The Long-Term Benefit of Sacubitril/Valsartan in Patients with HFrEF: A 5-Year Follow-Up Study in a Real World Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Kansas City Cardiomyopathy Questionnaire (KCCQ)
2.3. Echocardiography
2.4. Six Minutes Walking Test
2.5. Statistical Analysis
3. Results
Follow-Up
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Weston, S.A.; Jacobsen, S.J.; Roger, V.L. RRisk factors for heart failure: A population-based case-control study. Am. J. Med. 2009, 122, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, P.H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Anker, S.D.; Maggioni, A.P.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.; et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; Tuttle, M.S. Functional assessment of heart failurepatients. Heart Fail. Clin. 2015, 11, 29–36. [Google Scholar] [CrossRef]
- Gutekunst, D.J. Isokinetic torque timing parameters and ceramidesasmarkers of muscledysfunction in systolic heart failure. J. Card. Fail. 2016, 22, 356–357. [Google Scholar] [CrossRef]
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.; Packer, M.; Desai, A.S.; Rouleau, J.L.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. B-Type Natriuretic Peptide During Treatment with Sacubitril/Valsartan: The PARADIGM-HF Trial. J. Am. Coll. Cardiol. 2019, 73, 1264–1272. [Google Scholar] [CrossRef]
- Balmforth, C.; Simpson, J.; Shen, L.; Jhund, P.S.; Lefkowitz, M.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.; Solomon, S.D.; Swedberg, K.; et al. Outcomes and Effect of Treatment According to Etiology in HFrEF: An Analysis of PARADIGM-HF. JACC Heart Fail. 2019, 7, 457–465. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [PubMed]
- Visco, V.; Radano, I.; Campanile, A.; Ravera, A.; Silverio, A.; Masarone, D.; Pacileo, G.; Correale, M.; Mazzeo, P.; Dattilo, G.; et al. Predictors of sacubitril/valsartan high dose tolerability in a real world population with HFrEF. ESC Heart Fail. 2022, 9, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Dini, F.L.; Carluccio, E.; Bitto, R.; Ciccarelli, M.; Correale, M.; D’Agostino, A.; Dattilo, G.; Ferretti, M.; Grelli, A.; Guida, S.; et al. Echocardiographically defined haemodynamic categorization predicts prognosis in ambulatory heart failure patients treated with sacubitril/valsartan. ESC Heart Fail. 2022, 9, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Dini, F.L.; Bitto, R.; Ciccarelli, M.; Correale, M.; D’Agostino, A.; Dattilo, G.; Ferretti, M.; Grelli, A.; Guida, S.; et al. Benefit from sacubitril/valsartan is associated with hemodynamic improvement in heart failure with reduced ejection fraction: An echocardiographic study. Int. J. Cardiol. 2022, 350, 62–68. [Google Scholar] [CrossRef]
- Casale, M.; Mezzetti, M.; De Fazio, M.G.; Caccamo, L.; Busacca, P.; Dattilo, G. Novel active fixation lead guided by electrical delay can improve response to cardiac resynchronization therapy in heart failure. ESC Heart Fail. 2022, 9, 146–154. [Google Scholar] [CrossRef]
- Corrado, E.; Dattilo, G.; Coppola, G.; Morabito, C.; Bonni, E.; Zappia, L.; Novo, G.; de Gregorio, C. Low- vs high-dose ARNI effects on clinical status, exercise performance and cardiac function in real-life HFrEF patients. Eur. J. Clin. Pharmacol. 2022, 78, 19–25. [Google Scholar] [CrossRef]
- Dattilo, G.; Bitto, R.; Correale, M.; Morabito, C.; Vaccaro, V.; Laterra, G.; Casale, M.; Crea, P.; Di Bella, G.; Luzza, F.; et al. Trend of perceived quality of life and functional capacity in outpatients with chronic heart failure and in treatment with sacubitril/valsartan: A real-life experience. Minerva Cardiol. Angiol. 2022, 70, 555–562. [Google Scholar] [CrossRef]
- Casale, M.; Correale, M.; Laterra, G.; Vaccaro, V.; Morabito, C.; Crea, P.; Signorelli, S.S.; Katsiki, N.; Luzza, F.; de Gregorio, C.; et al. Effects of Sacubitril/Valsartan in Patients with High Arrhythmic Risk and an ICD: A Longitudinal Study. Clin. Drug Investig. 2021, 41, 169–176. [Google Scholar] [CrossRef]
- de Gregorio, C.; Laterra, G.; Vaccaro, V.; Bitto, R.; Dattilo, G. Time-based clinical and functional achievements in real-life HF patients on ARNI treatment. Eur. J. Intern. Med. 2020, 76, 115–117. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 American College of Cardiology/American Heart Association/Heart Failure Society of America Guideline for the Management of Heart Failure: Executive Summary. J. Card. Fail. 2022, 28, 810–830. [Google Scholar] [CrossRef]
- Pettersen, K.I.; Reikvam, A.; Rollag, A.; Stavem, K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur. J. Heart Fail. 2005, 7, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Green, C.P.; Porter, C.B.; Bresnahan, D.R.; Spertus, J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: A new health status measure for heart failure. J. Am. Coll. Cardiol. 2000, 35, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.V.; Spertus, J.A.; Lei, Y.; Allen, K.B.; Chhatriwalla, A.K.; Leon, M.B.; Smith, C.R.; Reynolds, M.R.; Webb, J.G.; Svensson, L.G. Use of the Kansas City cardiomyopathy questionnaire for monitoring health status in patients with aortic stenosis. Circ. Heart Fail. 2013, 6, 61–67. [Google Scholar] [CrossRef]
- Spertus, J.A.; Jones, P.G.; Kim, J.; Globe, D. Validity, reliability, and responsiveness of the Kansas City Cardiomyopathy Questionnaire in anemic heart failure patients. Qual. Life Res. 2008, 17, 291–298. [Google Scholar] [CrossRef]
- Spertus, J.; Peterson, E.; Conard, M.W.; Heidenreich, P.A.; Krumholz, H.M.; Jones, P.; McCullough, P.A.; Pina, I.; Tooley, J.; Weintraub, W.S.; et al. Monitoring clinical changes in patients with heart failure: A comparison of methods. Am. Heart J. 2005, 150, 707–715. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- McCabe, N.; Butler, J.; Dunbar, S.B.; Higgins, M.; Reilly, C. Six-minute walk distance predicts 30-day readmission after acute heart failure hospitalization. Heart Lung. 2017, 46, 287–292. [Google Scholar] [CrossRef]
- Zotter-Tufaro, C.; Mascherbauer, J.; Duca, F.; Koell, B.; Aschauer, S.; Kammerlander, A.A.; Panzenboeck, A.; Sadushi-Kolici, R.; Bangert, C.; Laimer, D.; et al. Prognostic Significance and Determinants of the 6-Min Walk Test in Patients with Heart Failure and Preserved Ejection Fraction. JACC Heart Fail. 2015, 3, 459–466. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.P.; Sato, N.; Shah, A.N.; et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef]
- Okumura, N.; Jhund, P.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Swedberg, K.; Zile, M.R.; Solomon, S.D.; et al. Importance of clinical worsening of heart failure treated in the outpatient setting: Evidence from the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM-HF). Circulation 2016, 133, 2254–2262. [Google Scholar] [CrossRef]
- Skali, H.; Dwyer, E.M.; Goldstein, R.; Haigney, M.; Krone, R.; Kukin, M.; Lichstein, E.; McNitt, S.; Moss, A.J.; Pfeffer, M.A.; et al. Prognosis and response to therapy of first inpatient and outpatient heart failure event in a heart failure clinical trial: MADIT-CRT. Eur. J. Heart Fail. 2014, 16, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Rame, J.; Sheffield, M.A.; Dries, D.L.; Gardner, E.B.; Toto, K.H.; Yancy, C.W.; Drazner, M.H. Outcomes after emergency department discharge with a primary diagnosis of heart failure. Am. Heart J. 2001, 142, 714–719. [Google Scholar] [CrossRef] [PubMed]


| General Features | Mean ± SD |
|---|---|
| Age, yrs | 65.6 ± 10.6 |
| Males, N (%) | 60 (85.7) |
| Weight, kg | 80.5 ± 16.1 |
| Height, cm | 168.8 ± 8 |
| Haemodynamic parameters | |
| SBP, mmHg | 125.6 ± 21.9 |
| DBP, mmHg | 75 ± 9.5 |
| Heart Rate, beats/min | 65.4 ± 12.7 |
| Aetiology | |
| Ischaemic, N (%) | 46 (65.7) |
| Idiopathic, N (%) | 24 (34.3) |
| Device | |
| CRT, N (%) | 10 (14.3) |
| ICD, N (%) | 44 (62.9) |
| No electrical therapy, N (%) | 16 (22.8) |
| Co-morbidities | |
| Diabetes, N (%) | 34 (50) |
| Hypertension, N (%) | 64 (94) |
| CKD, N (%) | 6 (8.6) |
| Atrial Fibrillation, N (%) | 18 (26) |
| Baseline | 12 Months | 60 Months | p Value | |
|---|---|---|---|---|
| SBP, mmHg | 125.6 (21.9) | 115.6 (12.8) | 119.4 (15.6) | *** |
| DBP, mmHg | 75 (9.5) | 65.4 (7.4) | 68.9 (10.8) | *** |
| Creatinine, mg/dL | 1.1 (0.3) | 1.1 (0.3) | 1.2 (0.4) | * |
| Sodium, mEq/L | 139.3 (5.3) | 138.6 (5.1) | 139.2 (4.3) | |
| Potassium, mEq/L | 4.4 (0.5) | 4.4 (0.4) | 4.6 (0.5) | ** |
| Haemoglobin, g/dL | 13.5 (1.9) | 13.4 (1.9) | 13.1 (2.7) | |
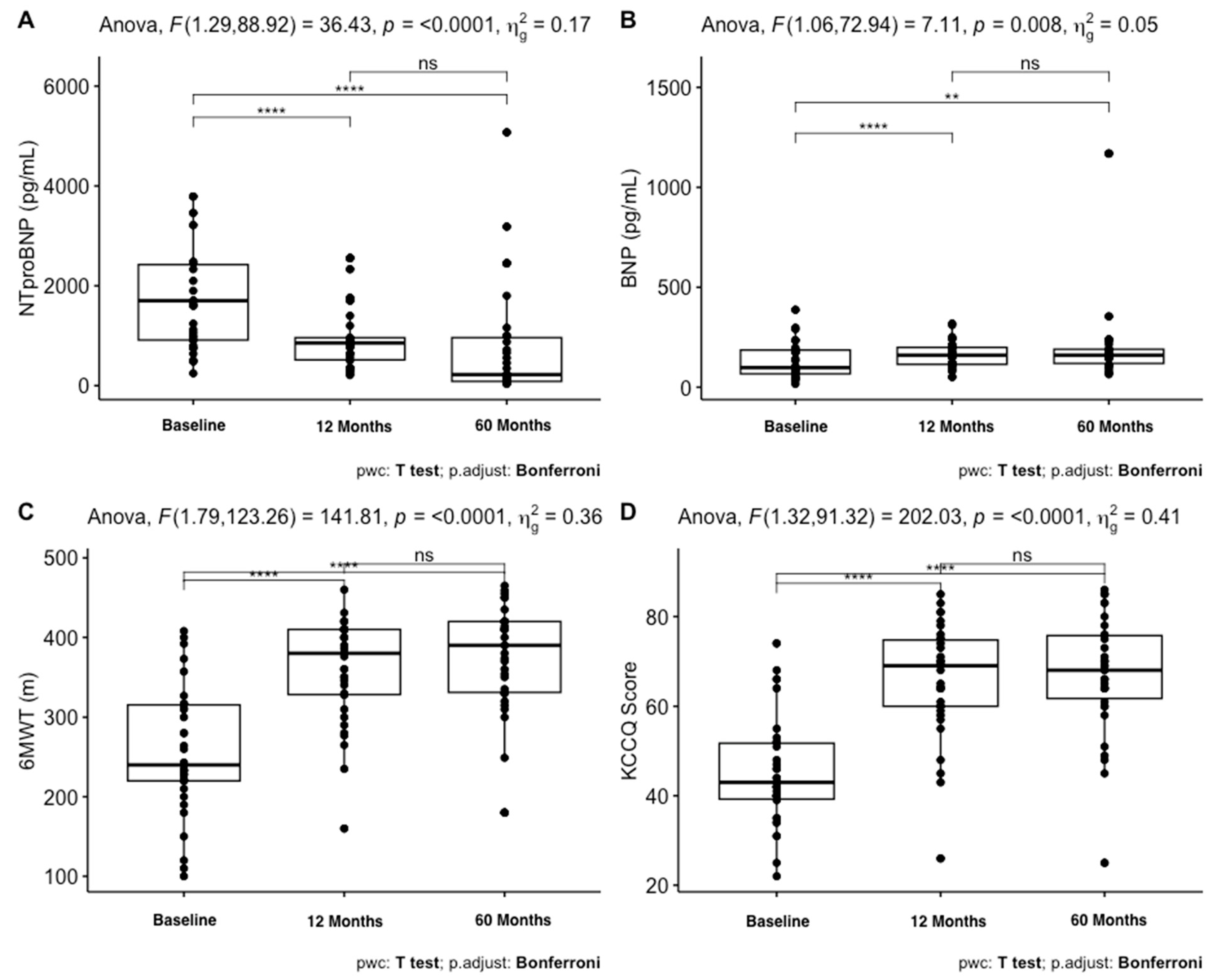
| NTproBNP, pg/mL | 1701/1510.8 | 854/444 | 220/876 | *** |
| BNP, pg/mL | 98/118.8 | 160/84.8 | 160/71 | ** |
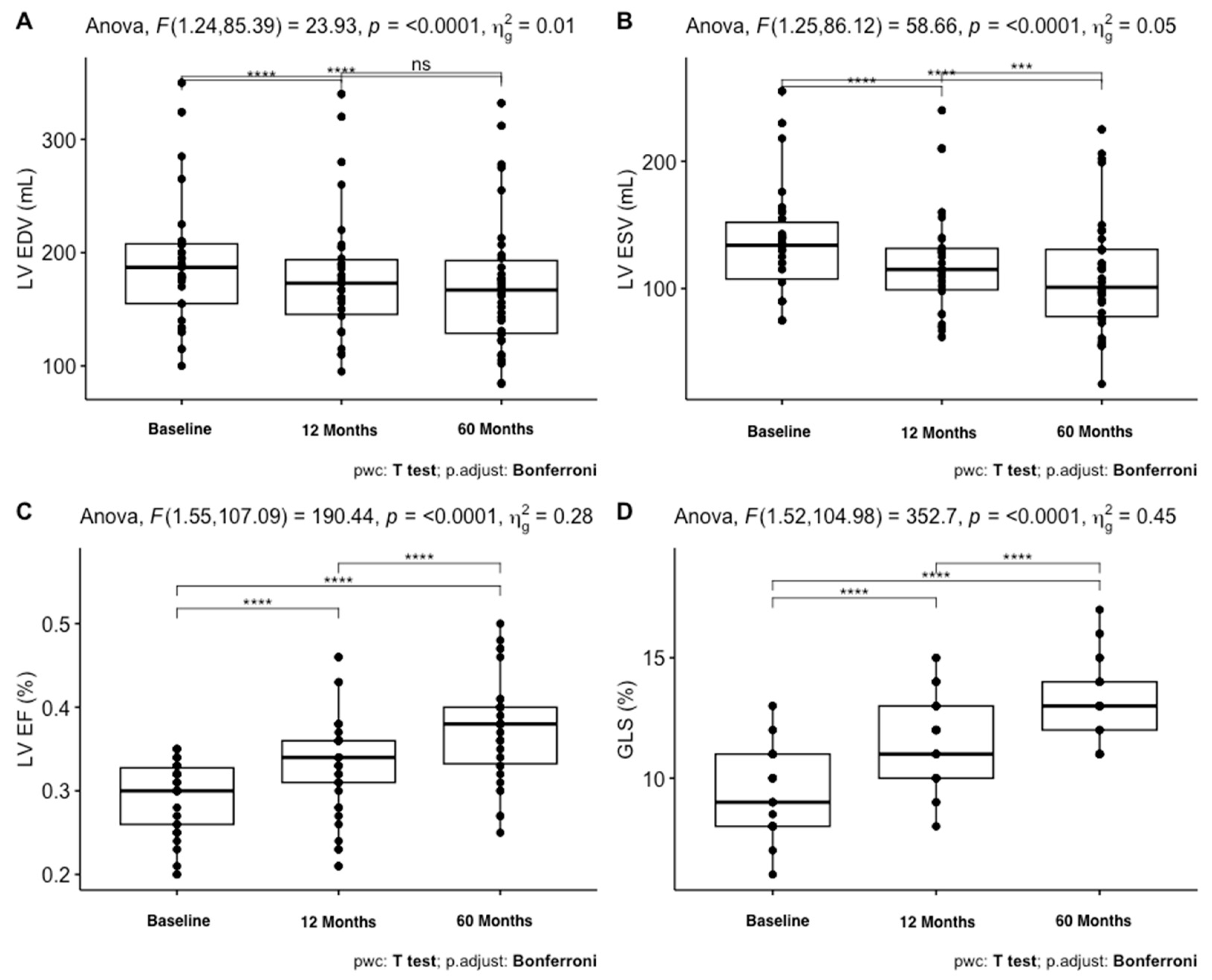
| LV EDV, mL | 188.6 (54.9) | 178.1 (54.9) | 171.7 (59.7) | * |
| LV ESV, mL | 134.5 (41) | 120.3 (41) | 110.2 (45.8) | *** |
| LVEF, % | 29.4 (4.3) | 33.4 (5.4) | 37.3 (5.9) | *** |
| E/e’ | 13 (2.9) | 11.6 (2.9) | 11.1 (3.4) | ** |
| TAPSE, mm | 20.3 (4.4) | 20.9 (4.4) | 21.4 (4.6) | |
| Tricuspid S’, cm/s | 10.7 (3.1) | 11.8 (2.7) | 12.3 (2.5) | ** |
| GLS, % | 9.4 (1.7) | 11.6 (1.8) | 13.2 (1.7) | *** |
| 6MWT, m | 256.9 (78.2) | 361 (64.2) | 373.8 (70.1) | *** |
| KCCQ | 45 (11.9) | 66 (12.3) | 67.2 (13) | *** |
| NYHA | *** | |||
| 1 | 0 (0.0%) | 24 (34.3%) | 40 (57.1%) | |
| 2 | 34 (48.6%) | 42 (60.0%) | 28 (40.0%) | |
| 3 | 36 (51.4%) | 4 (5.7%) | 2 (2.9%) |
| Baseline | 12 Months | 60 Months | p Value | |
|---|---|---|---|---|
| SBP, mmHg | # 0.478 | |||
| Ischaemic Idiopathic | 126.2 ± 25.6 124.5 ± 14.2 | 116.7 ± 14.7 112.7 ± 9.6 | 120.3 ± 16.9 118.1 ± 15.1 | * 0.010 * 0.016 |
| DBP, mmHg | # 0.003 | |||
| Ischaemic Idiopathic | 72.5 ± 7.9 79.5 ± 10.8 | 63.2 ± 7.9 68.6 ± 5.4 | 67 ± 10.3 70 ± 10.9 | * <0.001 * <0.001 |
| NTproBNP, pg/mL | # 0.53 | |||
| Ischaemic Idiopathic | 1767.5 ± 242.5 1733.1 ± 136.5 | 918.9 ± 178.4 867.3 ± 117.3 | 709.1 ± 273.1 438.2 ± 91.4 | * <0.001 * <0.001 |
| BNP | # 0.71 | |||
| Ischaemic Idiopathic | 132.7 ± 93.2 129.8 ± 80 | 159.5 ± 64.2 164.4 ± 70 | 203.6 ± 69.6 175.8 ± 75.9 | * <0.001 * 0.002 |
| LV EDV, mL | # 0.036 | |||
| Ischaemic Idiopathic | 183.4 ± 45.6 208.6 ± 65 | 170.3 ± 45.3 199.7 ± 67.6 | 165.1 ± 51.7 200.6 ± 72.9 | * <0.001 * 0.001 |
| LV EDVi, mL/m2 | # 0.024 | |||
| Ischaemic Idiopathic | 96.5 ± 24.3 110.7 ± 34.6 | 89.5 ± 23.3 106 ± 36.3 | 86.2 ± 24.5 105.5 ± 36.2 | * <0.001 * 0.001 |
| LV ESV, mL | # 0.016 | |||
| Ischaemic Idiopathic | 129.7 ± 32.5 152.7 ± 50.4 | 114.8 ± 32.3 138.4 ± 50.6 | 103.8 ± 35.4 133.5 ± 56.8 | * <0.001 * <0.001 |
| LV ESVi, mL/m2 | # 0.010 | |||
| Ischaemic Idiopathic | 68.3 ± 17.8 80.6 ± 25 | 60.3 ± 16.3 73.1 ± 25.6 | 54.2 ± 17.2 69.8 ± 26.8 | * <0.001 * <0.001 |
| LVEF, % | # 0.017 | |||
| Ischaemic Idiopathic | 29.9 ± 3.8 27.1 ± 4.8 | 33.9 ± 5 31 ± 5.7 | 38.3 ± 6.7 35.1 ± 6.6 | * <0.001 * <0.001 |
| E/e’ | #0.26 | |||
| Ischaemic Idiopathic | 14 ± 2.4 14.6 ± 2.7 | 12 ± 3.2 13.6 ± 2.7 | 11.7 ± 3.1 12.8 ± 3.3 | * <0.001 * 0.008 |
| TAPSE, mm | # 0.98 | |||
| Ischaemic Idiopathic | 20.1 ± 4.7 20 ± 3.9 | 20.7 ± 4.8 20.3 ± 3.9 | 20.7 ± 5.1 21.3 ± 4.5 | * <0.001 * <0.001 |
| Tricuspid S’, cm/s | # 0.97 | |||
| Ischaemic Idiopathic | 10.8 ± 3.3 10.7 ± 3.3 | 10.8 ± 3.3 10.8 ± 3.3 | 10.8 ± 3.3 10.8 ± 3.3 | * 0.99 * 0.015 |
| GLS, % | # 0.53 | |||
| Ischaemic Idiopathic | 9.7 ± 1.8 9.9 ± 1.6 | 11.9 ± 2.3 11.3 ± 1.4 | 11.5 ± 2.7 12.7 ± 2.6 | * <0.001 * <0.001 |
| 6MWT m | # 0.096 | |||
| Ischaemic Idiopathic | 226.9 ± 83.3 283.7 ± 85.7 | 352.9 ± 71.7 362 ± 50.5 | 364.1 ± 80.6 380.7 ± 51.7 | * <0.001 * <0.001 |
| KCCQ | # 0.12 | |||
| Ischaemic Idiopathic | 44.5 ± 10.4 50 ± 13.9 | 67.3 ± 10.5 69.6 ± 10.2 | 68.1 ± 10.9 72 ± 11.1 | * <0.001 * <0.001 |
| NYHA | # 0.037 | |||
| Ischaemic Idiopathic | 2.62 ± 0.5 2.3 ± 0.5 | 1.8 ± 0.6 1.55 ± 0.5 | 1.5 ± 0.6 1.36 ± 0.5 | * <0.001 * <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dattilo, G.; Laterra, G.; Licordari, R.; Parisi, F.; Pistelli, L.; Colarusso, L.; Zappia, L.; Vaccaro, V.; Demurtas, E.; Allegra, M.; et al. The Long-Term Benefit of Sacubitril/Valsartan in Patients with HFrEF: A 5-Year Follow-Up Study in a Real World Population. J. Clin. Med. 2023, 12, 6247. https://doi.org/10.3390/jcm12196247
Dattilo G, Laterra G, Licordari R, Parisi F, Pistelli L, Colarusso L, Zappia L, Vaccaro V, Demurtas E, Allegra M, et al. The Long-Term Benefit of Sacubitril/Valsartan in Patients with HFrEF: A 5-Year Follow-Up Study in a Real World Population. Journal of Clinical Medicine. 2023; 12(19):6247. https://doi.org/10.3390/jcm12196247
Chicago/Turabian StyleDattilo, Giuseppe, Giulia Laterra, Roberto Licordari, Francesca Parisi, Lorenzo Pistelli, Luigi Colarusso, Luca Zappia, Vittoria Vaccaro, Elisabetta Demurtas, Marta Allegra, and et al. 2023. "The Long-Term Benefit of Sacubitril/Valsartan in Patients with HFrEF: A 5-Year Follow-Up Study in a Real World Population" Journal of Clinical Medicine 12, no. 19: 6247. https://doi.org/10.3390/jcm12196247
APA StyleDattilo, G., Laterra, G., Licordari, R., Parisi, F., Pistelli, L., Colarusso, L., Zappia, L., Vaccaro, V., Demurtas, E., Allegra, M., Crea, P., Di Bella, G., Signorelli, S. S., Aspromonte, N., Imbalzano, E., & Correale, M. (2023). The Long-Term Benefit of Sacubitril/Valsartan in Patients with HFrEF: A 5-Year Follow-Up Study in a Real World Population. Journal of Clinical Medicine, 12(19), 6247. https://doi.org/10.3390/jcm12196247









