinger, J.; Serna-Higuita, L.; Winter, N.; Groeschel, S.; Grimm, A. Nerve ultrasound reference data in children from two to seven years. Clin. Neurophysiol. 2020, 131, 859–865. [Google Scholar] [CrossRef]
- Sindhu, D.; Huddar, A.; Saini, J.; Vengalil, S.; Nashi, S.; Bardhan, M.; Unnikrishnan, G.; Rajula, R.R.; Kandavel, T.; Bathala, L. Cross-sectional area reference values of nerves in the upper and lower extremities using ultrasonography in the Indian population. Ann. Indian Acad. Neurol. 2022, 25, 449. [Google Scholar] [CrossRef]
- Sreejith, K.; Sasidharanpillai, S.; Ajithkumar, K.; Mani, R.M.; Chathoth, A.T.; Menon, P.S.; George, B.; Manakkad, S.P.; Neerackal, R.J.; Menon, D.; et al. High-resolution ultrasound in the assessment of peripheral nerves in leprosy: A comparative cross-sectional study. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 199–206. [Google Scholar] [CrossRef]
- Tandon, A.; Khullar, T.; Maheshwari, S.; Bhatt, S.; Narang, S. High resolution ultrasound in subclinical diabetic neuropathy: A potential screening tool. Ultrasound 2020, 29, 150–161. [Google Scholar] [CrossRef]
- van Maurik, J.F.M.; Schouten, M.E.; ten Katen, I.; van Hal, M.; Peters, E.J.; Kon, M. Ultrasound findings after surgical decompression of the tarsal tunnel in patients with painful diabetic polyneuropathy: A prospective randomized study. Diabetes Care 2014, 37, 767–772. [Google Scholar] [CrossRef][Green Version]
- Yiu, E.M.; Brockley, C.R.; Lee, K.J.; Carroll, K.; de Valle, K.; Kennedy, R.; Rao, P.; Delatycki, M.B.; Ryan, M.M. Peripheral nerve ultrasound in pediatric Charcot-Marie-Tooth disease type 1A. Neurology 2015, 84, 569–574. [Google Scholar] [CrossRef]
- Pelosi, L.; Ghosh, A.; Leadbetter, R.; Lance, S.; Rodrigues, M.; Roxburgh, R. Nerve ultrasound detects abnormally small nerves in patients with spinal and bulbar muscular atrophy. Muscle Nerve 2022, 65, 599–602. [Google Scholar] [CrossRef]
- Grimm, A.; Décard, B.F.; Axer, H. Ultrasonography of the peripheral nervous system in the early stage of Guillain-Barré syndrome. J. Peripher. Nerv. Syst. 2014, 19, 234–241. [Google Scholar] [CrossRef]
- Tawfik, E.A.; El Zohiery, A.K.; Abouelela, A.A.K. Proposed sonographic criteria for the diagnosis of idiopathic tarsal tunnel syndrome. Arch. Phys. Med. Rehabil. 2016, 97, 1093–1099. [Google Scholar] [CrossRef]
- Senarai, T.; Pratipanawatr, T.; Yurasakpong, L.; Kruepunga, N.; Limwachiranon, J.; Phanthong, P.; Meemon, K.; Yammine, K.; Suwannakhan, A. Cross-Sectional area of the tibial nerve in diabetic peripheral neuropathy patients: A systematic review and meta-analysis of ultrasonography studies. Medicina 2022, 58, 1696. [Google Scholar] [CrossRef]
- Ranjan, T.; Chandak, S.; Malhotra, A.; Aggarwal, A.; Haria, J.; Singla, D. Role of high-resolution ultrasonography in the evaluation of the tibial and median nerves in diabetic peripheral neuropathy. J. Ultrason. 2022, 22, e209–e215. [Google Scholar] [CrossRef]
- Bueno-Gracia, E.; Ruiz-de-Escudero-Zapico, A.; Malo-Urriés, M.; Shacklock, M.; Estébanez-de-Miguel, E.; Fanlo-Mazas, P.; Caudevilla-Polo, S.; Jiménez-Del-Barrio, S. Dimensional changes of the carpal tunnel and the median nerve during manual mobilization of the carpal bones. Musculoskelet. Sci. Pract. 2018, 36, 12–16. [Google Scholar] [CrossRef]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef]
- Khadilkar, S.V.; Yadav, R.S.; Soni, G. A practical approach to enlargement of nerves, plexuses and roots. Pract. Neurol. 2015, 15, 105–115. [Google Scholar] [CrossRef]
- Weller, R.O.; Das Gupta, T.K. Experimental hypertrophic neuropathy: An electron microscope study. J. Neurol. Neurosurg. Psychiatry 1968, 31, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.; Trikalinos, T.A. The appropriateness of asymmetry tests for publication bias in meta-analyses: A large survey. CMAJ 2007, 176, 1091–1096. [Google Scholar] [CrossRef]

| Study | Year | Country | Location | Subjects 1 | CSA | SD | Age | Weight | BMI |
|---|---|---|---|---|---|---|---|---|---|
| Bae and An [23] | 2022 | South Korea | Popliteal fossa | 107 | 24.73 | 6.03 | N/A | 64.4 | 23.3 |
| Midcalf | 107 | 12.97 | 3.71 | N/A | 64.4 | 23.3 | |||
| Ankle | 107 | 15.55 | 3.8 | N/A | 64.4 | 23.3 | |||
| Bedewi et al. [24] | 2018 | Saudi Arabia | Ankle | 138 | 12.66 | 4.45 | 38.33 | 77.15 | 29.31 |
| Popliteal fossa | 138 | 19.08 | 6.88 | 38.33 | 77.15 | 29.31 | |||
| Bedewi et al. [25] | 2021 | Saudi Arabia | Popliteal fossa | 72 | 13.4 | 3.4 | 33.2 | 64.1 | 24.9 |
| Boehm et al. [26] | 2014 | Hungary and Germany | Ankle | 56 | 9.6 | 2.2 | N/A | N/A | 24.2 |
| Borire et al. [27] | 2018 | Australia | Ankle | 30 | 12.8 | 0.5 | 60.8 | N/A | 30.2 |
| Boyd and Dilley [28] | 2014 | USA | Ankle | 20 | 13.32 | 1.53 | 46.6 | 72.59 | 24.75 |
| Popliteal fossa | 20 | 25.37 | 4.41 | 46.4 | 72.59 | 24.75 | |||
| Breiner et al. [29] | 2016 | Canada | Ankle | 100 | 12.8 | 3.5 | 44.1 | N/A | 25.3 |
| Cartwright et al. [16] | 2008 | USA | Midcalf | 60 | 25.3 | 7.3 | 45.9 | N/A | N/A |
| Popliteal fossa | 60 | 35.5 | 10.3 | 45.9 | N/A | N/A | |||
| Ankle | 60 | 13.7 | 4.3 | 45.9 | N/A | N/A | |||
| Cartwright et al. [14] | 2013 | USA | Popliteal fossa | 60 | 11.2 | 3.3 | 42.4 | N/A | 26.2 |
| Popliteal fossa | 12 | 15.1 | 5.5 | 82.2 | N/A | 24.9 | |||
| Ankle | 5 | 12.6 | 2.1 | 13.8 | N/A | 22.8 | |||
| Ankle | 60 | 13.7 | 4.3 | 42.4 | N/A | 26.2 | |||
| Popliteal fossa | 3 | 6.7 | 3.1 | 13.8 | N/A | 22.8 | |||
| Popliteal fossa | 4 | 10.2 | 2.9 | 8.8 | N/A | 16.9 | |||
| Ankle | 4 | 7.5 | 2.5 | 8.8 | N/A | 16.9 | |||
| Chen et al. [30] | 2000 | China | Ankle | 33 | 8.31 | 2.32 | 51.51 | N/A | 23.28 |
| Dikici et al. [31] | 2017 | Türkiye | Ankle | 20 | 14.3 | 3.8 | 58 | N/A | N/A |
| Druzhinin et al. [13] | 2019 | Russia | Popliteal fossa | 7 | 9.71 | 2.82 | 5.61 | 27.5 | N/A |
| 5 | 12.46 | 1.69 | 8.47 | 34.3 | N/A | ||||
| 7 | 12.3 | 3.82 | 11.3 | 40.5 | N/A | ||||
| 22 | 12.7 | 8.3 | 24.5 | 64.9 | N/A | ||||
| 4 | 13.4 | 6.27 | 14.8 | 48.4 | N/A | ||||
| 12 | 8.41 | 2.39 | 3.27 | 18.1 | N/A | ||||
| Fantino et al. [33] | 2021 | France | Midcalf | 21 | 10.6 | 1.8 | 39 | 67 | N/A |
| Elfattah Hassan Gadalla et al. [32] | 2022 | Egypt | Ankle | 20 | 13.2 | 3.1 | 40 | N/A | N/A |
| Garg et al. [34] | 2018 | Australia | Ankle | 17 | 14.8 | 3.2 | N/A | N/A | N/A |
| Goyal et al. [35] | 2021 | India | Ankle | 70 | 5.7 | 1.3 | 44.2 | 66.9 | 24.95 |
| Grimm et al. [58] | 2014 | Germany | Ankle | 21 | 8.6 | 2.7 | 53.14 | 85.2 | N/A |
| Popliteal fossa | 21 | 21.5 | 4.4 | 53.14 | 85.2 | N/A | |||
| Grimm et al. [58] 2 | 2014 | Germany | Ankle | 8 | 10.3 | 2.5 | 49.71 | 76 | N/A |
| Popliteal fossa | 8 | 27.5 | 7 | 49.71 | 76 | N/A | |||
| Popliteal fossa | 21 | 8.6 | 2.7 | 53.14 | 64.29 | N/A | |||
| Grimm et al. [36] | 2018 | Germany | Ankle | 100 | 10.2 | 2 | N/A | N/A | N/A |
| He et al. [37] | 2019 | China | Ankle | 40 | 11.55 | 1.59 | 55.2 | 55.83 | 22.38 |
| Hobbelink et al. [38] | 2018 | Australia | Ankle | 5 | 5.8 | 0.9 | 8.2 | N/A | N/A |
| Hooper et al. [39] | 2011 | Canada | Ankle | 32 | 10.78 | 1.72 | 33.3 | 70.1 | 23.7 |
| Ibrahim [40] | 2022 | Egypt | Ankle | 50 | 10.26 | 1.86 | 54.23 | N/A | 28.81 |
| Ishibashi et al. [4] | 2016 | Japan | Ankle | 29 | 4.84 | 0.16 | 50.6 | N/A | 23 |
| Issar et al. [41] | 2022 | Australia | Ankle | 28 | 12.3 | 3.1 | 62 | N/A | 25 |
| Ito et al. [42] | 2007 | Japan | Ankle | 35 | 7.9 | 1.5 | N/A | N/A | N/A |
| Jain et al. [43] | 2009 | India | Ankle | 30 | 6.3 | 3.2 | 33 | N/A | N/A |
| Jang et al. [44] 3 | 2014 | South Korea | Ankle | 18 | 10 | 1.5 | 45.9 | 68.4 | 24.2 |
| 18 | 18 | 4 | 45.9 | 68.4 | 24.2 | ||||
| Jang et al. [44] | 2014 | South Korea | Popliteal fossa | 18 | 33.1 | 3.8 | 45.9 | 68.4 | 24.2 |
| Kang et al. [5] | South Korea | Ankle | 20 | 12.36 | 2.85 | 65 | 60.25 | 22.86 | |
| 2016 | Popliteal fossa | 20 | 33.14 | 4.92 | 65 | 60.25 | 22.86 | ||
| Midcalf | 20 | 16.39 | 2.95 | 65 | 60.25 | 22.86 | |||
| Kelle et al. [6] | 2016 | Turkey | Sciatic bifurcation | 53 | 18.43 | 5.79 | 57.8 | N/A | 30.22 |
| Kerasnoudis et al. [7] | 2013 | Germany | Ankle | 75 | 6.36 | 1.45 | N/A | N/A | N/A |
| Lothet et al. [46] | 2019 | USA | Ankle | 140 | 13.7 | 4.3 | N/A | N/A | 25.8 |
| Niu et al. [47] | 2021 | China | Ankle | 111 | 10.2 | 1.9 | 41.7 | 65.3 | 23.3 |
| Noto et al. [48] | 2018 | Australia | Popliteal fossa | 30 | 23.7 | 7.4 | 59.7 | 76.4 | N/A |
| Ankle | 30 | 14.1 | 3.2 | 59.7 | 76.4 | N/A | |||
| Pelosi et al. [57] | 2022 | New Zealand | Popliteal fossa | 18 | 34.46 | 11 | 52.3 | N/A | 25.7 |
| Pitarokoili et al. [49] | 2016 | Germany | Ankle | 55 | 9.14 | 2.41 | 64.1 | N/A | 26.64 |
| Qrimli et al. [17] | 2016 | Canada | Ankle | 98 | 12.7 | 3.1 | N/A | N/A | N/A |
| Razali et al. [50] | 2016 | Malaysia | Ankle | 17 | 12.6 | 5.4 | 50.5 | 70.5 | 27.5 |
| Schubert et al. [51] | 2020 | Germany | Ankle | 57 | 5.07 | 1.51 | 6 | N/A | 15.2 |
| 57 | 4.31 | 1.38 | 3 | N/A | 15.9 | ||||
| Seok et al. [15] | 2014 | South Korea | Ankle | 94 | 12.1 | 3.1 | 43.9 | 62.8 | 22.9 |
| Popliteal fossa | 94 | 24.4 | 4.4 | 43.9 | 62.8 | 22.9 | |||
| Sindhu et al. [52] | 2022 | India | Ankle | 100 | 10.1 | 2.23 | 40.7 | 58.6 | 22.41 |
| Popliteal fossa | 100 | 20.7 | 4.41 | 40.7 | 58.6 | 22.41 | |||
| Singh et al. [9] | 2017 | India | Ankle | 75 | 12.42 | 1.1 | 39.54 | 65.34 | N/A |
| Singh et al. [10] | 2022 | India | Midcalf | 200 | 19.6 | 1.4 | N/A | N/A | N/A |
| Ankle | 200 | 11.1 | 1.1 | N/A | N/A | N/A | |||
| Sreejith et al. [53] | 2021 | India | Ankle | 30 | 8.9 | 2.319 | 44 | N/A | N/A |
| Tandon et al. [54] | 2021 | India | Ankle | 30 | 3.01 | 0.61 | 51.26 | 61.5 | 25.46 |
| Tawfik et al. [59] | 2016 | Egypt | Ankle | 17 | 13.8 | 4.4 | N/A | N/A | N/A |
| van Maurik et al. [55] | 2014 | Netherlands | Ankle | 38 | 6.43 | 1.32 | 61.29 | 70.84 | 24.4 |
| Watanabe et al. [11] | 2010 | Japan | Ankle | 32 | 8.9 | 2.8 | 53.7 | 62.6 | N/A |
| Yiu et al. [56] | 2015 | Australia | Ankle | 29 | 6.3 | 1.9 | 11.3 | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senarai, T.; Suwannakhan, A.; Pratipanawatr, T.; Yammine, K.; Yurasakpong, L.; Sathapornsermsuk, T.; Janta, S.; Kittiboonya, A. Normative Reference Values of the Tibial Nerve in Healthy Individuals Using Ultrasonography: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 6186. https://doi.org/10.3390/jcm12196186
Senarai T, Suwannakhan A, Pratipanawatr T, Yammine K, Yurasakpong L, Sathapornsermsuk T, Janta S, Kittiboonya A. Normative Reference Values of the Tibial Nerve in Healthy Individuals Using Ultrasonography: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(19):6186. https://doi.org/10.3390/jcm12196186
Chicago/Turabian StyleSenarai, Thanyaporn, Athikhun Suwannakhan, Thongchai Pratipanawatr, Kaissar Yammine, Laphatrada Yurasakpong, Tanapat Sathapornsermsuk, Sirorat Janta, and Achiraya Kittiboonya. 2023. "Normative Reference Values of the Tibial Nerve in Healthy Individuals Using Ultrasonography: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 19: 6186. https://doi.org/10.3390/jcm12196186
APA StyleSenarai, T., Suwannakhan, A., Pratipanawatr, T., Yammine, K., Yurasakpong, L., Sathapornsermsuk, T., Janta, S., & Kittiboonya, A. (2023). Normative Reference Values of the Tibial Nerve in Healthy Individuals Using Ultrasonography: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(19), 6186. https://doi.org/10.3390/jcm12196186







