“De Novo” Psoriasis and Relapse of Psoriasis Induced by Dupilumab: Three New Cases and Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Literature Search
3. Results
3.1. Patients
3.2. Literature Search (Table 1, Table 2, Table 3 and Table 4)
| References | N° Cases | Age and Gender (Years) | Duration, (Month) | Type | Localization | Histology | Dupilumab Interruption | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Safa G, 2019 [6] | 1 | 55, M | 2 | Plaque | Trunk | Yes | No | Topical steroids | Improvement |
| Fowler E, 2019 [7] | 2 | 54, F | 4 | Plaque | Upper and lower extremities, chest, back, neck, abdomen, soles and palms | Yes | Yes | NA | Improvement |
| / | 49, F | 18 | Plaque | Bilateral upper and lower extremities, nails | NA | No | Topical steroids | Improvement | |
| Stout M, 2019 [8] | 1 | 59, F | 1 | Plaque | Bilateral upper and lower extremities | Yes | Yes | Topical steroids | Improvement |
| Gori N, 2019 [9] | 1 | 40, F | 3.5 | Guttate | Trunk and extremities | Yes | No | Topical calcipotriol and steroid | Improvement |
| Varma A, 2020 [10] | 1 | 73, M | 1 | Plaque | Bilateral upper extremities and right lower extremities | NA | NA | NA | NA |
| Schrom KP, 2020 [11] | 1 | 80, M | 2.5 | Plaque | Trunk, upper extremities and lower extremities | Yes | Yes | NB-UVB | Improvement |
| Ferrucci SM, 2020 [12] | 1 | 42, M | 3 | Plaque | Trunk and lower extremities | Yes | Yes | Topical calcipotriol and steroids | Improvement |
| Kim HS, 2020 [13] | 1 | 36, F | 5 | Plaque | Lower extremities | Yes | Yes | Topical steroids | Recurrence |
| Gambichler T, 2020 [14] | 1 | 59, M | 1 | Erythrodermic | Upper and lower extremities | Yes | Yes | NA | NA |
| Matsuda T, 2020 [15] | 1 | 60, M | 3.5 | Plaque | Upper and lower extremities, face | Yes | Yes | Topical steroids | Improvement |
| DeGrazia TM, 2020 [16] | 3 | 32, F | 1 | Plaque | Scalp, bilateral inguinal folds | NA | No | Topical steroids | NA |
| / | 67, M | 2 | Plaque | Scalp | NA | No | Topical steroids | NA | |
| / | 57, F | 9 | Plaque | Scalp | NA | No | Topical steroids | NA | |
| D’ambra I, 2020 [17] | 3 | 61, F | 1 | Guttate | Upper and lower extremities and trunk | NA | No | Topical steroids | Improvement |
| / | 56, F | 2 | Plaque | Upper extremities and scalp | Yes | No | Topical calcipotriol and steroids | Improvement | |
| / | 39, M | 1 | Plaque | Soles | NA | No | Topical steroids | Improvement | |
| Senner S, 2020 [18] | 1 | 40, M | 1.5 | Plaque | Lower extremities and palms | Yes | Yes | Topical steroids, oral steroids, cyclosporine | Improvement |
| Al-Janabi A, 2020 [19] | 1 | 72, M | 4 | Seborrheic | Scalp, face and ears | NA | No | Topical steroids | Improvement |
| Gallo R, 2020 [20] | 1 | 24, M | 1.5 | Reverse | Bilateral inguinal folds and scalp | No | Yes | Topical steroids and cyclosporine | Improvement |
| Mirza FN, 2021 [21] | 1 | 92, M | 8 | Plaque | Upper and lower extremities | Yes | No | Mycophenolate mofetil | Improvement |
| Beaziz J, 2021 [22] | 1 | 45, F | 12 | Plaque | Scalp | NA | No | Topical steroids | Improvement |
| Maiolini VM, 2021 [23] | 1 | 22, M | 5 | Plaque | Scalp, left upper extremity, nails | Yes | NA | Topical calcipotriol and steroid | Improvement |
| Russo F, 2021 [24] | 1 | 68, F | 1 | Plaque | Upper extremities and buttocks | Yes | Yes | Oral steroids | Improvement |
| Kurihara K, 2021 [25] | 2 | 34, M | 30 | Plaque | Upper extremities and trunk | Yes | No | Topical steroids, tacrolimus ointment 0.1%, delgocitinib ointment | Improvement |
| / | 23, M | 18 | Plaque | Face | Yes | No | Topical steroids, tacrolimus ointment 0.1%, delgocitinib ointment | Improvement | |
| Park J, 2021 [26] | 1 | 24, M | 2 | Plaque | Palms and soles | Yes | No | Topical steroids | Improvement |
| Juan-Carpena G, 2021 [27] | 1 | 46, M | 24 | Erythrodermic | Lower extremities and trunk | Yes | No | Topical steroids, tacrolimus ointment 0.1% | Persistence |
| Flanagan KE, 2022 [28] | 1 | 28, F | 5 | Plaque | Scalp and abdomen | NA | Yes | Topical steroids | Improvement |
| Fan J, 2022 [29] | 1 | 25, F | 2 | Inverse | Armpits | NA | Yes | Topical hormonal cream | Improvement |
| Incel Uysal P, 2022 [30] | 1 | 22, M | 4 | Pustular | Hands | Yes | Yes | Topical calcipotriol and steroids | Improvement |
| Jia X, 2022 [31] | 1 | 23, M | 2 | Pustular | Lower extremities | Yes | Yes | Topical steroids, cyclosporine | Improvement |
| Patruno C, 2022 [32] | 1 | 58, F | 5.5 | Plaque | Lower extremities, scalp, hands | Yes | Yes | Upadacitinib | Improvement |
| Zhong X, 2022 [33] | 1 | 51, F | 2 | Pustular | Upper extremities and trunk | Yes | Yes | Oral steroids | Improvement |
| Paolino G, 2022 [34] | 4 | 36, M | 25 | NA | NA | NA | No | Topical calcipotriol and steroids | Improvement |
| / | 36, F | 25 | NA | NA | NA | No | Topical calcipotriol and steroids | Improvement | |
| / | 36, F | 25 | NA | NA | NA | No | Topical calcipotriol and steroids | Improvement | |
| / | 36, F | 25 | NA | NA | NA | No | Topical calcipotriol and steroids | Improvement | |
| Casale F, 2022 [35] | 4 | 61, M | 10.5 | Plaque | Inferior extremities | Yes | Yes | Topical steroids | NA |
| / | 61, M | 10.5 | Plaque | Scalp | No | Yes | Topical steroids | NA | |
| / | 61, M | 10.5 | Plaque | Upper extremities | No | No | Topical steroids, cyclosporine | NA | |
| / | 61, F | 10.5 | Erythrodermic | NA | No | No | Cyclosporine | NA | |
| Grolleau C, 2023 [36] | 7 | 20, F | 1 | Plaque | NA | Yes | Yes | Topical steroids, baricitinib | Partial |
| / | 50, M | 4 | Pustular | NA | Yes | Yes | Topical steroids, methotrexate | Partial | |
| / | 25, F | 0.5 | Plaque, pustular | NA | Yes | Yes | Upadacitinib | NA | |
| / | 47, M | 6.5 | Plaque, pustular | NA | Yes | Yes | Upadacitinib | NA | |
| / | 39, M | 10 | Plaque | NA | Yes | No | NA | Partial | |
| / | 84, M | 1.5 | Plaque | NA | Yes | Yes | Methotrexate | Improvement | |
| / | 65, M | 2.5 | Pustular | NA | Yes | Yes | Methotrexate | Partial | |
| Napolitano M, 2023 [4] | 39 | 54, M (26) F (13) | 5 | NA | NA | Yes | Yes (10), No (29) | Topical steroids (26), NB-UVB (11), methotrexate (2) | Improvement (29), Worsening (6), Persistence (4) |
| Al Hawsawi K, 2023 [37] | 1 | 50, F | 1 | Plaque | Scalp | NA | Yes | Topical steroids | Recurrence |
| Chromy D, 2023 [38] | 4 | 30, M | 1 | Nummular | Upper and lower extremities | Yes | No | Topical calcipotriol and steroids | Recurrence |
| / | 48, F | 3.5 | Plaque | Upper and lower extremities, trunk, scalp, face | Yes | Yes | Topical calcipotriol and steroids and omalizumab | Improvement | |
| / | 36, M | 4.5 | Pustular | Face | No | No | NA | NA | |
| / | 82, M | 6 | Guttate | NA | Yes | No | Topical steroids | Improvement | |
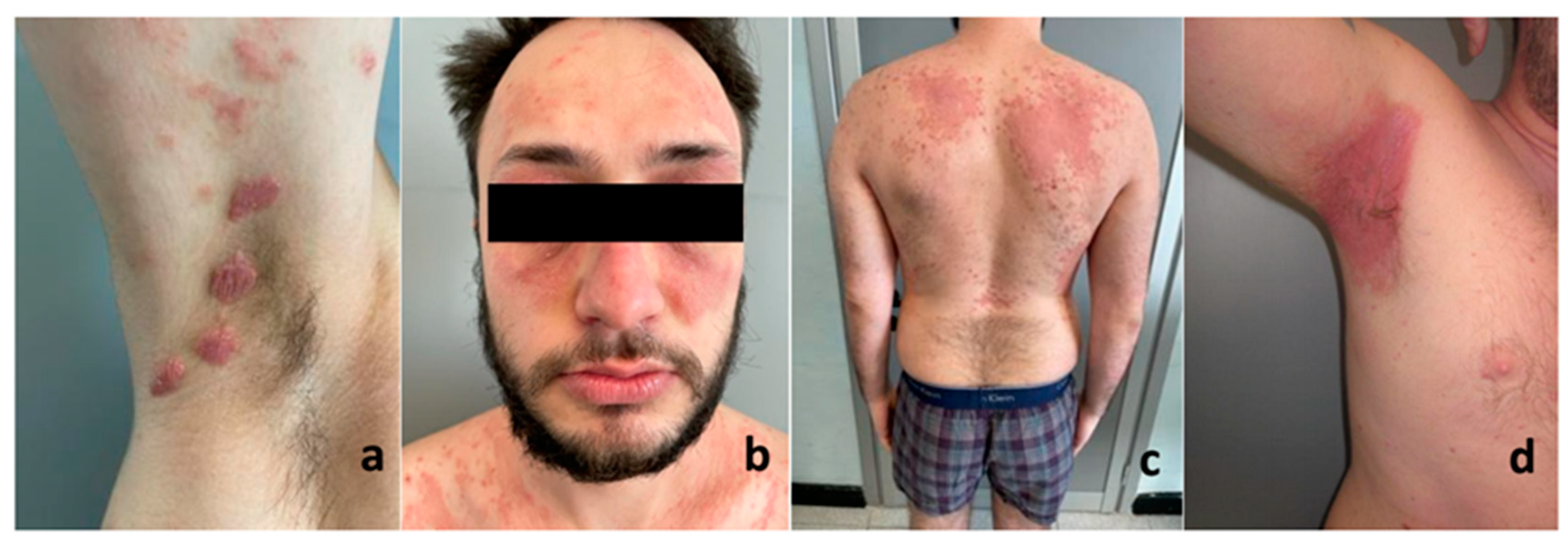
| Our case 3 | 1 | 25, M | 8 | Inverse | Armpits and scalp | No | Yes | Topical and systemic steroids and ciclosporine | Improvement |
| References | N° Cases | Age and Gender (Years) | Duration, (Month) | Type | Localization | Histology | Dupilumab Interruption | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Parker JJ, 2021 [39] | 4 | 4, M | 11 | Plaque and pustular | Lower extremities and trunk | No | Yes | Topical steroids, ustekinumab | Improvement |
| / | 14, F | 8 | Plaque | Face | No | No | Topical steroids | Improvement | |
| / | 12, F | 10 | Plaque | Lower extremities | No | No | Topical steroids | Improvement | |
| / | 16, M | 7 | Plaque | Lower extremities | No | No | Topical steroids | Improvement | |
| Park J, 2021 [26] | 1 | 17, M | 2 | Plaque | Palms and soles | Yes | No | Topical steroids, tacrolimus ointment 0.1% | Improvement |
| Ali K, 2022 [40] | 2 | 17, M | 5 | Plaque | Upper and lower extremities, back, face, scalp | Yes | Yes | Baricitinib | Improvement |
| / | 17, M | 5 | Plaque | Upper and lower extremities, back, face, scalp | Yes | Yes | Baricitinib | Improvement | |
| Colonna C, 2022 [41] | 1 | 9, M | 3 | Plaque | Upper extremities, trunk | Yes | NA | Topical steroids | Improvement |
| Our case 1 | 1 | 16, M | 1 | Plaque | Upper extremities, face and scalp | No | Yes | Topical steroids and cyclosporine | Improvement |
| References | N° Cases | Age and Gender (Years) | Duration, (Month) | Type | Localization | Histology | Dupilumab Interruption | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Tracey EH, 2018 [42] | 1 | 50, F | 2 | Erythrodermic | Upper and lower extremities, trunk and scalp | Yes | Yes | Topical steroids, methotrexate | Improvement |
| Dimitrov D, 2020 [42] | 1 | 35, M | 5 | Plaque | Upper and lower extremities | Yes | NA | NA | NA |
| Parker JJ, 2021 [39] | 2 | 18, M | 7 | Plaque | Scalp and face | NA | No | Topical steroids | Improvement |
| / | 18, F | 6 | Plaque | Upper extremities, scalp and trunk | NA | No | Topical steroids and pimecrolimus 1% cream | Improvement | |
| Casale F, 2022 [35] | 6 | 66, M (1), F (5) | 5 | Erythrodermic (2), plaque (5), guttate (1) | Scalp (2), face (1), upper extremities (5), inferior extremities (2), trunk (1), soles and palms (1) | Yes | Yes (2), No (4) | Topical steroids (6) and omalizumab (5) | NA |
| Our case 2 | 1 | 25, M | 1 | Plaque | Upper extremities, trunk | No | Yes | Topical steroids and methorexate | Improvement |
| References | N° Cases | Age and Gender (Years) | Duration, (Month) | Type | Localization | Histology | Dupilumab Interruption | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Parker JJ, 2021 [39] | 1 | 9, F | 3 | Plaque | Upper extremities | No | No | Topical steroids | Improvement |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cunliffe, A.; Gran, S.; Ali, U.; Grindlay, D.; Lax, S.J.; Williams, H.C.; Burden-Teh, E. Can atopic eczema and psoriasis coexist? A systematic review and meta-analysis. Skin Health Dis. 2021, 1, e29. [Google Scholar] [CrossRef] [PubMed]
- Guenova, E.; Skabytska, Y.; Hoetzenecker, W.; Weindl, G.; Sauer, K.; Tham, M.; Kim, K.W.; Park, J.H.; Seo, J.H.; Ignatova, D.; et al. IL-4 abrogates T(H)17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2015, 112, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Fabbrocini, G.; Patruno, C. Dupilumab-associated cutaneous adverse events among adult patients with atopic dermatitis: A retrospective study. J. Dermatol. 2023, 50, 880–887. [Google Scholar] [CrossRef]
- Tracey, E.H.; Elston, C.; Feasel, P.; Piliang, M.; Michael, M.; Vij, A. Erythrodermic presentation of psoriasis in a patient treated with dupilumab. JAAD Case Rep. 2018, 4, 708–710. [Google Scholar] [CrossRef]
- Safa, G.; Paumier, V. Psoriasis induced by dupilumab therapy. Clin. Exp. Dermatol. 2019, 44, e49–e50. [Google Scholar] [CrossRef]
- Fowler, E.; Silverberg, J.I.; Fox, J.D.; Yosipovitch, G. Psoriasiform Dermatitis After Initiation of Treatment with Dupilumab for Atopic Dermatitis. Dermatitis 2019, 30, 234–236. [Google Scholar] [CrossRef]
- Stout, M.; Guitart, J.; Tan, T.; Silverberg, J.I. Psoriasis-like Dermatitis Developing in a Patient with Atopic Dermatitis Treated with Dupilumab. Dermatitis 2019, 30, 376–378. [Google Scholar] [CrossRef]
- Gori, N.; Caldarola, G.; Pirro, F.; De Simone, C.; Peris, K. A case of guttate psoriasis during treatment with dupilumab. Dermatol. Ther. 2019, 32, e12998. [Google Scholar] [CrossRef]
- Varma, A.; Levitt, J. Dupilumab-induced phenotype switching from atopic dermatitis to psoriasis. JAAD Case Rep. 2020, 6, 217–218. [Google Scholar] [CrossRef]
- Schrom, K.P.; Kobs, A.; Nedorost, S. Clinical Psoriasiform Dermatitis Following Dupilumab Use for Autoeczematization Secondary to Chronic Stasis Dermatitis. Cureus 2020, 12, e7831. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, S.M.; Buffon, S.; Marzano, A.V.; Maronese, C.A. Phenotypic switch from atopic dermatitis to psoriasis during treatment with upadacitinib. Clin. Exp. Dermatol. 2022, 47, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Yeung, J. Psoriasis appearing after dupilumab therapy in atopic dermatitis: A case report. SAGE Open Med. Case Rep. 2020, 8, 2050313X20940458. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Reuther, J.; Richards, C.; Doerler, M. Dupilumab-induced psoriatic erythroderma with characteristic cytokine and antimicrobial peptide expression pattern in a patient with long standing atopic dermatitis. Int. J. Dermatol. 2020, 59, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Yamada, H.; Hida, N.; Nakaizumi, T.; Yamada, E.; Satoh, H.; Hizawa, N. An asthmatic case of psoriasiform eruption caused by administration of dupilumab. Allergol. Int. 2020, 69, 478–479. [Google Scholar] [CrossRef]
- DeGrazia, T.M.; Raji, K.; Alshamekh, S.; Chisolm, S. Psoriatic Plaques After Initiation of Dupilumab Therapy. Dermatitis 2020, 31, e36–e37. [Google Scholar] [CrossRef]
- D’Ambra, I.; Babino, G.; Fulgione, E.; Calabrese, G.; Ronchi, A.; Alfano, R.; Argenziano, G.; Piccolo, V. Psoriasis onset under dupilumab treatment in two patients affected by atopic dermatitis and one patient affected by alopecia areata: Clinical and dermoscopic patterns. Dermatol. Ther. 2020, 33, e14169. [Google Scholar] [CrossRef]
- Senner, S.; Eicher, L.; Aszodi, N.; Prinz, J.C.; French, L.E.; Wollenberg, A. Psoriasis bei Dupilumab-behandeltem atopischem Ekzem [Psoriasis in dupilumab-treated atopic dermatitis]. Hautarzt 2020, 71, 383–386. [Google Scholar] [CrossRef]
- Al-Janabi, A.; Marsland, A.M. Seborrhoeic dermatitis and sebopsoriasis developing in patients on dupilumab: Two case reports. Clin. Case Rep. 2020, 8, 1458–1460. [Google Scholar] [CrossRef]
- Gallo, R.; Trave, I.; Parodi, A. Massive Acute Alopecia of the Scalp in a Patient Treated with Dupilumab. Acta Derm. Venereol. 2020, 100, adv00191. [Google Scholar] [CrossRef]
- Mirza, F.N.; Wang, A.; Ramachandran, S.M.; Damsky, W.; Cohen, J.M. Dupilumab-induced phenotype switch from atopic dermatitis to psoriasis is characterized by de novo interleukin-17A expression: A case report. Br. J. Dermatol. 2021, 185, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Beaziz, J.; Bouaziz, J.D.; Jachiet, M.; Fite, C.; Lons-Danic, D. Dupilumab-induced psoriasis and alopecia areata: Case report and review of the literature. Ann. Dermatol. Venereol. 2021, 148, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Maiolini, V.M.; Sousa, N.A.; Marsillac, P.F.; Bressan, A.L. Alopecia areata-like and psoriasis after dupilumab use for atopic dermatitis. Annu. Bras. Dermatol. 2021, 96, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Provvidenziale, L.; Bruzziches, F.; Fiorani, D.; Santi, F.; Lamberti, A.; Lazzeri, L.; Flori, M.L.; Rubegni, P. Psoriasis-like Eruption triggered by Dupilumab Therapy. Dermatitis 2021, 32, e147–e148. [Google Scholar] [CrossRef]
- Kurihara, K.; Fujiyama, T.; Tokura, Y.; Honda, T. Two cases of psoriasiform dermatitis arising during dupilumab therapy and successfully treated with delgocitinib ointment. Eur. J. Dermatol. 2021, 31, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Park, S.J.; Koh, Y.G.; Park, K.Y.; Seo, S.J. Dupilumab-Induced Palmoplantar Psoriasis: Is It True Psoriasis? Dermatitis 2022, 33, e33–e35. [Google Scholar] [CrossRef]
- Juan-Carpena, G.; Palazón-Cabanes, J.C.; Bañuls, J.; Docampo-Simón, A.; Sánchez-Pujol, M.J.; Niveiro, M.; Silvestre, J.F. Dupilumab-Induced Extrafacial Paradoxical Persistent Erythema in an Atopic Dermatitis Patient. Dermatitis 2021, 32, e83–e85. [Google Scholar] [CrossRef]
- Flanagan, K.E.; Pupo Wiss, I.M.; Pathoulas, J.T.; Walker, C.J.; Senna, M.M. Dupilumab-induced psoriasis in a patient with atopic dermatitis and alopecia totalis: A case report and literature review. Dermatol. Ther. 2022, 35, e15255. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, L.; Lu, Y.; Zhou, P. A case of dupilumab-induced reverse psoriasis in a patient with atopic dermatitis. Dermatol. Ther. 2022, 35, e15345. [Google Scholar] [CrossRef]
- Incel Uysal, P.; Gunhan, O. De novo pustular psoriasis associated with dupilumab therapy in a young male with the diagnosis of atopic dermatitis. Dermatol. Ther. 2022, 35, e15399. [Google Scholar] [CrossRef]
- Jia, X.; Li, C.; Wu, J.; Liu, Q. Pustular Psoriasis Appearing Induced by Dupilumab Therapy in a Patient with Atopic Dermatitis. J. Drugs Dermatol. 2022, 21, 311–312. [Google Scholar] [PubMed]
- Patruno, C.; Fabbrocini, G.; De Lucia, M.; Picone, V.; Genco, L.; Napolitano, M. Psoriasiform dermatitis induced by dupilumab successfully treated with upadacitinib. Dermatol. Ther. 2022, 35, e15788. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, Y.; Gao, Y.; Zhang, Y.; Lu, J.; Yu, N.; Ding, Y.; Shi, Y. Pustular psoriasis appearing in a Chinese woman treated with dupilumab for atopic dermatitis: A case report. Dermatol. Ther. 2022, 35, e15851. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Di Nicola, M.R.; Brianti, P.; Bianchi, V.G.; Mercuri, S.R. New onset atopic dermatitis and psoriasis in the same patients under biologic treatments: The role of systemic treatments as a possible trigger. Dermatol. Ther. 2022, 35, e15814. [Google Scholar] [CrossRef]
- Casale, F.; Nguyen, C.; Dobry, A.; Smith, J.; Mesinkovska, N.A. Dupilumab-associated psoriasis and psoriasiform dermatitis in patients with atopic dermatitis. Australas. J. Dermatol. 2022, 63, 394–397. [Google Scholar] [CrossRef]
- Grolleau, C.; Calugareanu, A.; Demouche, S.; Nosbaum, A.; Staumont-Sallé, D.; Aubert, H.; Cassius, C.; Jachiet, M.; Saussine, A.; Bagot, M.; et al. IL-4/IL-13 Inhibitors for Atopic Dermatitis Induce Psoriatic Rash Transcriptionally Close to Pustular Psoriasis. J. Investig. Dermatol. 2023, 143, 711–721.e7. [Google Scholar] [CrossRef]
- Al Hawsawi, K.; AlDoboke, A.W.; Alsulami, S.A.; Alamri, G.E.; Alsufi, R.F. Dupilumab-Induced Scalp Psoriasis in a Patient with Prurigo Nodularis: A Case Report. Cureus 2023, 15, e37992. [Google Scholar] [CrossRef]
- Chromy, D.; Bartosik, T.; Brkic, F.F.; Quint, T.; Tu, A.; Eckl-Dorna, J.; Schneider, S.; Bangert, C. Dupilumab-induced skin-associated side effects in patients with chronic rhinosinusitis with nasal polyposis. J. Dermatol. 2023, 50, 89–93. [Google Scholar] [CrossRef]
- Parker, J.J.; Sugarman, J.L.; Silverberg, N.B.; Gonzalez, M.E.; Ramien, M.L.; Teng, J.M.C.; Paller, A.S. Psoriasiform dermatitis during dupilumab treatment for moderate-to-severe atopic dermatitis in children. Pediatr. Dermatol. 2021, 38, 1500–1505. [Google Scholar] [CrossRef]
- Ali, K.; Wu, L.; Qiu, Y.; Li, M. Case report: Clinical and histopathological characteristics of psoriasiform erythema and de novo IL-17A cytokines expression on lesioned skin in atopic dermatitis children treated with dupilumab. Front. Med. 2022, 9, 932766. [Google Scholar] [CrossRef]
- Colonna, C.; Zussino, M.; Monzani, N.A.; Ponziani, A.; Cavalli, R. Treatment of children from 6 to 11 years old affected by moderate-severe atopic dermatitis with dupilumab: A first perspective from a single-center real-life experience in Italy. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e478–e480. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.; Al Adawi, M.; Abdelhadi, Z.; Jafferany, M. Exacerbation of psoriasis after initiation of dupilumab in atopic dermatitis patient. Dermatol. Ther. 2020, 33, e13572. [Google Scholar] [CrossRef] [PubMed]
- Jaulent, L.; Staumont-Sallé, D.; Tauber, M.; Paul, C.; Aubert, H.; Marchetti, A.; Sassolas, B.; Valois, A.; Nicolas, J.F.; Nosbaum, A.; et al. De novo psoriasis in atopic dermatitis patients treated with dupilumab: A retrospective cohort. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e296–e297. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Scalvenzi, M.; Fabbrocini, G.; Cinelli, E.; Patruno, C. Occurrence of psoriasiform eruption during dupilumab therapy for adult atopic dermatitis: A case series. Dermatol. Ther. 2019, 32, e13142. [Google Scholar] [CrossRef] [PubMed]
- Agache, I.; Akdis, C.; Jutel, M.; Virchow, J.C. Untangling asthma phenotypes and endotypes. Allergy 2012, 67, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Lötvall, J.; Akdis, C.A.; Bacharier, L.B.; Bjermer, L.; Casale, T.B.; Custovic, A.; Lemanske, R.F., Jr.; Wardlaw, A.J.; Wenzel, S.E.; Greenberger, P.A. Asthma endotypes: A new approach to classification of disease entities within the asthma syndrome. J. Allergy Clin. Immunol. 2011, 127, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef]
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Investig. Dermatol. 2008, 128, 2625–2630. [Google Scholar] [CrossRef]
- Salo, P.M.; Arbes, S.J., Jr.; Jaramillo, R.; Calatroni, A.; Weir, C.H.; Sever, M.L.; Hoppin, J.A.; Rose, K.M.; Liu, A.H.; Gergen, P.J.; et al. Prevalence of allergic sensitization in the United States: Results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J. Allergy Clin. Immunol. 2014, 134, 350–359. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Lowes, M.A.; Fuentes-Duculan, J.; Zaba, L.C.; Cardinale, I.; Nograles, K.E.; Khatcherian, A.; Novitskaya, I.; Carucci, J.A.; Bergman, R.; et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J. Immunol. 2008, 181, 7420–7427. [Google Scholar] [CrossRef]
- Prahalad, S. Atopy, autoimmunity, and the T(H)1/T(H)2 balance. J. Pediatr. 2000, 137, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, J.; Huh, Y.J.; Kim, K.; Chaparala, V.; Krueger, J.G.; Kim, J. Genomic profiling of the overlap phenotype between psoriasis and atopic dermatitis. J. Investig. Dermatol. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.; Zancanaro, P.; Casseres, R.; Abdat, R.; Dumont, N.; Rosmarin, D. Concomitant atopic dermatitis and psoriasis—A retrospective review. J. Dermatolog. Treat. 2021, 32, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Traidl, S.; Freimooser, S.; Werfel, T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol. Select. 2021, 5, 293–304. [Google Scholar] [CrossRef] [PubMed]

| Histology Features | “De Novo” PsO in Adults (71/93) | “De Novo” PsO in Pediatric Patients (4/8) | Relapsed PsO in Adults (8/10) |
|---|---|---|---|
| Parakeratosis | 22 | 4 | 1 |
| Intracorneal microabscesses | 16 | 0 | 1 |
| Elimination of granular layer | 11 | 3 | 1 |
| Lymphocytic infiltrate in the upper dermis | 11 | 1 | 0 |
| Psoriasiform hyperplasia | 6 | 2 | 2 |
| Hyperkeratosis | 8 | 2 | 0 |
| Acanthosis | 9 | 2 | 1 |
| Infiltrates of macrophages or neutrophils in dermis | 10 | 0 | 0 |
| Spongiosis | 10 | 0 | 1 |
| Dilated capillaries | 5 | 0 | 3 |
| Thinning of supra-papillary plates | 3 | 2 | 0 |
| Exocytosis lymphocytes | 5 | 0 | 0 |
| Rete ridge elongation | 3 | 1 | 0 |
| Perivascular infiltrates | 4 | 0 | 0 |
| “De Novo” Pso in Adults (n = 94) | “De Novo” Pso in Pediatric Patients (n = 9) | Relapses of Pso in Adults (n = 11) | Relapses of Pso in Pediatric Patients (n = 1) | |
|---|---|---|---|---|
| Sex | ||||
| M | 58 (61.7) | 7 (77.8) | 4 (36.4) | 0 (0) |
| F | 36 (38.3) | 2 (22.2) | 7 (63.6) | 1 (100) |
| Age (average) | 45.8 | 13.6 | 27.5 | 9 |
| Time to psoriasis presentation (average months) | 5.6 | 6.4 | 4.8 | 4.8 |
| Type of psoriasis | ||||
| Plaque | 35 (68.6) | 9 (100) | 10 (90.9) | 1 (100) |
| Pustular | 8 (15.7) | 1 (11.1) | 0 | 0 |
| Erythrodermic | 3 (5.9) | 0 | 3 (27.3) | 0 |
| Guttate | 3 (5.9) | 0 | 1 (9.1) | 0 |
| Inverse | 3 (5.9) | 0 | 0 | 0 |
| Nummular | 1 (1.9) | 0 | 0 | 0 |
| Localization | ||||
| Face and scalp | 18 (41.9) | 4 (44.4) | 6 (54.5) | 0 |
| Trunk | 14 (32.6) | 4 (44.4) | 4 (36.4) | 0 |
| Upper extremities | 21 (48.8) | 5 (55.6) | 10 (90.9) | 1 (100) |
| Lower extremities | 22 (51.2) | 6 (66.7) | 5 (45.5) | 0 |
| Histology | ||||
| Parakeratosis | 22 (30.9) | 4 (100) | 1 (12.5) | 0 |
| Intracorneal microabscesses | 16 (22.5) | 0 | 1 (12.5) | 0 |
| Elimination of granular layer | 11 (15.5) | 3 (75) | 1 (12.5) | 0 |
| Lymphocytic infiltrate in the upper dermis | 11 (15.5) | 1 (25) | 0 | 0 |
| Psoriasiform hyperplasia | 6 (8.5) | 2 (50) | 2 (25) | 0 |
| Hyperkeratosis | 8 (11.3) | 2 (50) | 0 | 0 |
| Acanthosis | 9 (12.7) | 2 (50) | 1 (12.5) | 0 |
| Infiltrates of macrophages or neutrophils in dermis | 10 (14.1) | 0 | 0 | 0 |
| Spongiosis | 10 (14.1) | 0 | 1 (12.5) | 0 |
| Dilated capillaries | 5 (7.0) | 0 | 3 (37.5) | 0 |
| Thinning of supra-papillary plates | 3 (4.2) | 2 (50) | 0 | 0 |
| Exocytosis lymphocytes | 5 (7.0) | 0 | 0 | 0 |
| Rete ridge elongation | 3 (4.2) | 1 (25) | 0 | 0 |
| Perivascular infiltrates | 4 (5.6) | 0 | 0 | 0 |
| Dupilumab interruption | 37 (40.2) | 4 (50) | 4 (36.4) | 0 |
| Remission of Pso | 64 (78.0) | 9 (100) | 4 (100) | 1 (100) |
| Management | 0 | |||
| Topical steroids | 54 (60.7) | 8 (88.9) | 5 (50.0) | 1 (100) |
| NB-UVB | 12 (13.5) | 0 | 0 | 0 |
| Topical calcipotriol and steroid | 11 (12.4) | 0 | 0 | 0 |
| Methotrexate | 5 (5.6) | 0 | 2 (20.0) | 0 |
| Cyclosporin | 6 (6.7) | 2 (22.2) | 0 | 0 |
| Oral steroids | 4 (4.5) | 0 | 0 | 0 |
| Tacrolimus | 3 (3.4) | 1 (11.1) | 0 | 0 |
| Pimecrolimus | 0 | 0 | 1 (10.0) | 0 |
| Omalizumab | 1 (1.1) | 0 | 5 (50.0) | 0 |
| Mycophenolate mofetil | 1 (1.1) | 0 | 0 | 0 |
| JAKi | 5 (5.6) | 2 (22.2) | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trave, I.; Salvi, I.; Burlando, M.; Cozzani, E.; Parodi, A. “De Novo” Psoriasis and Relapse of Psoriasis Induced by Dupilumab: Three New Cases and Review of the Literature. J. Clin. Med. 2023, 12, 6291. https://doi.org/10.3390/jcm12196291
Trave I, Salvi I, Burlando M, Cozzani E, Parodi A. “De Novo” Psoriasis and Relapse of Psoriasis Induced by Dupilumab: Three New Cases and Review of the Literature. Journal of Clinical Medicine. 2023; 12(19):6291. https://doi.org/10.3390/jcm12196291
Chicago/Turabian StyleTrave, Ilaria, Ilaria Salvi, Martina Burlando, Emanuele Cozzani, and Aurora Parodi. 2023. "“De Novo” Psoriasis and Relapse of Psoriasis Induced by Dupilumab: Three New Cases and Review of the Literature" Journal of Clinical Medicine 12, no. 19: 6291. https://doi.org/10.3390/jcm12196291





