The Association of APOE ε4 Allele with Retinal Layer Thickness and Microvasculature in Older Adults: Optic Nerve Decline and Cognitive Change Study
Abstract
:1. Introduction
2. Methods
2.1. Participant Recruitment
2.2. Ophthalmic Examination and Optical Coherence Tomography (OCT)
2.3. Neuropsychological Evaluation
2.4. Multifocal Visual Evoked Potential (mfVEP)
2.5. Magnetic Resonance Imaging (MRI) of the Brain
2.6. Quantification of Brain Volumetric Measures and Quality Control
2.7. Blood Collection and DNA Extraction
2.8. APOE Analysis
2.9. Statistical Analysis
3. Results
3.1. mfVEP and RNFL Thickness Analysis
3.2. Optical Coherence Tomography–Angiography Analysis (OCT-A)
3.3. Neuropsychological Test Scores Analysis
| Carrier Status | APOE ε4− (n = 85) | APOE ε4+ (n = 24) | B-Value | 95% CI | p-Value |
|---|---|---|---|---|---|
| Mean | Mean | ||||
| Cognitive Test (Score) | |||||
| Predicted WAIS-IV_FSIQ | 112 | 113.3 | −1.47 | −4.906 to 1.967 | 0.402 |
| LM Immediate | 12.6 | 14.8 | −2.246 | −4.030 to −0.461 | 0.014 * |
| LM Delay | 11.3 | 12.8 | −1.447 | −3.524 to 0.631 | 0.172 |
| LM Recognition | 12 | 12.2 | −0.22 | −1.009 to 0.570 | 0.586 |
| CVLT_TL | 41.3 | 45 | −3.547 | −8.980 to 1.886 | 0.201 |
| CVLT sdfr | 8.8 | 9.6 | −0.585 | −2.282 to 1.112 | 0.499 |
| CVLT ldfr | 9.3 | 9.8 | −0.273 | −2.101 to 1.554 | 0.769 |
| Letter Fluency | 42.2 | 44.4 | −1.817 | −6.948 to 3.314 | 0.488 |
| Category Fluency | 19.7 | 19.5 | 0.147 | −1.679 to 1.972 | 0.875 |
| SDMT | 48.5 | 48.9 | −0.283 | −4.653 to 4.087 | 0.899 |
| DSF | 11 | 10.9 | 0.169 | −0.847 to 1.185 | 0.744 |
| DSB | 9.2 | 8.4 | 0.905 | −0.038 to 1.848 | 0.06 |
| DS Seq | 8 | 8.4 | −0.348 | −1.055 to 0.358 | 0.334 |
| DS Total | 28.2 | 27.6 | 0.726 | −1.265 to 2.717 | 0.475 |
| TMA | 32.6 | 33.4 | −0.277 | −4.030 to 3.476 | 0.885 |
| TMB | 79 | 92.7 | −11.14 | −34.891 to 12.610 | 0.358 |
| BNT_NCS | 27.4 | 27.7 | −0.414 | −1.468 to 0.640 | 0.441 |
| MMSE | 28.4 | 28.7 | −0.363 | −1.099 to 0.372 | 0.333 |
| GDS | 1.9 | 1.4 | 0.504 | −1.062 to 0.923 | 0.288 |
| RCFT_1 | 33.7 | 34.2 | −0.619 | −1.962 to 0.723 | 0.366 |
| RCFT_2 | 17.3 | 19.2 | −1.97 | −5.351 to 1.411 | 0.253 |
| RCFT_3 | 16.6 | 17.5 | −0.813 | −4.600 to 2.973 | 0.674 |
| Visual Evoked Potential | |||||
| VEP Amplitude (nV) | 141 | 154.1 | −5.601 | −29.539 to 18.337 | 0.647 |
| VEP Latency (ms) | 143.6 | 139.4 | 4.241 | −2.981 to 11.462 | 0.25 |
| MRI Brain Volumes (mL) | |||||
| ETICV | 1,567,758 | 1,548,422 | −39,335.344 | −112,315.810 to 33,645.121 | 0.291 |
| TGMV | 600,412 | 597,664 | −14,160.752 | −40,569.022 to 12,247.518 | 0.293 |
| Cortex Volume | 438,318 | 437,714 | −12,689.852 | −34,193.327 to 8813.623 | 0.247 |
| CWMV | 447,808 | 444,803 | −5479.851 | −20,893.032 to 9933.330 | 0.486 |
| GM Cingulate | 18,396 | 17,873 | −180.946 | −1182.660 to 820.768 | 0.723 |
| GM Parietal | 102,065 | 100,950 | −1798.391 | −7333.500 to 3736.717 | 0.524 |
| Entorhinal Volume | 3798 | 3840 | −132.203 | −472.440 to 208.034 | 0.446 |
| Third Ventricle | 1534 | 1552 | −148.685 | −400.656 to 103.286 | 0.247 |
| Forth Ventricle | 1970 | 1796 | 116.368 | −68.410 to 301.146 | 0.217 |
| Thalamus | 13,368 | 13,225 | −127.904 | −810.665 to 554.857 | 0.713 |
| Putamen | 9110 | 8935 | 3.211 | −748.604 to 755.026 | 0.993 |
| Hippocampus | 7962 | 7795 | 69.839 | −333.016 to 472.694 | 0.734 |
3.4. MRI Brain Volume Analysis
4. Discussion
4.1. Our Findings
4.2. Comparisons to Previous Literature
4.3. Strengths of Our Study
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Mukadam, N. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- López-Cuenca, I.; de Hoz, R.; Salobrar-García, E.; Elvira-Hurtado, L.; Rojas, P.; Fernández-Albarral, J.A.; Ramírez, J.M. Macular thickness decrease in asymptomatic subjects at high genetic risk of developing Alzheimer’s disease: An OCT study. J. Clin. Med. 2020, 9, 1728. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Strickland, M.R.; Soranno, A.; Holtzman, D.M. Apolipoprotein E: Structural insights and links to Alzheimer disease pathogenesis. Neuron 2021, 109, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Small, B.J.; Rosnick, C.B.; Fratiglioni, L.; Bäckman, L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychol. Aging 2004, 19, 592. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Bienias, J.L.; Berry-Kravis, E.; Evans, D.A.; Bennett, D.A. The apolipoprotein E ε2 allele and decline in episodic memory. J. Neurol. Neurosurg. Psychiatry 2002, 73, 672–677. [Google Scholar] [CrossRef]
- Elahi, F.M. Retinal imaging demonstrates reduced capillary density in clinically unimpaired APOE ε4 gene carriers. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12181. [Google Scholar] [CrossRef]
- Salobrar-Garcia, E.; Méndez-Hernández, C.; Hoz, R.D.; Ramírez, A.I.; López-Cuenca, I.; Fernández-Albarral, J.A.; Ramírez, J.M. Ocular vascular changes in mild alzheimer’s disease patients: Foveal avascular zone, choroidal thickness, and onh hemoglobin analysis. J. Pers. Med. 2020, 10, 231. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Albert, M.S.; Alonso, A.; Coker, L.H.; Coresh, J.; Davis, S.M.; Knopman, D.S. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017, 74, 1246–1254. [Google Scholar] [CrossRef]
- Ma, Y.; Sajeev, G.; VanderWeele, T.J.; Viswanathan, A.; Sigurdsson, S.; Eiriksdottir, G.; Blacker, D. APOE ε4 and late-life cognition: Mediation by structural brain imaging markers. Eur. J. Epidemiol. 2022, 37, 591–601. [Google Scholar] [CrossRef]
- Saks, D.; Schulz, A.; Sheriff, S.; Shen, T.; Gupta, V.; Qassim, A. Quantification of localised vascular wedge-shaped defects in glaucoma. Clin. Exp. Ophthalmol. 2022, 50, 724–735. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Q.; Tao, R.; Lu, H.; Xiao, Z.; Zheng, L.; Xiao, Y. Decreased retinal vascular density in Alzheimer’s disease (AD) and mild cognitive impairment (MCI): An optical coherence tomography angiography (OCTA) study. Front. Aging Neurosci. 2021, 12, 572484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, Y.; Wei, Y.; Shi, Y.; Wright, C.B.; Sun, X.; Wang, J. Impaired retinal microcirculation in patients with Alzheimer’s disease. PLoS ONE 2018, 13, e0192154. [Google Scholar] [CrossRef]
- Den Haan, J.; Janssen, S.F.; van de Kreeke, J.A.; Scheltens, P.; Verbraak, F.D.; Bouwman, F.H. Retinal thickness correlates with parietal cortical atrophy in early-onset Alzheimer’s disease and controls. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018, 10, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Grewal, D.S.; Polascik, B.W.; Hoffmeyer, G.C.; Fekrat, S. Assessment of differences in retinal microvasculature using OCT angiography in Alzheimer’s disease: A twin discordance report. Ophthalmic Surg. Lasers Imaging Retin. 2018, 49, 440–444. [Google Scholar] [CrossRef] [PubMed]
- López-Cuenca, I.; Salobrar-García, E.; Elvira-Hurtado, L.; Fernández-Albarral, J.A.; Sánchez-Puebla, L.; Salazar, J.J.; de Hoz, R. The value of oct and octa as potential biomarkers for preclinical Alzheimer’s disease: A review study. Life 2021, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Sheriff, S.; You, Y.; Jiang, J.; Schulz, A. Evaluating associations of RNFL thickness and multifocal VEP with cognitive assessment and brain MRI volumes in older adults: Optic nerve decline and cognitive change (ONDCC) initiative. Aging Brain 2022, 2, 100049. [Google Scholar] [CrossRef]
- Tewarie, P.; Balk, L.; Costello, F.; Green, A.; Martin, R.; Schippling, S.; Petzold, A. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS ONE 2012, 7, e34823. [Google Scholar] [CrossRef]
- Elfarnawany, M.H.E.-K. Signal Processing Methods for Quantitative Power Doppler Microvascular Angiography; The University of Western Ontario: London, ON, Canada, 2015. [Google Scholar]
- Bright, P.; Hale, E.; Gooch, V.J.; Myhill, T.; van der Linde, I. The National Adult Reading Test: Restandardisation against the Wechsler adult intelligence scale—Fourth edition. Neuropsychol. Rehabil. 2018, 28, 1019–1027. [Google Scholar] [CrossRef]
- Delis, D.C.; Freeland, J.; Kramer, J.H.; Kaplan, E. Integrating clinical assessment with cognitive neuroscience: Construct validation of the California Verbal Learning Test. J. Consult. Clin. Psychol. 1988, 56, 123–130. [Google Scholar] [CrossRef]
- Ahn, Y.D.; Yi, D.; Joung, H.; Seo, E.H.; Lee, Y.H.; Byun, M.S.; Lee, J.H.; Jeon, S.Y.; Lee, J.-Y.; Sohn, B.K.; et al. Normative Data for the Logical Memory Subtest of the Wechsler Memory Scale-IV in Middle-Aged and Elderly Korean People. Psychiatry Investig. 2020, 17, 1247. [Google Scholar] [CrossRef]
- Ross, T.P.; Calhoun, E.; Cox, T.; Wenner, C.; Kono, W.; Pleasant, M. The reliability and validity of qualitative scores for the Controlled Oral Word Association Test. Arch. Clin. Neuropsychol. 2007, 22, 475–488. [Google Scholar] [CrossRef]
- Smith, A. Symbol Digit Modalities Test; Western Psychological Services Los Angeles: Los Angeles, CA, USA, 1973. [Google Scholar]
- Bowie, C.R.; Harvey, P.D. Administration and interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Adult Intelligence Scale, 3rd ed.; (WAIS-III); Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Lumpkin, J.; Sheerin, C.M. Digit span sequencing as a neurocognitive screening tool in an aging, veteran population. Psychol. Neurosci. 2019, 12, 180. [Google Scholar] [CrossRef]
- Kaplan, E.F.; Goodglass, H.; Weintraub, S. The Boston Naming Test, 2nd ed.; Lea&Febiger: Philadelphia, PA, USA, 1982. [Google Scholar]
- Wilson, N.-A.; Batchelor, J. Examining Rey complex figure test organization in healthy adults. J. Clin. Exp. Neuropsychol. 2015, 37, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Greenberg, S.A. The geriatric depression scale (GDS). Best Pract. Nurs. Care Older Adults 2012, 4, 1–2. [Google Scholar]
- Shen, T.; You, Y.; Arunachalam, S.; Fontes, A.; Liu, S.; Gupta, V. Differing structural and functional patterns of optic nerve damage in multiple sclerosis and neuromyelitis optica spectrum disorder. Ophthalmology 2019, 126, 445–453. [Google Scholar] [CrossRef]
- Ip, S.C.; Lin, S.-W.; Lai, K.-M. An evaluation of the performance of five extraction methods: Chelex® 100, QIAamp® DNA blood mini kit, QIAamp® DNA investigator kit, QIAsymphony® DNA Investigator® kit and DNA IQ™. Sci. Justice 2015, 55, 200–208. [Google Scholar] [CrossRef]
- Su, Y.; Dong, J.; Sun, J.; Zhang, Y.; Ma, S.; Li, M. Cognitive function assessed by Mini-mental state examination and risk of all-cause mortality: A community-based prospective cohort study. BMC Geriatr. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Lehtovirta, M.; Soininen, H.; Helisalmi, S.; Mannermaa, A.; Helkala, E.L.; Hartikainen, P. Clinical and neuropsychological characteristics in familial and sporadic Alzheimer’s disease: Relation to apolipoprotein E polymorphism. Neurology 1996, 46, 413–419. [Google Scholar] [CrossRef]
- Salobrar-García, E.; Rodrigues-Neves, A.C.; Ramírez, A.I.; de Hoz, R.; Fernández-Albarral, J.A.; López-Cuenca, I. Microglial activation in the retina of a triple-transgenic alzheimer’s disease mouse model (3xTg-AD). Int. J. Mol. Sci. 2020, 21, 816. [Google Scholar] [CrossRef] [PubMed]
- Zabel, P.; Kaluzny, J.J.; Wilkosc-Debczynska, M.; Gebska-Toloczko, M. Comparison of retinal microvasculature in patients with Alzheimer’s disease and primary open-angle glaucoma by optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3447–3455. [Google Scholar] [CrossRef] [PubMed]
- Mullany, S.; Marshall, H.; Diaz-Torres, S.; Berry, E.C.; Schmidt, J.M.; Thomson, D. The APOE E4 allele is associated with faster rates of neuroretinal thinning in a prospective cohort study of suspect and early glaucoma. Ophthalmol. Sci. 2022, 2, 100159. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Calippe, B.; Lavalette, S.; Hu, S.J.; Raoul, W. Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age-related macular degeneration. EMBO Mol. Med. 2015, 7, 211–226. [Google Scholar] [CrossRef]
- Mormino, E.C.; Betensky, R.A.; Hedden, T.; Schultz, A.P.; Ward, A. Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology 2014, 82, 1760–1767. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Y.; Li, X.; Bai, Q. Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch. Gerontol. Geriatr. 2015, 60, 162–167. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, X.; Hu, J.; Jiang, L.; Mei, X.; Zheng, H. Retinal nerve fiber layer thickness is associated with hippocampus and lingual gyrus volumes in nondemented older adults. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 99, 109824. [Google Scholar] [CrossRef]
- Gold, B.T.; Powell, D.K.; Andersen, A.H.; Smith, C.D. Alterations in multiple measures of white matter integrity in normal women at high risk for Alzheimer’s disease. Neuroimage 2010, 52, 1487–1494. [Google Scholar] [CrossRef]
- Salat, D.; Tuch, D.S.; Van der Kouwe, A.J.W.; Greve, D.N.; Pappu, V.; Lee, S.Y. White matter pathology isolates the hippocampal formation in Alzheimer’s disease. Neurobiol. Aging 2010, 31, 244–256. [Google Scholar] [CrossRef]
- Kanamori, A.; Nakamura, M.; Escano, M.F.; Seya, R. Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness measured by optical coherence tomography. Am. J. Ophthalmol. 2003, 135, 513–520. [Google Scholar] [CrossRef]
- Miki, A.; Medeiros, F.A.; Weinreb, R.N.; Jain, S.; He, F. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology 2014, 121, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lin, C.; Weinreb, R.N.; Lai, G.; Chiu, V. Risk of visual field progression in glaucoma patients with progressive retinal nerve fiber layer thinning: A 5-year prospective study. Ophthalmology 2016, 123, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Klistorner, A.; Garrick, R.; Barnett, M.H.; Graham, S.L.; Arvind, H.; Sriram, P. Axonal loss in non–optic neuritis eyes of patients with multiple sclerosis linked to delayed visual evoked potential. Neurology 2013, 80, 242–245. [Google Scholar] [CrossRef]
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
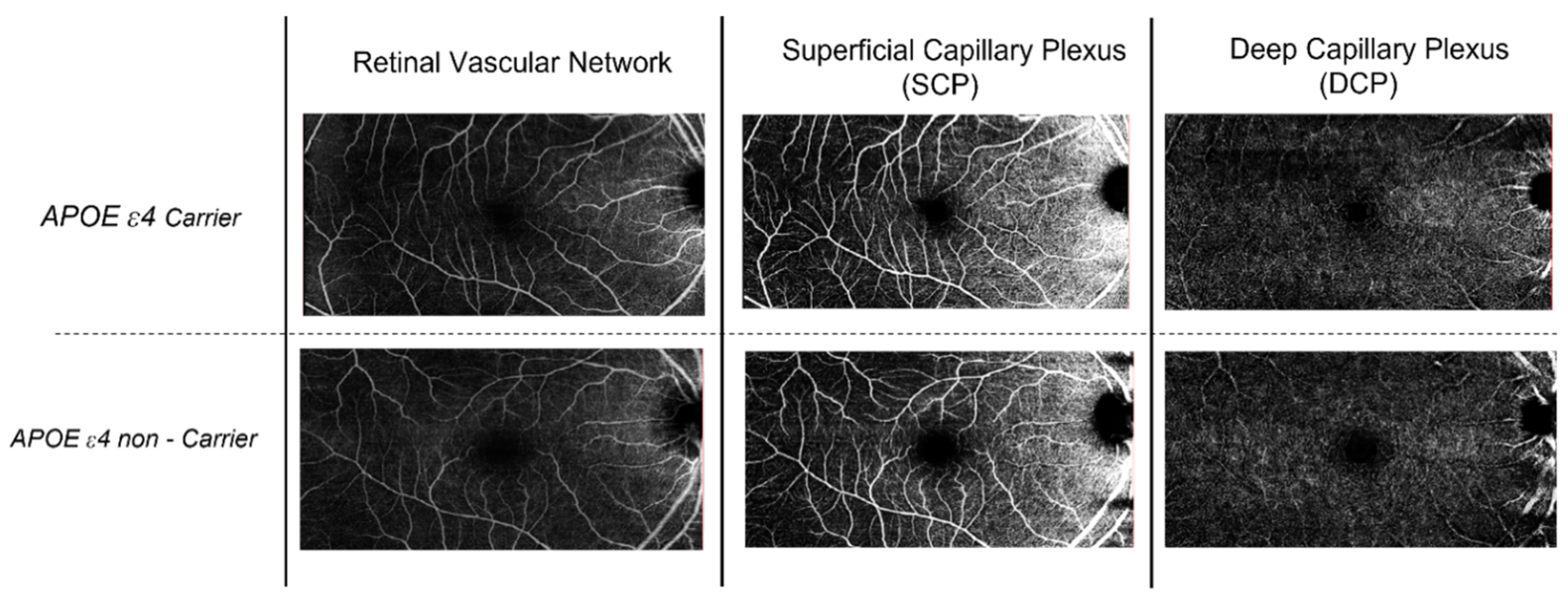
| Controls (n = 109) | |
|---|---|
| Age, mean ± SD, years | 61.1 ± 9.0 |
| Sex (M/F), (n/n) | 53/56 |
| Male No. (%) | 48.60% |
| Female No. (%) | 51.4% |
| APOE ε4 carrier (n/%) | (24/22.3%) |
| APOE ε4 non-carrier (n/%) | (85/77.9%) |
| Education Level (n) | |
| Missing | 8 |
| Secondary School | 15 |
| Bachelor or TAFE | 70 |
| Master’s | 11 |
| PhD | 7 |
| General Health Status (n/% of Cohort) | |
| Diabetes Mellitus | 5/4.6% |
| Hypertension | 39/36% |
| Hypercholesterolemia | 40/36.6% |
| Systolic Blood Pressure, mm Hg mean—(SD) | 132.1 ± 15.2 |
| Diastolic Blood Pressure, mm Hg mean—(SD) | 85.9 ± 9.1 |
| VA, mean ± SD, LogMAR | 0.03 ± 0.05 |
| MMSE, mean ± SD | 28.42 ± 2. |
| APOE ε4− | APOE ε4+ | p-Value | |
|---|---|---|---|
| N | 85 | 24 | |
| Age, mean ± SD, years | 67.01 (9.1) | 67.6 (8.9) | 0.68 |
| Sex (M/F) (n/n) | 43/42 | 9/15 | 0.11 |
| Retinal Microvasculature, mean | |||
| FAZ area (mm2) SCP | 0.40 (0.2) | 0.30 (0.1) | <0.001 * |
| FAZ area (mm2) DCP | 0.39 (0.2) | 0.29 (0.1) | 0.003 * |
| FAZ perimeter (mm)—SCP | 2.31 (0.5) | 1.90 (0.2) | 0.001 * |
| FAZ perimeter (mm)—DCP | 2.32 (0.4) | 2.01 (0.1) | 0.004 * |
| FAZ circularity index—SCP | 0.91 (0.1) | 0.90 (0.0) | 0.111 |
| FAZ circularity index—DCP | 0.89 (0.01) | 0.92 (0.02) | 0.034 * |
| VD (%)—SCP | 23.07 (5.5) | 24.59 (5.2) | 0.592 |
| VD (%)—DCP | 20.82 (8.0) | 21.71 (3.3) | 0.771 |
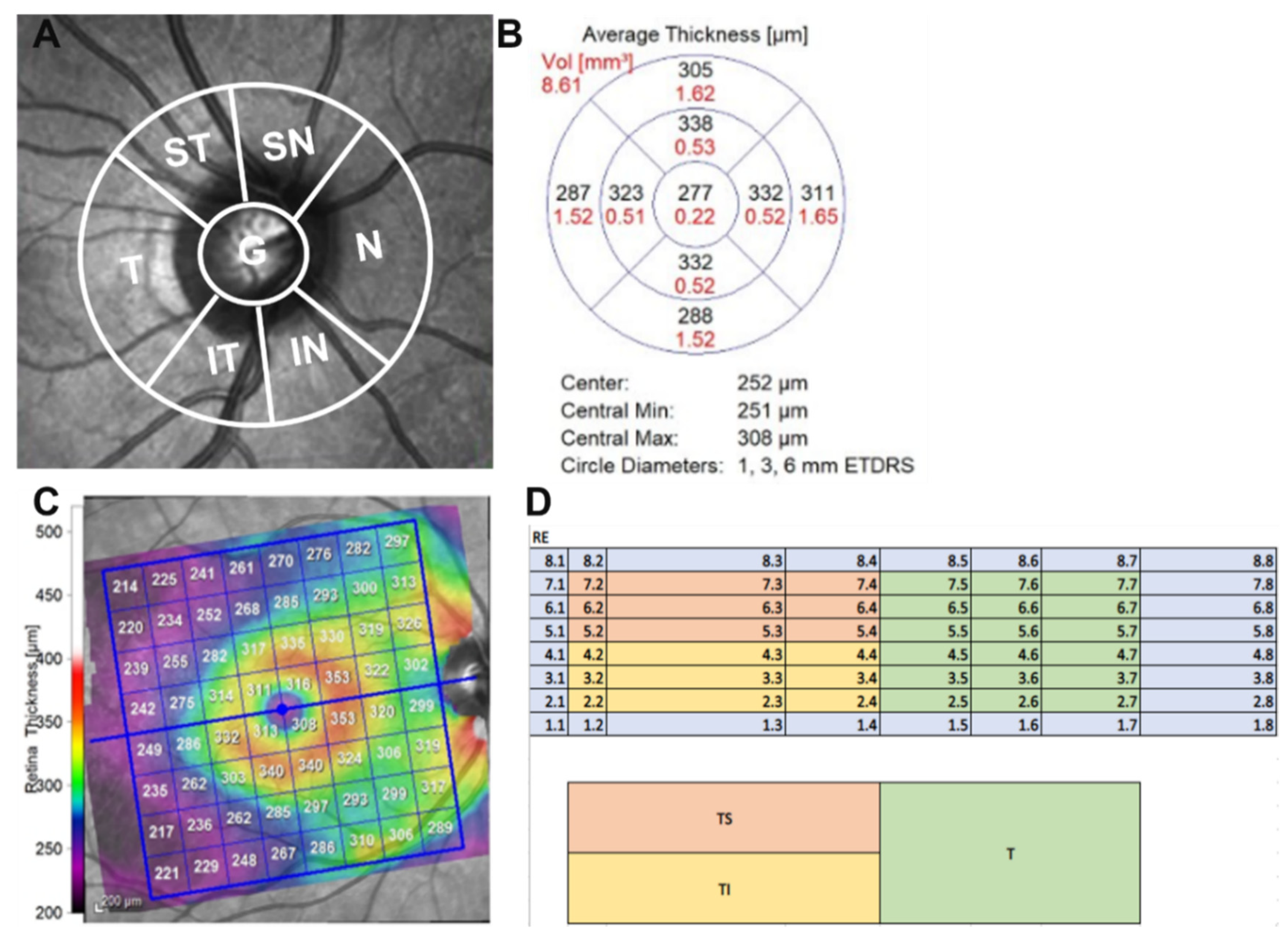
| Retinal layer thickness, (μm) ± SD | |||
| RNFL G | 96.5 (9.7) | 96.0 (7.6) | 0.698 |
| RNFL T | 71.7 (12.3) | 66.7 (8.3) | 0.01 * |
| RNFL TS | 130.8 (20.9) | 130 (21.4) | 0.758 |
| RNFL TI | 145.2 (20.6) | 145.6 (20.4) | 0.904 |
| RNFL N | 77.3 (12.1) | 76.6 (12.5) | 0.857 |
| RNFL NS | 108.1 (21.4) | 105.3 (22.1) | 0.641 |
| RNFL NI | 110.9 (22.3) | 117.1 (19.5) | 0.229 |
| RNFL S | 119.5 (14.8) | 118.9 (13.5) | 0.787 |
| RNFL I | 128.1 (18.4) | 131.8 (16.8) | 0.462 |
| P Pole T | 66.7 (5.2) | 66.3 (6.0) | 0.823 |
| P Pole TS | 67.5 (5.5) | 65.6 (6.1) | 0.227 |
| P Pole TI | 67.1 (5.8) | 65.4 (5.8) | 0.202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheriff, S.; Shen, T.; Saks, D.; Schultz, A.; Francis, H.; Wen, W.; Jiang, J.; Mirzaei, M.; Gupta, V.; Fiatarone Singh, M.; et al. The Association of APOE ε4 Allele with Retinal Layer Thickness and Microvasculature in Older Adults: Optic Nerve Decline and Cognitive Change Study. J. Clin. Med. 2023, 12, 6219. https://doi.org/10.3390/jcm12196219
Sheriff S, Shen T, Saks D, Schultz A, Francis H, Wen W, Jiang J, Mirzaei M, Gupta V, Fiatarone Singh M, et al. The Association of APOE ε4 Allele with Retinal Layer Thickness and Microvasculature in Older Adults: Optic Nerve Decline and Cognitive Change Study. Journal of Clinical Medicine. 2023; 12(19):6219. https://doi.org/10.3390/jcm12196219
Chicago/Turabian StyleSheriff, Samran, Ting Shen, Danit Saks, Angela Schultz, Heather Francis, Wei Wen, Jiyang Jiang, Mehdi Mirzaei, Veer Gupta, Maria Fiatarone Singh, and et al. 2023. "The Association of APOE ε4 Allele with Retinal Layer Thickness and Microvasculature in Older Adults: Optic Nerve Decline and Cognitive Change Study" Journal of Clinical Medicine 12, no. 19: 6219. https://doi.org/10.3390/jcm12196219
APA StyleSheriff, S., Shen, T., Saks, D., Schultz, A., Francis, H., Wen, W., Jiang, J., Mirzaei, M., Gupta, V., Fiatarone Singh, M., Sachdev, P. S., Graham, S. L., & Gupta, V. (2023). The Association of APOE ε4 Allele with Retinal Layer Thickness and Microvasculature in Older Adults: Optic Nerve Decline and Cognitive Change Study. Journal of Clinical Medicine, 12(19), 6219. https://doi.org/10.3390/jcm12196219








