Geriatric Surgery Produces a Hypoactive Molecular Phenotype in the Monocyte Immune Gene Transcriptome
Abstract
:1. Introduction
2. Methods
2.1. Study Participants
2.2. Sample Collection
2.3. Isolation of CD14+ Monocytes
2.4. RNA isolation and Quality Assessment
2.5. NanoString
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alazawi, W.; Pirmadjid, N.; Lahiri, R.; Bhattacharya, S. Inflammatory and Immune Responses to Surgery and Their Clinical Impact. Ann. Surg. 2016, 264, 73–80. [Google Scholar] [CrossRef]
- Aono, H.; Ohwada, T.; Kaneko, N.; Fuji, T.; Iwasaki, M. The post-operative changes in the level of inflammatory markers after posterior lumbar interbody fusion. J. Bone Joint Surg. Br. 2007, 89, 1478–1481. [Google Scholar] [CrossRef]
- Baigrie, R.J.; Lamont, P.M.; Kwiatkowski, D.; Dallman, M.J.; Morris, P.J. Systemic cytokine response after major surgery. Br. J. Surg. 1992, 79, 757–760. [Google Scholar] [CrossRef]
- Fong, T.G.; Chan, N.Y.; Dillon, S.T.; Zhou, W.; Tripp, B.; Ngo, L.H.; Otu, H.H.; Inouye, S.K.; Vasunilashorn, S.M.; Cooper, Z.; et al. Identification of Plasma Proteome Signatures Associated with Surgery Using SOMAscan. Ann. Surg. 2021, 273, 732–742. [Google Scholar] [CrossRef]
- Lin, E.; Calvano, S.E.; Lowry, S.F. Inflammatory cytokines and cell response in surgery. Surgery 2000, 127, 117–126. [Google Scholar] [CrossRef]
- Rosenberger, P.H.; Ickovics, J.R.; Epel, E.; Nadler, E.; Jokl, P.; Fulkerson, J.P.; Tillie, J.M.; Dhabhar, F.S. Surgical stress-induced immune cell redistribution profiles predict short-term and long-term postsurgical recovery. A prospective study. J. Bone Joint Surg. Am. 2009, 91, 2783–2794. [Google Scholar] [CrossRef]
- Xiao, W.; Mindrinos, M.N.; Seok, J.; Cuschieri, J.; Cuenca, A.G.; Gao, H.; Hayden, D.L.; Hennessy, L.; Moore, E.E.; Minei, J.P.; et al. A genomic storm in critically injured humans. J. Exp. Med. 2011, 208, 2581–2590. [Google Scholar] [CrossRef]
- Allen, C.J.; Griswold, A.J.; Schulman, C.I.; Sleeman, D.; Levi, J.U.; Livingstone, A.S.; Proctor, K.G. Global Gene Expression Change Induced by Major Thoracoabdominal Surgery. Ann. Surg. 2017, 266, 981–987. [Google Scholar] [CrossRef]
- Gaudilliere, B.; Fragiadakis, G.K.; Bruggner, R.V.; Nicolau, M.; Finck, R.; Tingle, M.; Silva, J.; Ganio, E.A.; Yeh, C.G.; Maloney, W.J.; et al. Clinical recovery from surgery correlates with single-cell immune signatures. Sci. Transl. Med. 2014, 6, 255ra131. [Google Scholar] [CrossRef]
- Deiner, S.; Westlake, B.; Dutton, R.P. Patterns of surgical care and complications in elderly adults. J. Am. Geriatr. Soc. 2014, 62, 829–835. [Google Scholar] [CrossRef]
- Finlayson, E.; Wang, L.; Landefeld, C.S.; Dudley, R.A. Major abdominal surgery in nursing home residents: A national study. Ann. Surg. 2011, 254, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Gajdos, C.; Kile, D.; Hawn, M.T.; Finlayson, E.; Henderson, W.G.; Robinson, T.N. Advancing age and 30-day adverse outcomes after nonemergent general surgeries. J. Am. Geriatr. Soc. 2013, 61, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Segev, D.L.; Pronovost, P.J.; Syin, D.; Bandeen-Roche, K.; Patel, P.; Takenaga, R.; Devgan, L.; Holzmueller, C.G.; Tian, J.; et al. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010, 210, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.B.; Rosenthal, R.A.; Merkow, R.P.; Ko, C.Y.; Esnaola, N.F. Optimal preoperative assessment of the geriatric surgical patient: A best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J. Am. Coll. Surg. 2012, 215, 453–466. [Google Scholar] [CrossRef]
- Finlayson, E.; Zhao, S.; Boscardin, W.J.; Fries, B.E.; Landefeld, C.S.; Dudley, R.A. Functional status after colon cancer surgery in elderly nursing home residents. J. Am. Geriatr. Soc. 2012, 60, 967–973. [Google Scholar] [CrossRef]
- Fragiadakis, G.K.; Gaudillière, B.; Ganio, E.A.; Aghaeepour, N.; Tingle, M.; Nolan, G.P.; Angst, M.S. Patient-specific Immune States before Surgery Are Strong Correlates of Surgical Recovery. Anesthesiology 2015, 123, 1241–1255. [Google Scholar] [CrossRef]
- Culley, D.J.; Flaherty, D.; Fahey, M.C.; Rudolph, J.L.; Javedan, H.; Huang, C.C.; Wright, J.; Bader, A.M.; Hyman, B.T.; Blacker, D.; et al. Poor Performance on a Preoperative Cognitive Screening Test Predicts Postoperative Complications in Older Orthopedic Surgical Patients. Anesthesiology 2017, 127, 765–774. [Google Scholar] [CrossRef]
- Susano, M.J.; Grasfield, R.H.; Friese, M.; Rosner, B.; Crosby, G.; Bader, A.M.; Kang, J.D.; Smith, T.R.; Lu, Y.; Groff, M.W.; et al. Brief Preoperative Screening for Frailty and Cognitive Impairment Predicts Delirium after Spine Surgery. Anesthesiology 2020, 133, 1184–1191. [Google Scholar] [CrossRef]
- Gomez, C.R.; Boehmer, E.D.; Kovacs, E.J. The aging innate immune system. Curr. Opin. Immunol. 2005, 17, 457–462. [Google Scholar] [CrossRef]
- Arnardottir, H.H.; Dalli, J.; Colas, R.A.; Shinohara, M.; Serhan, C.N. Aging delays resolution of acute inflammation in mice: Reprogramming the host response with novel nano-proresolving medicines. J. Immunol. 2014, 193, 4235–4244. [Google Scholar] [CrossRef]
- Mahbub, S.; Brubaker, A.L.; Kovacs, E.J. Aging of the Innate Immune System: An Update. Curr. Immunol. Rev. 2011, 7, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Nikolich-Zugich, J. The twilight of immunity: Emerging concepts in aging of the immune system. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Shih, L.; Guler, N.; Syed, D.; Hopkinson, W.; McComas, K.N.; Walborn, A.; Hoppensteadt, D.; Fareed, J.; Rondina, M.T. Postoperative Changes in the Systemic Inflammatory Milieu in Older Surgical Patients. Clin. Appl. Thromb. Hemost. 2018, 24, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Stervbo, U.; Meier, S.; Mälzer, J.N.; Baron, U.; Bozzetti, C.; Jürchott, K.; Nienen, M.; Olek, S.; Rachwalik, D.; Schulz, A.R.; et al. Effects of aging on human leukocytes (part I): Immunophenotyping of innate immune cells. Age 2015, 37, 92. [Google Scholar] [CrossRef]
- Seidler, S.; Zimmermann, H.W.; Bartneck, M.; Trautwein, C.; Tacke, F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010, 11, 30. [Google Scholar] [CrossRef]
- Nyugen, J.; Agrawal, S.; Gollapudi, S.; Gupta, S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J. Clin. Immunol. 2010, 30, 806–813. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef]
- Solana, R.; Tarazona, R.; Gayoso, I.; Lesur, O.; Dupuis, G.; Fülöp, T. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Semin. Immunol. 2012, 24, 331–341. [Google Scholar] [CrossRef]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Sutherland, A.G.; Cook, A.; Miller, C.; Duncan, L.; Yuecel, R.; Heys, S.D.; Hutchison, J.D.; Liversidge, J. Older Patients Are Immunocompromised by Cytokine Depletion and Loss of Innate Immune Function After HIP Fracture Surgery. Geriatr. Orthop. Surg. Rehabil. 2015, 6, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kolseth, I.B.; Forland, D.T.; Risoe, P.K.; Flood-Kjeldsen, S.; Agren, J.; Reseland, J.E.; Lyngstadaas, S.P.; Johnson, E.; Dahle, M.K. Human monocyte responses to lipopolysaccharide and 9-cis retinoic acid after laparoscopic surgery for colon cancer. Scand. J. Clin. Lab. Investig. 2012, 72, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, I.R.; Hess, A.; Fuchs, A.; Frazier, E.P.; Ghosh, S.; Hughes, S.W.; Kelly, M.P. Cellular immunophenotype of major spine surgery in adults. Spine Deform. 2022, 10, 1375–1384. [Google Scholar] [CrossRef]
- Saresella, M.; Marventano, I.; Calabrese, E.; Piancone, F.; Rainone, V.; Gatti, A.; Alberoni, M.; Nemni, R.; Clerici, M. A complex proinflammatory role for peripheral monocytes in Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 38, 403–413. [Google Scholar] [CrossRef]
- Wohleb, E.S.; McKim, D.B.; Sheridan, J.F.; Godbout, J.P. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 2015, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, N.; Walker, S.; Galandiuk, S.; Gardner, S.; Polk, H.C. The Significance and Challenges of Monocyte Impairment: For the Ill Patient and the Surgeon. Surg. Infect. 2016, 17, 303–312. [Google Scholar] [CrossRef]
- He, H.J.; Wang, Y.; Le, Y.; Duan, K.M.; Yan, X.B.; Liao, Q.; Liao, Y.; Tong, J.B.; Terrando, N.; Ouyang, W. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci. Ther. 2012, 18, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, C.; Le, T.; Swain, M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 2009, 29, 2089–2102. [Google Scholar] [CrossRef]
- D’Mello, C.; Swain, M.G. Liver-brain inflammation axis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G749–G761. [Google Scholar] [CrossRef]
- Vacas, S.; Degos, V.; Tracey, K.J.; Maze, M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology 2014, 120, 1160–1167. [Google Scholar] [CrossRef]
- Degos, V.; Vacas, S.; Han, Z.; Van Rooijen, N.; Gressens, P.; Su, H.; Young, W.L.; Maze, M. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology 2013, 118, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef]
- Spies, C.; Luetz, A.; Lachmann, G.; Renius, M.; von Haefen, C.; Wernecke, K.D.; Bahra, M.; Schiemann, A.; Paupers, M.; Meisel, C. Influence of Granulocyte-Macrophage Colony-Stimulating Factor or Influenza Vaccination on HLA-DR, Infection and Delirium Days in Immunosuppressed Surgical Patients: Double Blind, Randomised Controlled Trial. PLoS ONE 2015, 10, e0144003. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.E.; Yamada, K.J.; Fallet, R.; Odvody, J.; Schwarz, D.M.; Lyden, E.R.; Anderson, M.J.; Alter, R.; Vidlak, D.; Hartman, C.W.; et al. Orthopaedic Surgery Elicits a Systemic Anti-Inflammatory Signature. J. Clin. Med. 2020, 9, 2123. [Google Scholar] [CrossRef] [PubMed]
- Torrance, H.D.T.; Longbottom, E.R.; Vivian, M.E.; Lalabekyan, B.; Abbott, T.E.F.; Ackland, G.L.; Hinds, C.J.; Pearse, R.M.; O’Dwyer, M.J. Post-operative immune suppression is mediated via reversible, Interleukin-10 dependent pathways in circulating monocytes following major abdominal surgery. PLoS ONE 2018, 13, e0203795. [Google Scholar] [CrossRef] [PubMed]
- Bocsi, J.; Hanzka, M.C.; Osmancik, P.; Hambsch, J.; Dahnert, I.; Sack, U.; Bellinghausen, W.; Schneider, P.; Janousek, J.; Kostelka, M.; et al. Modulation of the cellular and humoral immune response to pediatric open heart surgery by methylprednisolone. Cytom. B Clin. Cytom. 2011, 80, 212–220. [Google Scholar] [CrossRef]
- Forsberg, A.; Cervenka, S.; Jonsson Fagerlund, M.; Rasmussen, L.S.; Zetterberg, H.; Erlandsson Harris, H.; Stridh, P.; Christensson, E.; Granstrom, A.; Schening, A.; et al. The immune response of the human brain to abdominal surgery. Ann. Neurol. 2017, 81, 572–582. [Google Scholar] [CrossRef]
- Majai, G.; Sarang, Z.; Csomos, K.; Zahuczky, G.; Fesus, L. PPARgamma-dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur. J. Immunol. 2007, 37, 1343–1354. [Google Scholar] [CrossRef]
- Cai, W.; Yang, T.; Liu, H.; Han, L.; Zhang, K.; Hu, X.; Zhang, X.; Yin, K.J.; Gao, Y.; Bennett, M.V.L.; et al. Peroxisome proliferator-activated receptor gamma (PPARgamma): A master gatekeeper in CNS injury and repair. Prog. Neurobiol. 2018, 163–164, 27–58. [Google Scholar] [CrossRef]
- Feng, M.-J.; Ning, W.-B.; Wang, W.; Lv, Z.-H.; Liu, X.-B.; Zhu, Y.; Gao, W.; Jin, H.-Z.; Gao, S.-S. Serum S100A12 as a prognostic biomarker of severe traumatic brain injury. Clin. Chim. Acta 2018, 480, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chibnik, L.B.; Shulman, J.M.; Leurgans, S.E.; Schneider, J.A.; Wilson, R.S.; Tran, D.; Aubin, C.; Buchman, A.S.; Heward, C.B.; Myers, A.J.; et al. CR1 is associated with amyloid plaque burden and age-related cognitive decline. Ann. Neurol. 2011, 69, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.I.; Chu, S.; Pierce, A.L.; Brubaker, W.D.; Hauhart, R.E.; Mastroeni, D.; Clarke, E.V.; Rogers, J.; Atkinson, J.P.; Tenner, A.J. Analysis of the Putative Role of CR1 in Alzheimer’s Disease: Genetic Association, Expression and Function. PLoS ONE 2016, 11, e0149792. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, L.E.; Jukema, J.W.; Mooijaart, S.P.; de Vries, M.A.; Stott, D.J.; Castro Cabezas, M.; Trompet, S. Association of complement receptor 1 gene polymorphisms with cognitive function. Physiol. Genom. 2018, 50, 102–103. [Google Scholar] [CrossRef]
- Wild, A.B.; Krzyzak, L.; Peckert, K.; Stich, L.; Kuhnt, C.; Butterhof, A.; Seitz, C.; Mattner, J.; Grüner, N.; Gänsbauer, M.; et al. CD83 orchestrates immunity toward self and non-self in dendritic cells. JCI Insight 2019, 4, 735. [Google Scholar] [CrossRef]
- Oren, R.L.; Kim, E.J.; Leonard, A.K.; Rosner, B.; Chibnik, L.B.; Das, S.; Grodstein, F.; Crosby, G.; Culley, D.J. Age-dependent differences and similarities in the plasma proteomic signature of postoperative delirium. Sci. Rep. 2023, 13, 7431. [Google Scholar] [CrossRef]
- Nazarov, P.V.; Muller, A.; Kaoma, T.; Nicot, N.; Maximo, C.; Birembaut, P.; Tran, N.L.; Dittmar, G.; Vallar, L. RNA sequencing and transcriptome arrays analyses show opposing results for alternative splicing in patient derived samples. BMC Genom. 2017, 18, 443. [Google Scholar] [CrossRef]
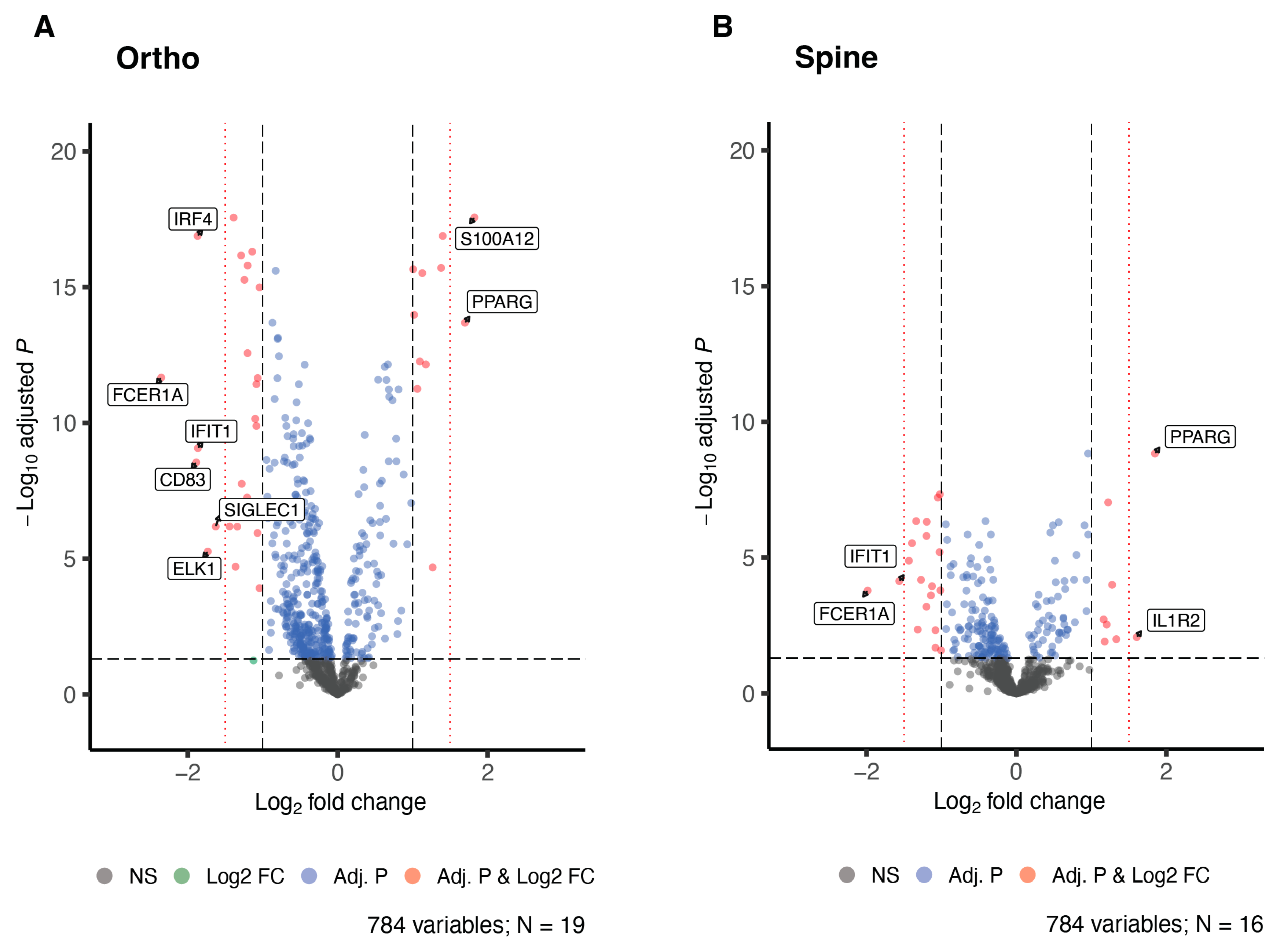
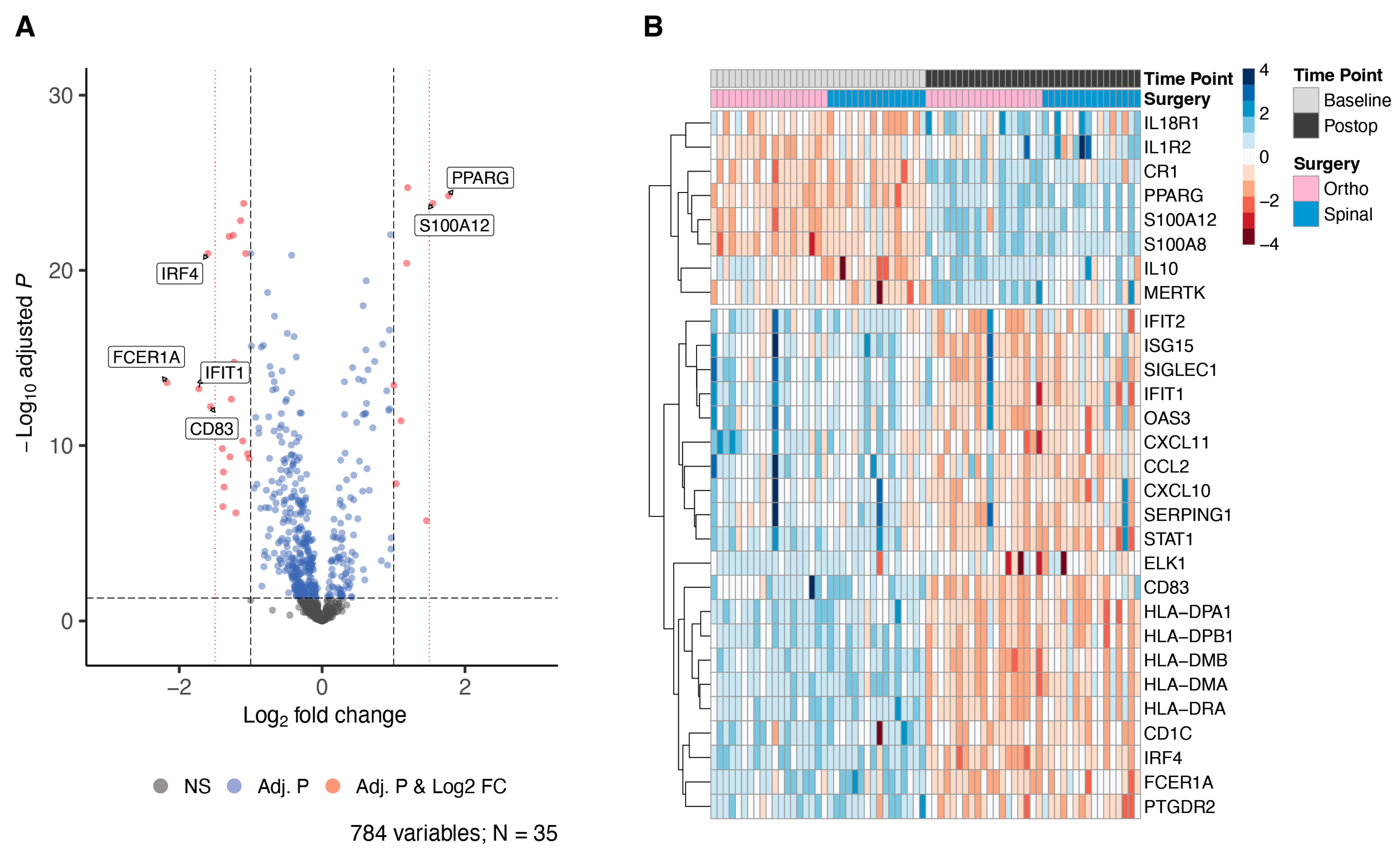
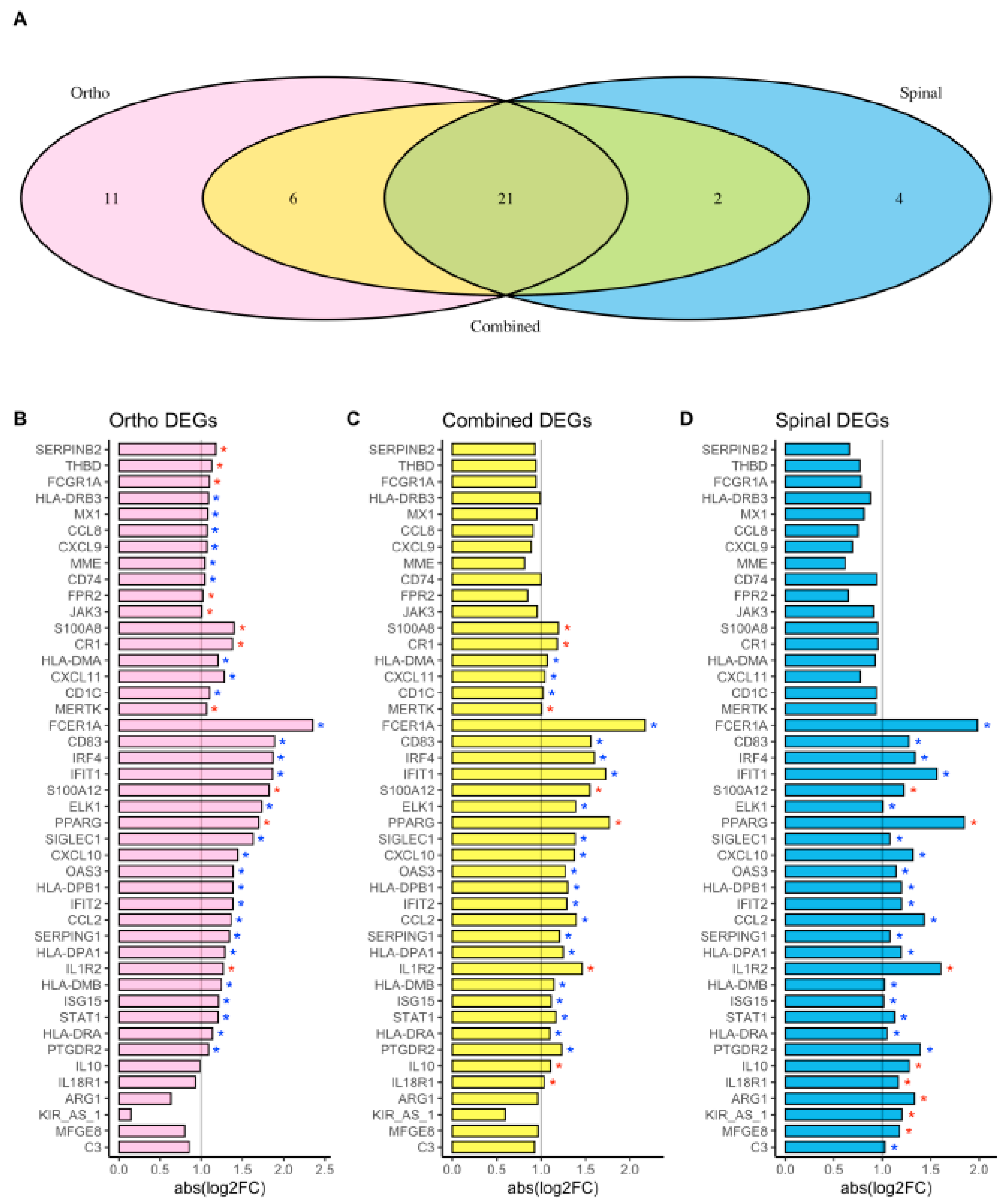
| Hip or Knee Replacement | Spinal Surgery | Combined Analysis | ||||
|---|---|---|---|---|---|---|
| BH-adj. p-val | log2FC | BH-adj. p-val | log2FC | BH-adj. p-val | log2FC | |
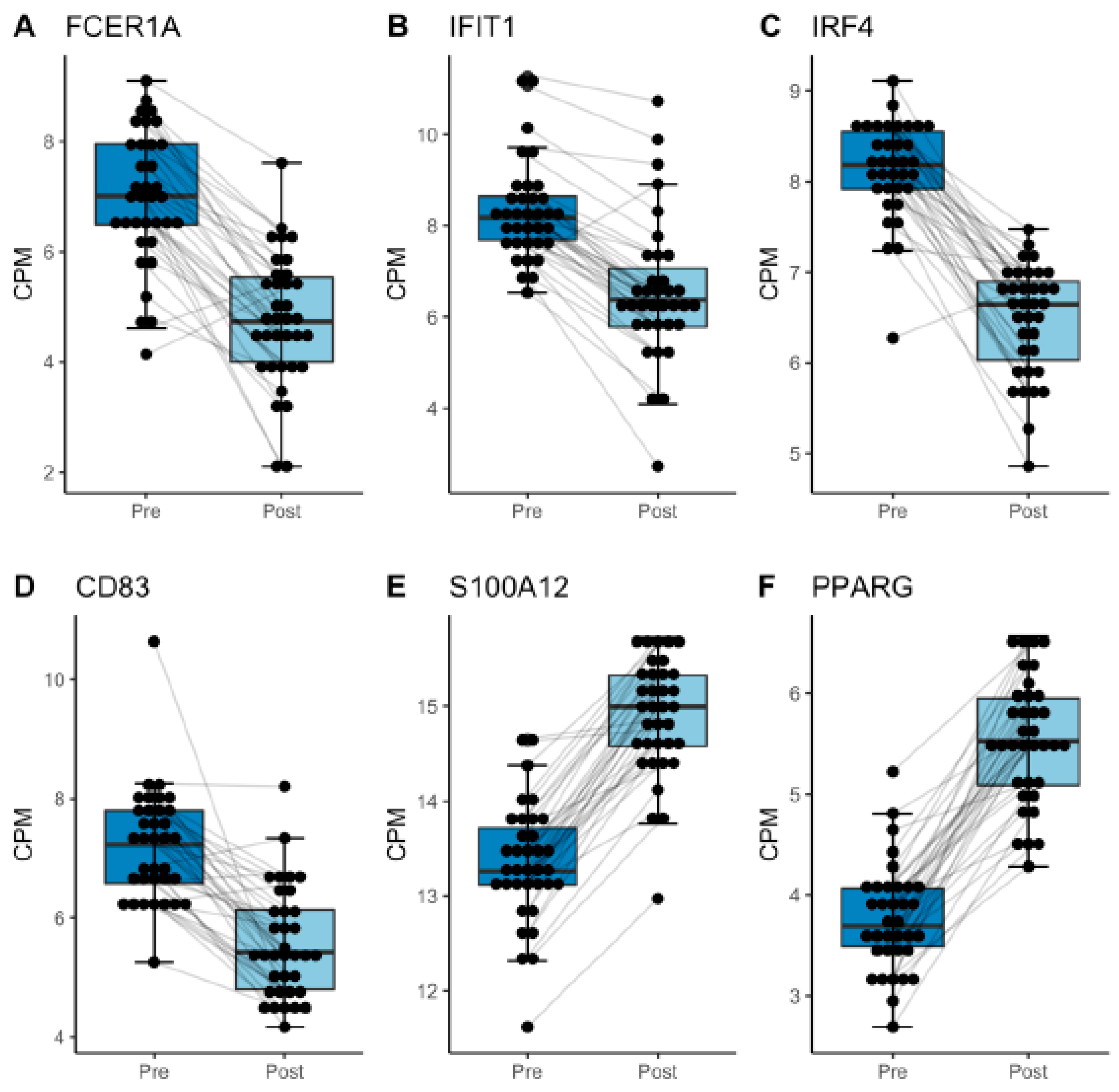
| FCER1A | 2.13 × 10−12 | −2.35 | 1.64 × 10−4 | −1.98 | 2.54 × 10−14 | −2.17 |
| CD83 | 2.83 × 10−9 | −1.89 | 6.58 × 10−5 | −1.27 | 5.94 × 10−13 | −1.56 |
| IRF4 | 1.30 × 10−17 | −1.87 | 4.53 × 10−7 | −1.34 | 1.08 × 10−21 | −1.6 |
| IFIT1 | 8.52 × 10−10 | −1.86 | 7.32 × 10−5 | −1.56 | 5.67 × 10−14 | −1.72 |
| S100A12 | 2.70 × 10−18 | 1.82 | 9.25 × 10−8 | 1.22 | 1.49 × 10−24 | 1.55 |
| ELK1 | 5.44 × 10−6 | −1.73 | 2.60 × 10−2 | −1.01 | 3.10 × 10−7 | −1.39 |
| PPARG | 2.04 × 10−14 | 1.7 | 1.46 × 10−9 | 1.85 | 5.56 × 10−25 | 1.77 |
| SIGLEC1 | 6.55 × 10−7 | −1.63 | 4.72 × 10−3 | −1.08 | 3.24 × 10−9 | −1.38 |
| CXCL10 | 6.51 × 10−7 | −1.44 | 4.46 × 10−3 | −1.32 | 2.33 × 10−8 | −1.37 |
| OAS3 | 1.53 × 10−10 | −1.39 | 2.46 × 10−4 | −1.14 | 2.24 × 10−13 | −1.27 |
| HLA-DPB1 | 2.74 × 10−18 | −1.39 | 1.59 × 10−6 | −1.2 | 1.17 × 10−22 | −1.3 |
| IFIT2 | 2.13 × 10−7 | −1.39 | 6.46 × 10−4 | −1.2 | 4.43 × 10−10 | −1.29 |
| CCL2 | 1.98 × 10−5 | −1.36 | 1.30 × 10−5 | −1.43 | 1.48 × 10−10 | −1.4 |
| SERPING1 | 6.62 × 10−7 | −1.34 | 2.08 × 10−2 | −1.08 | 6.94 × 10−7 | −1.21 |
| HLA-DPA1 | 6.79 × 10−17 | −1.29 | 4.80 × 10−7 | −1.2 | 9.90 × 10−23 | −1.25 |
| IL1R2 | 2.10 × 10−5 | 1.27 | 8.50 × 10−3 | 1.6 | 1.94 × 10−6 | 1.46 |
| HLA-DMB | 5.36 × 10−16 | −1.24 | 4.63 × 10−8 | −1.02 | 1.44 × 10−23 | −1.14 |
| ISG15 | 5.60 × 10−8 | −1.21 | 1.60 × 10−4 | −1.01 | 5.51 × 10−11 | −1.11 |
| STAT1 | 2.69 × 10−13 | −1.2 | 1.13 × 10−4 | −1.13 | 5.57 × 10−15 | −1.17 |
| HLA-DRA | 4.94 × 10−17 | −1.14 | 6.12 × 10−8 | −1.05 | 1.49 × 10−24 | −1.1 |
| PTGDR2 | 1.29 × 10−10 | −1.08 | 2.94 × 10−6 | −1.39 | 1.75 × 10−15 | −1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oren, R.L.; Grasfield, R.H.; Friese, M.B.; Chibnik, L.B.; Chi, J.H.; Groff, M.W.; Kang, J.D.; Xie, Z.; Culley, D.J.; Crosby, G. Geriatric Surgery Produces a Hypoactive Molecular Phenotype in the Monocyte Immune Gene Transcriptome. J. Clin. Med. 2023, 12, 6271. https://doi.org/10.3390/jcm12196271
Oren RL, Grasfield RH, Friese MB, Chibnik LB, Chi JH, Groff MW, Kang JD, Xie Z, Culley DJ, Crosby G. Geriatric Surgery Produces a Hypoactive Molecular Phenotype in the Monocyte Immune Gene Transcriptome. Journal of Clinical Medicine. 2023; 12(19):6271. https://doi.org/10.3390/jcm12196271
Chicago/Turabian StyleOren, Rachel L., Rachel H. Grasfield, Matthew B. Friese, Lori B. Chibnik, John H. Chi, Michael W. Groff, James D. Kang, Zhongcong Xie, Deborah J. Culley, and Gregory Crosby. 2023. "Geriatric Surgery Produces a Hypoactive Molecular Phenotype in the Monocyte Immune Gene Transcriptome" Journal of Clinical Medicine 12, no. 19: 6271. https://doi.org/10.3390/jcm12196271
APA StyleOren, R. L., Grasfield, R. H., Friese, M. B., Chibnik, L. B., Chi, J. H., Groff, M. W., Kang, J. D., Xie, Z., Culley, D. J., & Crosby, G. (2023). Geriatric Surgery Produces a Hypoactive Molecular Phenotype in the Monocyte Immune Gene Transcriptome. Journal of Clinical Medicine, 12(19), 6271. https://doi.org/10.3390/jcm12196271





