Cochleo-Vestibular Disorders in Herpes Zoster Oticus: A Literature Review and a Case of Bilateral Vestibular Hypofunction in Unilateral HZO
Abstract
1. Introduction
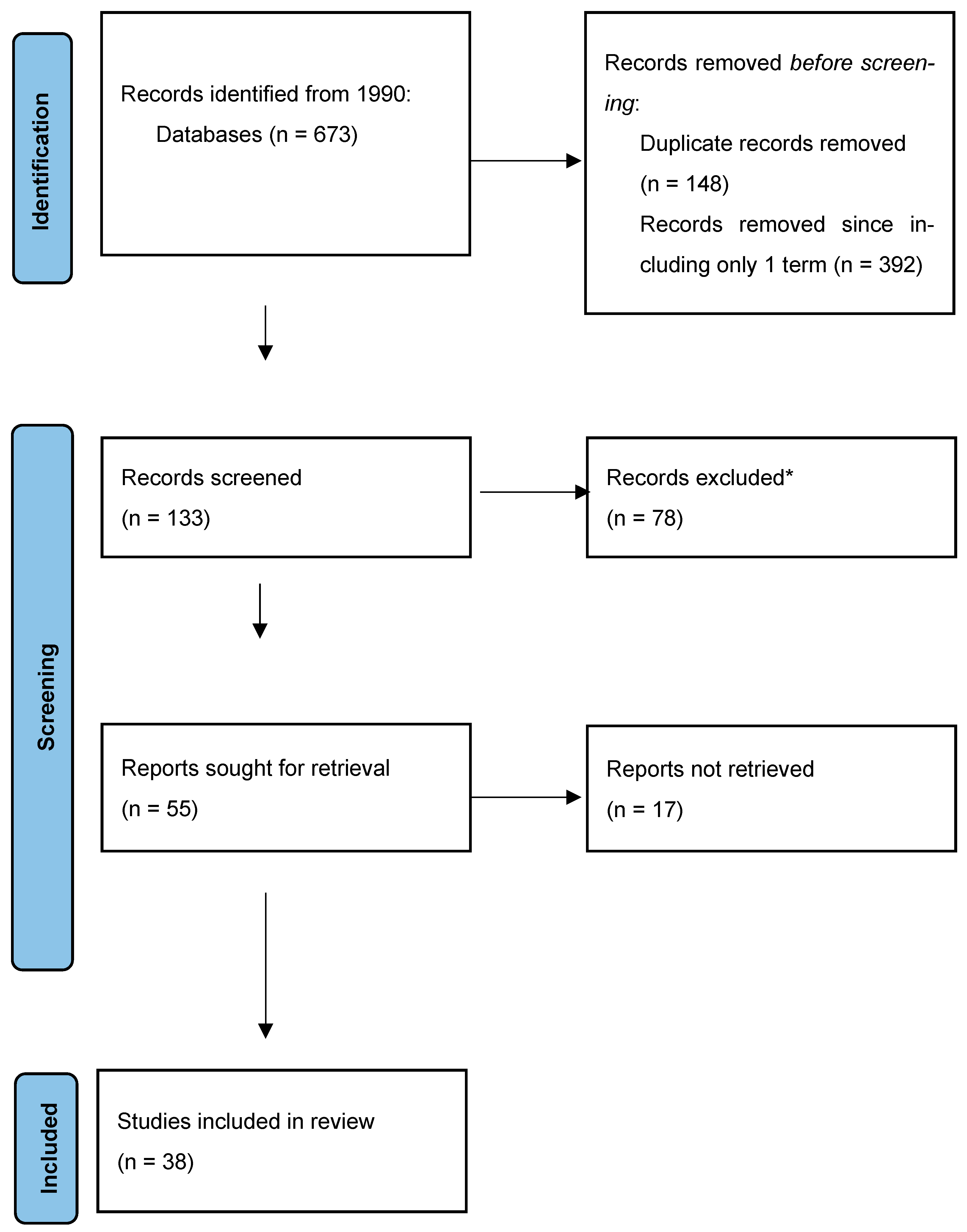
2. Materials and Methods
3. Results
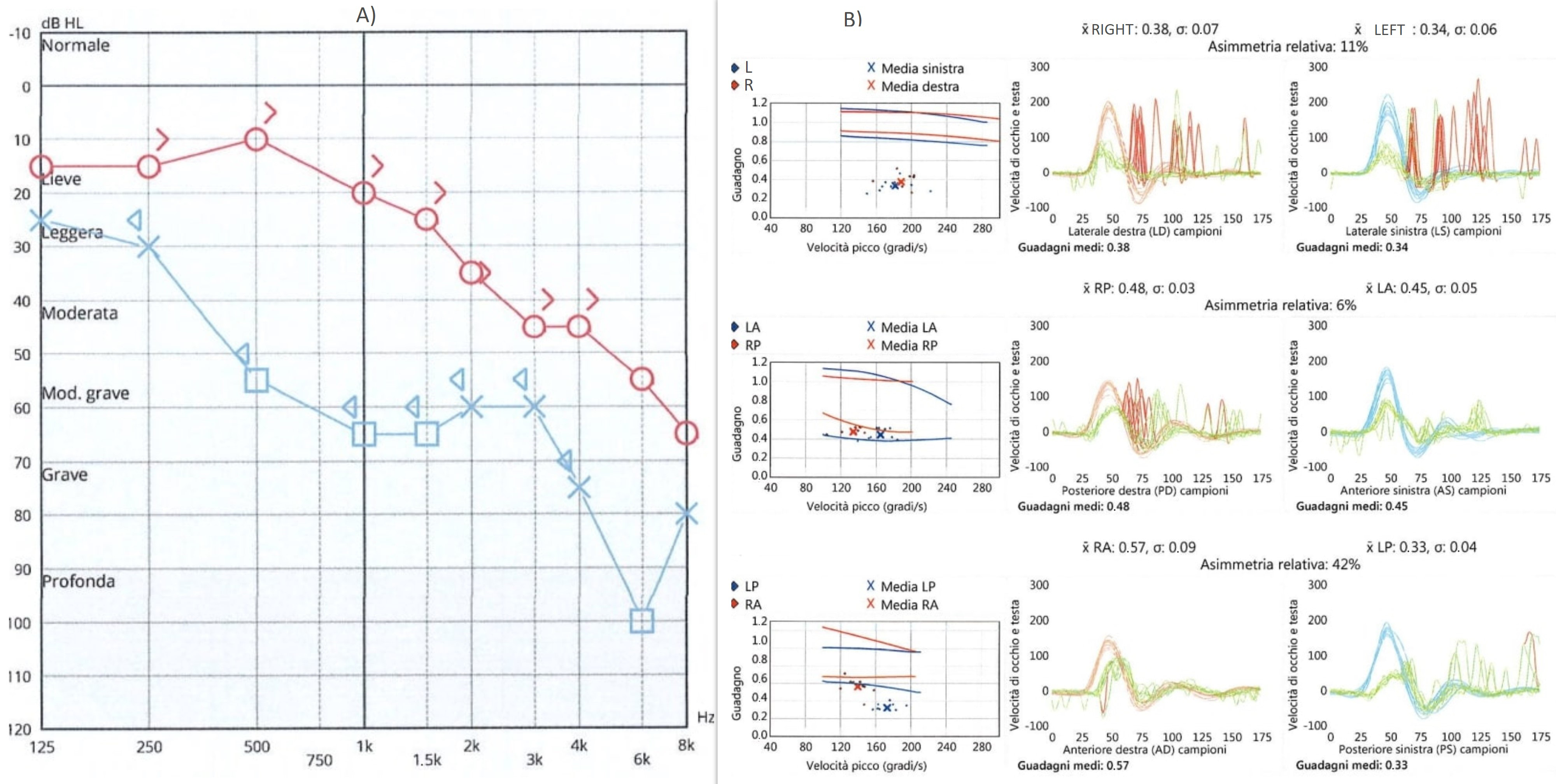
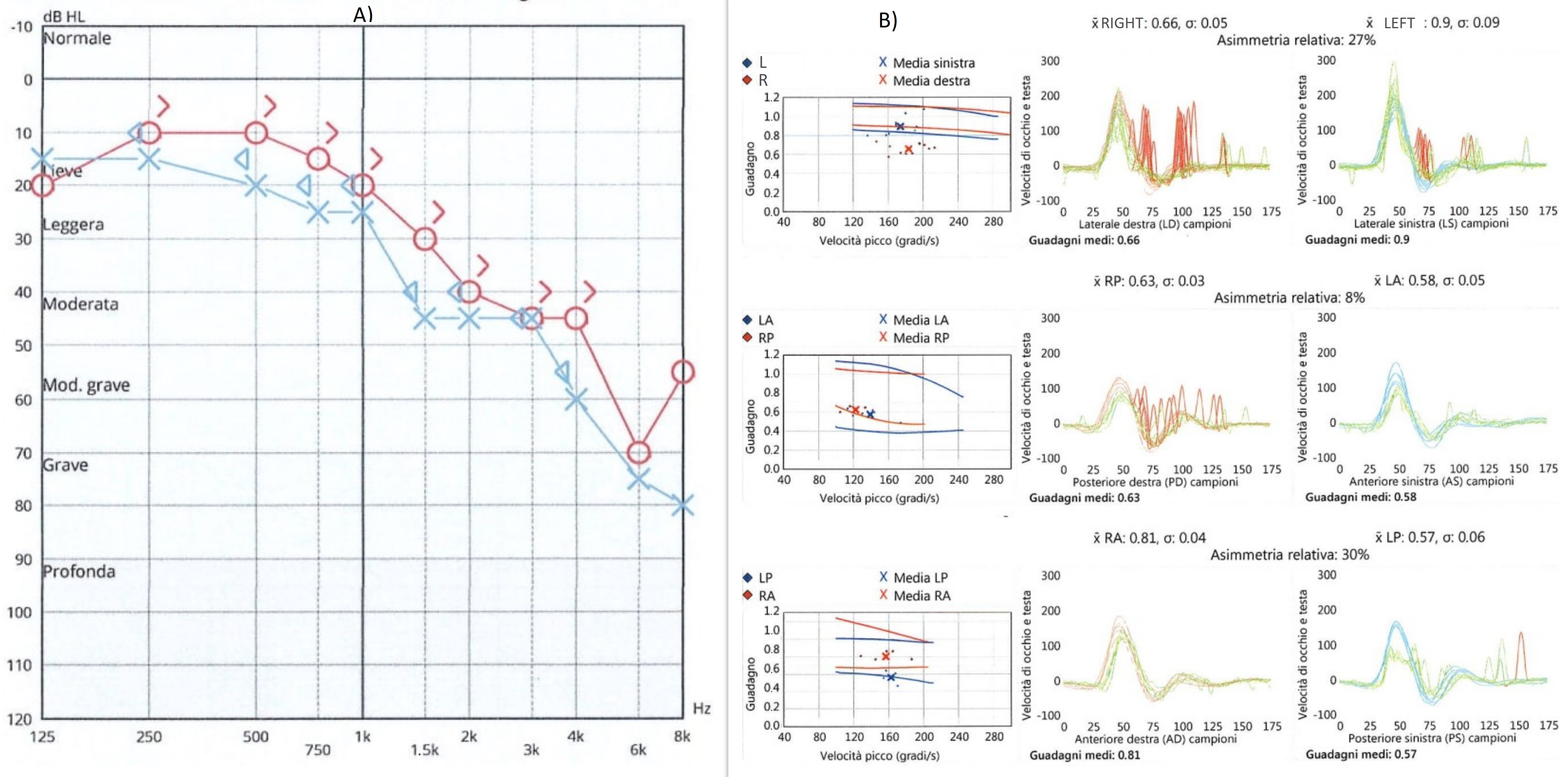
Case Report
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, D.H.; Kim, B.-R.; Shin, J.E.; Kim, C.-H. Clinical manifestations in patients with herpes zoster oticus. Eur. Arch. Otorhinolaryngol. 2016, 273, 1739–1743. [Google Scholar] [CrossRef]
- Muecke, M.; Amedee, R.G. Herpes zoster oticus: Diagnosis and management. J. La. State Med. Soc. 1993, 145, 333–335. [Google Scholar]
- Furuta, Y.; Aizawa, H.; Sawa, H.; Ohtani, F.; Fukuda, S. Varicella-zoster virus DNA level and facial paralysis in Ramsay Hunt syndrome. Ann. Otol. Rhinol. Laryngol. 2004, 113, 700–705. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, S.; Soodin, D.; Sritharan, N.; Sivapathasingam, V. Authors reply to letter to the editor considering “Ramsay Hunt Syndrome with multiple cranial neuropathy: A literature review”. Eur. Arch Otorhinolaryngol. 2022, 279, 2709–2712. [Google Scholar] [CrossRef]
- Yadav, R.; Gohel, A.; Vengalil, S.; Mundlamuri, R.; Nalini, A.; Kulanthaivelu, K. Ramsay Hunt Syndrome Leading to the Brainstem and Cerebellar Involvement: A Case Report of a Rare Co-occurrence. Ann. Indian Acad. Neurol. 2022, 25, 159–161. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, S.; Soodin, D.; Sritharan, N.; Sivapathasingam, V. Ramsay Hunt syndrome with multiple cranial neuropathy: A literature review. Eur. Arch. Otorhinolaryngol. 2022, 279, 2239–2244. [Google Scholar] [CrossRef]
- Bakshi, S.S.; Ramesh, S.; Annam, C.S. Ramsay Hunt Syndrome. Am. J. Med. 2022, 135, e125–e126. [Google Scholar] [CrossRef]
- Wackym, P.A. Molecular temporal bone pathology: II. Ramsay Hunt syndrome (herpes zoster oticus). Laryngoscope 1997, 107, 1165–1175. [Google Scholar] [CrossRef]
- Murakami, S.; Nakashiro, Y.; Mizobuchi, M.; Hato, N.; Honda, N.; Gyo, K. Varicella-zoster virus distribution in Ramsay Hunt syndrome revealed by polymerase chain reaction. Acta. Otolaryngol. 1998, 118, 145–149. [Google Scholar] [CrossRef]
- Furuta, Y.; Fukuda, S.; Suzuki, S.; Takasu, T.; Inuyama, Y.; Nagashima, K. Detection of varicella-zoster virus DNA in patients with acute peripheral facial palsy by the polymerase chain reaction, and its use for early diagnosis of zoster sine herpete. J. Med. Virol. 1997, 52, 316–319. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Yanagihara, N.; Kurata, T. Middle ear mucosa in Ramsay Hunt syndrome. Ann. Otol. Rhinol. Laryngol. 1990, 99, 359–362. [Google Scholar] [CrossRef]
- Kim, C.H.; Choi, H.; Shin, J.E. Characteristics of hearing loss in patients with herpes zoster oticus. Medicine 2016, 95, e5438. [Google Scholar] [CrossRef]
- Abramovich, S.; Prasher, D.K. Electrocochleography and brain-stem potentials in Ramsay Hunt syndrome. Arch. Otolaryngol. Head Neck Surg. 1986, 112, 925–928. [Google Scholar] [CrossRef]
- Wayman, D.M.; Pham, H.N.; Byl, F.M.; Adour, K.K. Audiological manifestations of Ramsay Hunt syndrome. J. Laryngol. Otol. 1990, 104, 104–108. [Google Scholar] [CrossRef]
- Shinha, T.; Krishna, P. Ramsay Hunt syndrome and zoster laryngitis with multiple cranial nerve involvement. IDCases 2015, 2, 47–48. [Google Scholar] [CrossRef][Green Version]
- Kaberos, A.; Balatsouras, D.G.; Kandiloros, D.; Korres, S.G.; Economou, C. Audiological assessment in Ramsay Hunt syndrome. Ann. Otol. Rhinol. Laryngol. 2002, 111, 68–76. [Google Scholar] [CrossRef]
- Huang, Y.C.; Wang, S.J.; Young, Y.H. Test battery of cranial nerves VII and VIII for assessing herpes zoster oticus. Otolaryngol. Head Neck Surg. 2015, 152, 143–148. [Google Scholar] [CrossRef]
- Yeo, S.-W.; Lee, D.-H.; Jun, B.-C.; Chang, K.-H.; Park, Y.-S. Analysis of prognostic factors in Bell’s palsy and Ramsay Hunt syndrome. Auris Nasus Larynx. 2007, 34, 159–164. [Google Scholar] [CrossRef]
- Martin-Sanz, E.; Rueda, A.; Esteban-Sanchez, J.; Yanes, J.; Rey-Martinez, J.; Sanz-Fernandez, R. Vestibular Restoration and Adaptation in Vestibular Neuritis and Ramsay Hunt Syndrome with Vertigo. Otol. Neurotol. 2017, 38, e203–e208. [Google Scholar] [CrossRef]
- Kim, C.; Jeong, K.; Ahn, S.H.; Shin, D.H.; Kim, Y.W.; Shin, J.E. Vibration- and hyperventilation-induced nystagmus in patients with Ramsay Hunt syndrome with vertigo. Otolaryngol. Head Neck Surg. 2015, 152, 912–918. [Google Scholar] [CrossRef]
- Yokose, M.; Shimizu, T. A Case of Ramsay Hunt Syndrome That Began with Vestibular Symptoms: A Great Mimicker. Am. J. Med. 2021, 134, e271–e272. [Google Scholar] [CrossRef]
- Saito, S.; Ochi, K.; Kobayashi, T.; Sugiura, N.; Komatsuzaki, Y.; Ohashi, T. Vestibular-evoked myogenic potentials in two patients with Ramsay Hunt syndrome. Auris Nasus Larynx. 2003, 30, S89–S92. [Google Scholar] [CrossRef]
- Takahashi, M.; Sato, G.; Toda, N.; Azuma, T.; Nakamura, K.; Iwasaki, H.; Miyoshi, H.; Matsuda, K.; Kitamura, Y.; Abe, K.; et al. Vestibular and cochlear nerve enhancement on MRI and its correlation with vestibulocochlear functional deficits in patients with Ramsay Hunt syndrome. Auris Nasus Larynx. 2021, 48, 347–352. [Google Scholar] [CrossRef]
- Ozeki, H.; Iwasaki, S.; Ushio, M.; Takeuchi, N.; Murofushi, T. The lesion site of vestibular dysfunction in Ramsay Hunt syndrome: A study by click and galvanic VEMP. J. Vestib. Res. 2006, 16, 217–222. [Google Scholar] [CrossRef]
- Kuhweide, R.; Van de Steene, V.; Vlaminck, S.; Casselman, J.W. Ramsay Hunt syndrome: Pathophysiology of cochleovestibular symptoms. J. Laryngol. Otol. 2002, 116, 844–848. [Google Scholar] [CrossRef]
- Lu, Y.C.; Young, Y.H. Vertigo from herpes zoster oticus: Superior or inferior vestibular nerve origin? Laryngoscope 2003, 113, 307–311. [Google Scholar] [CrossRef]
- Lee, J.; Choi, B.; Noh, H.; Jeong, H.; Shin, J.E.; Kim, C.-H. Nystagmus in Ramsay Hunt syndrome with or without dizziness. Neurol. Sci. 2021, 42, 193–198. [Google Scholar] [CrossRef]
- Ohtani, F.; Furuta, Y.; Aizawa, H.; Fukuda, S. Varicella-zoster virus load and cochleovestibular symptoms in Ramsay Hunt syndrome. Ann. Otol. Rhinol. Laryngol. 2006, 115, 233–238. [Google Scholar] [CrossRef]
- Kim, C.-H.; Choi, J.W.; Han, K.J.; Lee, Y.S.; Shin, J.E. Direction-fixed and Direction-changing Positional Nystagmus in Ramsay Hunt Syndrome. Otol. Neurotol. 2018, 39, e209–e213. [Google Scholar] [CrossRef]
- Shim, H.J.; Jung, H.; Park, D.C.; Lee, J.H.; Yeo, S.G. Ramsay Hunt syndrome with multicranial nerve involvement. Acta. Otolaryngol. 2011, 131, 210–215. [Google Scholar] [CrossRef]
- Berrettini, S.; Bianchi, M.C.; Segnini, G.; Sellari-Franceschini, S.; Bruschini, P.; Montanaro, D. Herpes zoster oticus: Correlations between clinical and MRI findings. Eur. Neurol. 1998, 39, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Toda, N.; Takahashi, M.; Azuma, T.; Nakamura, K.; Takao, S.-I.; Harada, M.; Takeda, N. Vestibular and cochlear neuritis in patients with Ramsay Hunt syndrome: A Gd-enhanced MRI study. Acta. Otolaryngol. 2013, 133, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.W.; Kim, C.H. Features of Audio-Vestibular Deficit and 3D-FLAIR Temporal Bone MRI in Patients with Herpes Zoster Oticus. Viruses 2022, 14, 2568. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Arbusow, V.; Strupp, M.; Dieterich, M.; Sautier, W.; Brandt, T. Sympathetic contralateral vestibulopathy after unilateral zoster oticus. J. Neurol. Neurosurg. Psychiatry 1999, 66, 672–676. [Google Scholar] [CrossRef][Green Version]
- Sung-Won, C.; Jae, Y.J.; Chung-Ku, R.; Myung Whan, S. Bilateral Vestibular Hypofunction Induced by Unilateral Herpes Zoster. J. Korean Bal. Soc. 2008, 7, 207–212. [Google Scholar]
- Young-Hun, Y.; Jeong-Ho, P. A Case of Bilateral Vestibulopathy Caused by Varicella-Zoster Meningitis. J. Korean Bal. Soc. 2007, 6, 230–233. [Google Scholar]
- Karbassi, M.; Raizman, M.B.; Schuman, J.S. Herpes zoster ophthalmicus. Surv. Ophthalmol. 1992, 36, 395–410. [Google Scholar] [CrossRef]
- Sanders, E.A.C.M.; Peters, A.C.B.; Gratana, J.W.; Hughes, R.A.C. Guillain-Barré syndrome after varicella-zoster infection. Report of two cases. J. Neurol. 1987, 234, 437–439. [Google Scholar] [CrossRef]



| N. | Exams Performed | Main Findings | |
|---|---|---|---|
| Wayman, 1990 [14] | 7 | Audiometry, ABR | 6 presented cochlear pattern |
| Kaberos, 2002 [16] | 15 | Audiometry, ABR, Otoacoustic emissions | 12 presented hearing loss 8 with retrocochlear involvement 3 with pure cochlear hearing loss |
| Huang, 2015 [17] | 20 | Caloric tests, c VEMPs | 8 abnormal calorics 13 pathological VEMPs |
| Kim, 2015 [20] | 17 | Vestibular tests | Positive skull vibration in 94% Hyperventilation-positive in 59% |
| Saito, 2003 [22] | 2 with canal paresis | Caloric tests, c VEMPs | 1 with pathological VEMPs |
| Takahashi, 2021 [23] | 19 | Caloric tests, o and c VEMPs | 79% pathological caloric tests 53% pathological o-VEMPs 17% pathological c-VEMPs |
| Ozeki, 2006 [24] | 10 | Caloric tests, c VEMPs | 10 abnormal caloric tests 7 pathological VEMPs |
| Lee, 2021 [27] | 36; 27 with and 9 without dizziness | Vestibular tests | 67% of subjects without dizziness presented spontaneous nystagmus |
| Kim, 2018 [29] | 28 | Vestibular tests | 7 of them presented a bipositional direction changing nystagmus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teggi, R.; Del Poggio, A.; Cangiano, I.; Nobile, A.; Gatti, O.; Bussi, M. Cochleo-Vestibular Disorders in Herpes Zoster Oticus: A Literature Review and a Case of Bilateral Vestibular Hypofunction in Unilateral HZO. J. Clin. Med. 2023, 12, 6206. https://doi.org/10.3390/jcm12196206
Teggi R, Del Poggio A, Cangiano I, Nobile A, Gatti O, Bussi M. Cochleo-Vestibular Disorders in Herpes Zoster Oticus: A Literature Review and a Case of Bilateral Vestibular Hypofunction in Unilateral HZO. Journal of Clinical Medicine. 2023; 12(19):6206. https://doi.org/10.3390/jcm12196206
Chicago/Turabian StyleTeggi, Roberto, Anna Del Poggio, Iacopo Cangiano, Alessandro Nobile, Omar Gatti, and Mario Bussi. 2023. "Cochleo-Vestibular Disorders in Herpes Zoster Oticus: A Literature Review and a Case of Bilateral Vestibular Hypofunction in Unilateral HZO" Journal of Clinical Medicine 12, no. 19: 6206. https://doi.org/10.3390/jcm12196206
APA StyleTeggi, R., Del Poggio, A., Cangiano, I., Nobile, A., Gatti, O., & Bussi, M. (2023). Cochleo-Vestibular Disorders in Herpes Zoster Oticus: A Literature Review and a Case of Bilateral Vestibular Hypofunction in Unilateral HZO. Journal of Clinical Medicine, 12(19), 6206. https://doi.org/10.3390/jcm12196206






