Influence of Aortic Valve Stenosis and Wall Shear Stress on Platelets Function
Abstract
:1. Introduction
2. Mechanisms of Hemodynamics and Wall Shear Stress in Aortic Stenosis
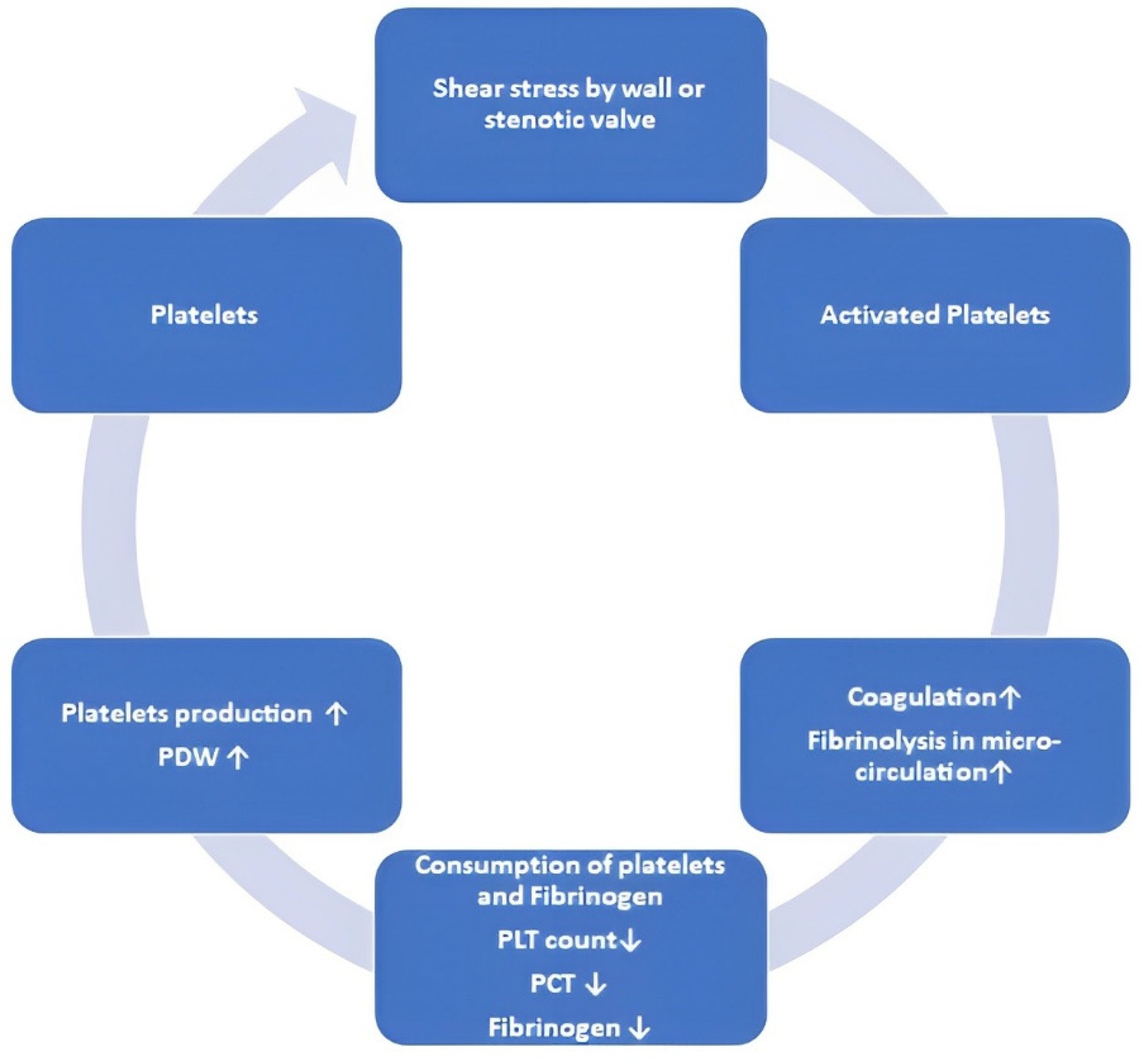
3. Influence of Shear Stress in Aortic Stenosis on Platelet Function
4. Platelets Function in High- and Low-Gradient Aortic Stenosis
5. Platelets Function in Aortic Stenosis and Cerebrovascular Events
6. Aortic Stenosis and Antiplatelet Therapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.-L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis: The Tronso study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.T.; Srigunapalan, S.; Simmons, C.A.; Anseth, K.S. Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ. Res. 2013, 113, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Bouchareb, R.; Boulanger, M.-C.; Tastet, L.; Mkannez, G.; Nsaibia, M.J.; Hadji, F.; Dahou, A.; Messadeq, Y.; Arsenault, B.J.; Pibarot, P.; et al. Activated platelets promote an osteogenic programme and the progression of calcific aortic valve stenosis. Eur. Heart J. 2019, 40, 1362–1373. [Google Scholar] [CrossRef]
- Vahidkhah, K.; Cordasco, D.; Abbasi, M.; Ge, L.; Tseng, E.; Bagchi, P.; Azadani, A.N. Flow-Induced Damage to Blood Cells in Aortic Valve Stenosis. Ann. Biomed. Eng. 2016, 44, 2724–2736. [Google Scholar] [CrossRef]
- Sriram, K.; Intaglietta, M.; Tartakovsky, D.M. Non-newtonian flow of blood in arterioles: Consequences for wall shear stress measurements. Microcirculation 2014, 21, 628–639. [Google Scholar] [CrossRef]
- Baeyens, N.; Bandyopadhyay, C.; Coon, B.G.; Yun, S.; Schwartz, M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig. 2016, 126, 821–828. [Google Scholar] [CrossRef]
- Russo, G.; Pedicino, D.; Chiastra, C.; Vinci, R.; Rizzini, M.L.; Genuardi, L.; Sarraf, M.; D’Aiello, A.; Bologna, M.; Aurigemma, C.; et al. Coronary artery plaque rupture and erosion: Role of wall shear stress profiling and biological patterns in acute coronary syndromes. Int. J. Cardiol. 2022, 370, 356–365. [Google Scholar] [CrossRef]
- Clark, C. Turbulent velocity-measurements in a model of aortic-stenosis. J. Biomech. 1976, 9, 677–687. [Google Scholar] [CrossRef]
- Bird, J.J.; Murgo, J.P.; Pasipoularides, A. Fluid dynamics of aortic stenosis: Subvalvular gradients without subvalvular obstruction. Circulation 1982, 66, 835–840. [Google Scholar] [CrossRef]
- Pasipoularides, A.; Murgo, J.P.; Bird, J.J.; Craig, W.E. Fluid dynamics of aortic stenosis: Mechanisms for the presence of subvalvular pressure gradients. Am. J. Physiol. 1984, 246, H542–H550. [Google Scholar] [CrossRef] [PubMed]
- Yoganathan, A.P. Fluid mechanics of aortic stenosis. Eur. Heart J. 1988, 9 (Suppl. E), 13–17. [Google Scholar] [CrossRef] [PubMed]
- Yearwood, T.L.; Misbach, G.A.; Chandran, K.B. Experimental fluid dynamics of aortic stenosis in a model of the human aorta. Clin. Phys. Physiol. Meas. 1989, 10, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Anupindi, K.; Delorme, Y.; Ghaisas, N.; Shetty, D.A.; Frankel, S.H. Large eddy simulation of transitional flow in an idealized stenotic blood vessel: Evaluation of subgrid scale models. J. Biomech. Eng. 2014, 136, 071009. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Lantz, J.; Haraldsson, H.; Casas, B.; Ziegler, M.; Karlsson, M.; Saloner, D.; Dyverfeldt, P.; Ebbers, T. Assessment of turbulent viscous stress using ICOSA 4D Flow MRI for prediction of hemodynamic blood damage. Sci. Rep. 2016, 6, 39773. [Google Scholar] [CrossRef]
- Jhun, C.-S.; Newswanger, R.; Cysyk, J.P.; Ponnaluri, S.; Good, B.; Manning, K.B.; Rosenberg, G. Dynamics of Blood Flows in Aortic Stenosis: Mild, Moderate, and Severe. ASAIO J. 2021, 67, 666–674. [Google Scholar] [CrossRef]
- Komoriyama, H.; Kamiya, K.; Nagai, T.; Oyama-Manabe, N.; Tsuneta, S.; Kobayashi, Y.; Kato, Y.; Sarashina, M.; Omote, K.; Konishi, T.; et al. Blood flow dynamics with four-dimensional flow cardiovascular magnetic resonance in patients with aortic stenosis before and after transcatheter aortic valve replacement. J. Cardiovasc. Magn. Reson. 2021, 23, 81. [Google Scholar] [CrossRef]
- Rosenberg, G.; Siedlecki, C.A.; Jhun, C.; Weiss, W.J.; Manning, K.; Deutsch, S.; Pierce, W. Acquired Von Willebrand Syndrome and Blood Pump Design. Artif. Organs 2018, 42, 1119–1124. [Google Scholar] [CrossRef]
- Vincentelli, A.; Susen, S.; Tourneau, T.L.; Six, I.; Fabre, O.; Juthier, F.; Bauters, A.; Decoene, C.; Goudemand, J.; Prat, A.; et al. Acquired von Willebrand syndrome in aortic stenosis. N. Engl. J. Med. 2003, 349, 343–349. [Google Scholar] [CrossRef]
- Blackshear, J.L.; Wysokinska, E.M.; Safford, R.E.; Thomas, C.S.; Shapiro, B.P.; Ung, S.; Stark, M.E.; Parikh, P.; Johns, G.S.; Chen, D. Shear stress-associated acquired von Willebrand syndrome in patients with mitral regurgitation. J. Thromb. Haemost. 2014, 12, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Bortot, M.; Ashworth, K.; Sharifi, A.; Walker, F.; Crawford, N.C.; Neeves, K.B.; Bark, D.; Di Paola, J. Turbulent flow promotes cleavage of VWF (von Willebrand factor) by ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type-1 motif, member 13). Arter. Thromb. Vasc. Biol. 2019, 39, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Waldschmidt, L.; Seiffert, M. Heyde syndrome: Treat aortic valve disease to stop gastrointestinal bleeding? Eur. Heart J. 2023, 44, 3178–3180. [Google Scholar] [CrossRef] [PubMed]
- Natorska, J.; Bykowska, K.; Hlawaty, M.; Marek, G.; Sadowski, J.; Undas, A. Increased thrombin generation and platelet activation are associated with deficiency in high molecular weight multimers of von Willebrand factor in patients with moderate-to-severe aortic stenosis. Heart 2011, 97, 2023–2028. [Google Scholar] [CrossRef]
- Natorska, J.; Marek, G.; Hlawaty, M.; Sobczyk, D.; Sadowski, J.; Tracz, W.; Undas, A. Evidence for tissue factor expression in aortic valves in patients with aortic stenosis. Pol. Arch. Intern. Med. 2009, 119, 636–643. [Google Scholar] [CrossRef]
- Breyne, J.; Juthier, F.; Corseaux, D.; Marechaux, S.; Zawadzki, C.; Jeanpierre, E.; Ung, A.; Ennezat, P.-V.; Susen, S.; Van Belle, E.; et al. Atherosclerotic-like process in aortic stenosis: Activation of the tissue factor–thrombin pathway and potential role through osteopontin alteration. Atherosclerosis 2010, 213, 369–376. [Google Scholar] [CrossRef]
- Bolen, A.L.; Naren, A.P.; Yarlagadda, S.; Beranova-Giorgianni, S.; Chen, L.; Norman, D.; Baker, D.L.; Rowland, M.M.; Best, M.D.; Sano, T.; et al. The phospholipase A1 activity of lysophospholipase A-I links platelet activation to LPA production during blood coagulation. J. Lipid Res. 2011, 52, 958–970. [Google Scholar] [CrossRef]
- Fulkerson, Z.; Wu, T.; Sunkara, M.; Kooi, C.V.; Morris, A.J.; Smyth, S.S. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J. Biol. Chem. 2011, 286, 34654–34663. [Google Scholar] [CrossRef]
- Bouchareb, R.; Mahmut, A.; Nsaibia, M.J.; Boulanger, M.-C.; Dahou, A.; Lépine, J.-L.; Laflamme, M.-H.; Hadji, F.; Couture, C.; Trahan, S.; et al. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation 2015, 132, 677–690. [Google Scholar] [CrossRef]
- Côté, N.; El Husseini, D.; Pépin, A.; Guauque-Olarte, S.; Ducharme, V.; Bouchard-Cannon, P.; Audet, A.; Fournier, D.; Gaudreault, N.; Derbali, H.; et al. ATP acts as a survival signal and prevents the mineralization of aortic valve. J. Mol. Cell. Cardiol. 2012, 52, 1191–1202. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Malashicheva, A.; Sullivan, G.; Bogdanova, M.; Kostareva, A.; Stensløkken, K.; Fiane, A.; Vaage, J. Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification. J. Am. Heart Assoc. 2017, 6, e006339. [Google Scholar] [CrossRef] [PubMed]
- Osman, L.; Yacoub, M.H.; Latif, N.; Amrani, M.; Chester, A.H. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation 2006, 114, I547–I552. [Google Scholar] [CrossRef] [PubMed]
- Osman, L.; Chester, A.H.; Amrani, M.; Yacoub, M.H.; Smolenski, R.T. A novel role of extracellular nucleotides in valve calcification: A potential target for atorvastatin. Circulation 2006, 114, I566–I572. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fullerton, D.A.; Su, X.; Ao, L.; Cleveland, J.C.; Meng, X. Pro-osteogenic phenotype of human aortic valve interstitial cells is associated with higher levels of toll-like receptors 2 and 4 and enhanced expression of bone morphogenetic protein 2. J. Am. Coll. Cardiol. 2009, 53, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Meng, X.; Su, X.; Mauchley, D.C.; Ao, L.; Cleveland, J.C.; Fullerton, D.A. Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: Role of Smad1 and extracellular signal-regulated kinase 1/2. J. Thorac. Cardiovasc. Surg. 2009, 138, 1008–1015. [Google Scholar] [CrossRef]
- Yu, Z.; Seya, K.; Daitoku, K.; Motomura, S.; Fukuda, I.; Furukawa, K.-I. Tumor necrosis factor-α accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2-Dlx5 pathway. J. Pharmacol. Exp. Ther. 2011, 337, 16–23. [Google Scholar] [CrossRef]
- Carthy, J.M.; Boroomand, S.; McManus, B.M. Versican and CD44 in in vitro valvular interstitial cell injury and repair. Cardiovasc. Pathol. 2012, 21, 74–82. [Google Scholar] [CrossRef]
- Song, R.; Zeng, Q.; Ao, L.; Jessica, A.Y.; Cleveland, J.C.; Zhao, K.-S.; Fullerton, D.A.; Meng, X. Biglycan induces the expression of osteogenic factors in human aortic valve interstitial cells via toll-like receptor-2. Atertio. Thromb. Vasc. Biol. 2012, 32, 2711–2720. [Google Scholar] [CrossRef]
- Zeng, Q.; Jin, C.; Ao, L.; Cleveland, J.C., Jr.; Song, R.; Xu, D.; Fullerton, D.A.; Meng, X. Cross-talk between the toll-like receptor 4 and Notch1 pathways augments the inflammatory response in the interstitial cells of stenotic human aortic valves. J. Pharmacol. Exp. Ther. 2012, 126, S222–S230. [Google Scholar] [CrossRef]
- Poggio, P.; Sainger, R.; Branchetti, E.; Grau, J.B.; Lai, E.K.; Gorman, R.C.; Sacks, M.S.; Parolari, A.; Bavaria, J.E.; Ferrari, G. Noggin attenuates the osteogenic activation of human valve interstitial cells in aortic valve sclerosis. Cardiovasc. Res. 2013, 98, 402–410. [Google Scholar] [CrossRef]
- Zeng, Q.; Song, R.; Ao, L.; Weyant, M.J.; Lee, J.; Xu, D.; Fullerton, D.A.; Meng, X. Notch1 promotes the pro-osteogenic response of human aortic valve interstitial cells via modulation of ERK1/2 and nuclear factor-κB activation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Nadlonek, N.; Lee, J.H.; Reece, T.B.; Weyant, M.J.; Cleveland, J.C.; Meng, X.; Fullerton, D.A. Interleukin-1 beta induces an inflammatory phenotype in human aortic valve interstitial cells through nuclear factor kappa beta. Ann. Thorac. Surg. 2013, 96, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, X.; Zhang, X.; Song, Z.; Han, L.; He, Y.; Xu, Z. MicroRNA-30b is a multifunctional regulator of aortic valve interstitial cells. J. Thorac. Cardiovasc. Surg. 2014, 147, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Galeone, A.; Brunetti, G.; Oranger, A.; Greco, G.; Di Benedetto, A.; Mori, G.; Colucci, S.; Zallone, A.; Paparella, D.; Grano, M. Aortic valvular interstitial cells apoptosis and calcification are mediated by TNF-related apoptosis-inducing ligand. Int. J. Cardiol. 2013, 169, 296–304. [Google Scholar] [CrossRef]
- El Husseini, D.; Boulanger, M.-C.; Mahmut, A.; Bouchareb, R.; Laflamme, M.-H.; Fournier, D.; Pibarot, P.; Bossé, Y.; Mathieu, P. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: Implication for calcific aortic valve disease. J. Mol. Cell. Cardiol. 2014, 72, 146–156. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Zhang, B.-Y.; Wang, S.-W.; Gong, D.-J.; Han, L.; Xu, Z.-Y.; Liu, X.-H. Twist-related protein 1 negatively regulated osteoblastic transdifferentiation of human aortic valve interstitial cells by directly inhibiting runt-related transcription factor 2. J. Thorac. Cardiovasc. Surg. 2014, 148, 1700–1708. [Google Scholar] [CrossRef]
- Carrion, K.; Dyo, J.; Patel, V.; Sasik, R.; Mohamed, S.A.; Hardiman, G.; Nigam, V. The long non-coding HOTAIR is modulated by cyclic stretch and WNT/β-CATENIN in human aortic valve cells and is a novel repressor of calcification genes. PLoS ONE 2014, 9, e96577. [Google Scholar] [CrossRef]
- Zeng, Q.; Song, R.; Ao, L.; Xu, D.; Venardos, N.; Fullerton, D.A.; Meng, X. Augmented osteogenic responses in human aortic valve cells exposed to oxLDL and TLR4 agonist: A mechanistic role of Notch1 and NF-κB interaction. PLoS ONE 2014, 9, e95400. [Google Scholar] [CrossRef]
- Witt, W.; Büttner, P.; Jannasch, A.; Matschke, K.; Waldow, T. Reversal of myofibroblastic activation by polyunsaturated fatty acids in valvular interstitial cells from aortic valves. Role of RhoA/G-actin/MRTF signalling. J. Mol. Cell. Cardiol. 2014, 74, 127–138. [Google Scholar] [CrossRef]
- Song, R.; Ao, L.; Zhao, K.S.; Zheng, D.; Venardos, N.; Fullerton, D.A.; Meng, X. Soluble biglycan induces the production of ICAM-1 and MCP-1 in human aortic valve interstitial cells through TLR2/4 and the ERK1/2 pathway. Inflamm. Res. 2014, 63, 703–710. [Google Scholar] [CrossRef]
- Parra-Izquierdo, I.; Castaños-Mollor, I.; López, J.; Gómez, C.; San Román, J.A.; Sánchez Crespo, M.; García-Rodríguez, C. Lipopolysaccharide and interferon-γ team up to activate HIF-1α via STAT1 in normoxia and exhibit sex differences in human aortic valve interstitial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, H.; Gao, J.; Chen, J.; He, P.; Zhong, H.; Tan, X.; Staines, K.A.; Macrae, V.E.; Fu, X.; et al. PALMD regulates aortic valve calcification via altered glycolysis and NF-κB-mediated inflammation. J. Biol. Chem. 2022, 298, 101887. [Google Scholar] [CrossRef] [PubMed]
- Voicu, G.; Rebleanu, D.; Mocanu, C.A.; Tanko, G.; Droc, I.; Uritu, C.M.; Pinteala, M.; Manduteanu, I.; Simionescu, M.; Calin, M. VCAM-1 Targeted Lipopolyplexes as Vehicles for Efficient Delivery of shRNA-Runx2 to Osteoblast-Differentiated Valvular Interstitial Cells; Implications in Calcific Valve Disease Treatment. Int. J. Mol. Sci. 2022, 23, 3824. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, R.; Cao, Z.; Guo, Q.; Huang, H.; Liu, L.; Xiao, Y.; Duan, C.; Ma, R. Identification of MMP9 as a Novel Biomarker to Mitochondrial Metabolism Disorder and Oxidative Stress in Calcific Aortic Valve Stenosis. Oxidative Med. Cell. Longev. 2022, 2022, 3858871. [Google Scholar] [CrossRef]
- Iqbal, F.; Schlotter, F.; Becker-Greene, D.; Lupieri, A.; Goettsch, C.; Hutcheson, J.D.; A Rogers, M.; Itoh, S.; Halu, A.; Lee, L.H.; et al. Sortilin enhances fibrosis and calcification in aortic valve disease by inducing interstitial cell heterogeneity. Eur. Heart J. 2023, 44, 885–898. [Google Scholar] [CrossRef]
- Sedaghat, A.; Kulka, H.; Sinning, J.-M.; Falkenberg, N.; Driesen, J.; Preisler, B.; Hammerstingl, C.; Nickenig, G.; Pötzsch, B.; Oldenburg, J.; et al. Transcatheter aortic valve implantation leads to a restoration of von Willebrand factor (VWF) abnormalities in patients with severe aortic stenosis–Incidence and relevance of clinical and subclinical VWF dysfunction in patients undergoing transfemoral TAVI. Thromb. Res. 2017, 151, 23–28. [Google Scholar] [CrossRef]
- Kibler, M.; Marchandot, B.; Messas, N.; Caspar, T.; Vincent, F.; Von Hunolstein, J.-J.; Grunebaum, L.; Reydel, A.; Rauch, A.; Crimizade, U.; et al. CT-ADP point-of-care assay predicts 30-day paravalvular aortic regurgitation and bleeding events following transcatheter aortic valve replacement. Thromb. Haemost. 2018, 118, 893–905. [Google Scholar] [CrossRef]
- Grodecki, K.; Zbroński, K.; Przybyszewska-Kazulak, E.; Olasińska-Wiśniewska, A.; Wilimski, R.; Rymuza, B.; Scisło, P.; Czub, P.; Koper, D.; Kochman, J.; et al. Pre-procedural abnormal function of von Willebrand Factor is predictive of bleeding after surgical but not transcatheter aortic valve replacement. J. Thromb. Thrombolysis 2019, 48, 610–618. [Google Scholar] [CrossRef]
- Kanda, H.; Yamakuchi, M.; Matsumoto, K.; Mukaihara, K.; Shigehisa, Y.; Tachioka, S.; Okawa, M.; Takenouchi, K.; Oyama, Y.; Hashiguchi, T.; et al. Dynamic changes in platelets caused by shear stress in aortic valve stenosis. Clin. Hemorheol. Microcirc. 2021, 77, 71–81. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Stein, P.D.; Sabbah, H.N.; Pitha, J.V. Continuing disease process of calcific aortic stenosis: Role of microthrombi and turbulent flow. Am. J. Cardiol. 1977, 39, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, T.; Ma, S. Impact of leaflet thrombosis on hemodynamics and clinical outcomes after bioprosthetic aortic valve replacement: A meta-analysis. Clin. Cardiol. 2020, 43, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, C.; Gislason, G.H.; Køber, L.; Abdulla, J.; Martinsson, A.; Smith, J.G.; Torp-Pedersen, C.; Andersson, C. Incidence of Ischemic Stroke in Individuals with and without Aortic Valve Stenosis: A Danish Retrospective Cohort Study. Stroke 2020, 51, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.F.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar]
- Dangas, G.D.; De Backer, O.; Windecker, S. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N. Engl. J. Med. 2020, 383, e8. [Google Scholar] [CrossRef]
- Brouwer, J.; Nijenhuis, V.J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.; et al. Aspirin with or without clopidogrel after transcatheter aortic-valve implantation. N. Engl. J. Med. 2020, 383, 1447–1457. [Google Scholar] [CrossRef]

| Type of AS Model | Mild | Moderate | Severe |
|---|---|---|---|
| Peak velocities | 2.0 m/s | 4.0 m/s | 8.0 m/s |
| Transvalvular pressure gradients | 14 mmHg | 30 mmHg | 113 mmHg |
| Mean WSS | 39.6 ± 12.4 Pa | 104.7 ± 23.0 Pa | 180.8 ± 95.2 Pa |
| RSSmax at centerline | 260 Pa | 490 Pa | 2500 Pa |
| Author, Year | Factor | Key Result, End Point |
|---|---|---|
| Osman, 2006 [32] | TGF β family cytokines and statins | Cytokines from the TGFβ family promote the differentiation of osteoblasts, whereas atorvastatin inhibits this process. |
| Osman, 2006 [33] | Adenosine triphosphate and statins | The activation of osteoblast differentiation is facilitated by adenosine triphosphate, but this effect is counteracted by atorvastatin. |
| Yang, 2009 [34] | LPS and peptidoglycan | Osteoblast differentiation is prompted by lipopolysaccharides (LPS) and peptidoglycan through the activation of toll-like receptors 2 and 4. |
| Yang, 2009 [35] | BMP 2 | BMP2 triggers the early phases of osteoblast differentiation through both canonical and non-canonical pathways. |
| Yu, 2011 [36] | TNF α and BMP2 | Tumor necrosis factor α exclusively triggers osteoblast differentiation in calcified VICs via BMP2 and NFkB signaling. |
| Carthy, 2012 [37] | Versican | VICs secrete versican in the wound assay; inhibiting its receptor CD44 leads to a reduction in stress fiber (αSMA) formation during VIC migration and inhibits collagen gel contraction. |
| Song, 2012 [38] | Biglycan | VICs derived from calcified valves exhibit elevated levels of biglycan expression. Biglycan, in turn, promotes osteoblast differentiation through the toll-like receptor 2 and ERK signaling pathways. The expression of biglycan and the calcification process are further stimulated by oxidized low-density lipopolysaccharides. |
| Zeng, 2012 [39] | LPS, toll-like receptor 4, and Notch | LPS activates an inflammatory phenotype through toll-like receptor 4 (TLR4). In calcified VICs, Notch1 enhances the responsiveness of toll-like receptor 4 to LPS through NFκB signaling. |
| Poggio, 2013 [40] | BMP 4 | Bone morphogenetic protein 4 exclusively initiates osteoblast differentiation in non-calcified VICs, leading to higher levels of differentiation compared to osteogenic medium alone. |
| Zeng, 2013 [41] | LPS, Notch1 | LPS stimulates the cleavage and nuclear translocation of the Notch1 intracellular domain, which subsequently triggers osteoblast differentiation via the activation of ERK and NFκB signaling pathways. |
| Nadlonek, 2013 [42] | Interleukin-1β | Interleukin-1β induces an inflammatory phenotype in VIC via NFκB. |
| Zhang, 2014 [43] | MicroRNA 30b | BMP2 initiates osteoblastic differentiation in VICs and suppresses the expression of microRNA 30b. MicroRNA 30b, in turn, inhibits osteoblastic differentiation and apoptosis. |
| Galeone, 2013 [44] | TNF-related apoptosis-inducing ligand (TRAIL) | Calcified VICs exhibit the presence of TRAIL receptors. The addition of TRAIL to the osteogenic medium enhances the formation of calcified nodules and promotes apoptosis. |
| El Husseini, 2014 [45] | AKT kinase and P2Y2 receptor | NFκB pathway is involved in inhibiting the expression of IL-6, which is a necessary factor for mineralization. Both AKT kinase and P2Y2 receptor activate this pathway, thereby suppressing IL-6 expression. Cells derived from P2Y2−/− mice are prone to osteoblast differentiation. |
| Zhang, 2014 [46] | Transcription factor Twist | The osteogenic medium leads to the upregulation of Twist. This process leads to a decrease in the expression of other calcification-related genes. Conversely, the use of Twist siRNA induces osteoblast differentiation. |
| Carrion, 2014 [47] | Long noncoding RNA HOTAIR | Stretching downregulates HOTAIR through the Wnt signaling pathway. When siRNA is used to target HOTAIR, it leads to the upregulation of BMP2 and alkaline phosphatase expression. |
| Zeng, 2014 [48] | Oxidized low-density lipoproteins, LPS, and Notch1 | Oxidized low-density lipoproteins enhance LPS-induced osteoblastic differentiation through the activation of NFκB and cleavage of Notch1. |
| Witt, 2014 [49] | Polyunsaturated fatty acids | Several polyunsaturated fatty acids can temporarily inhibit myofibroblast activation through the suppression of Rho kinase and ROCK kinase. |
| Song, 2014 [50] | Biglycan | Biglycan acts as a ligand for toll-like receptors 2 and 4, contributing to the activation of inflammation in VICs. This effect is mediated through NFκB and ERK pathways |
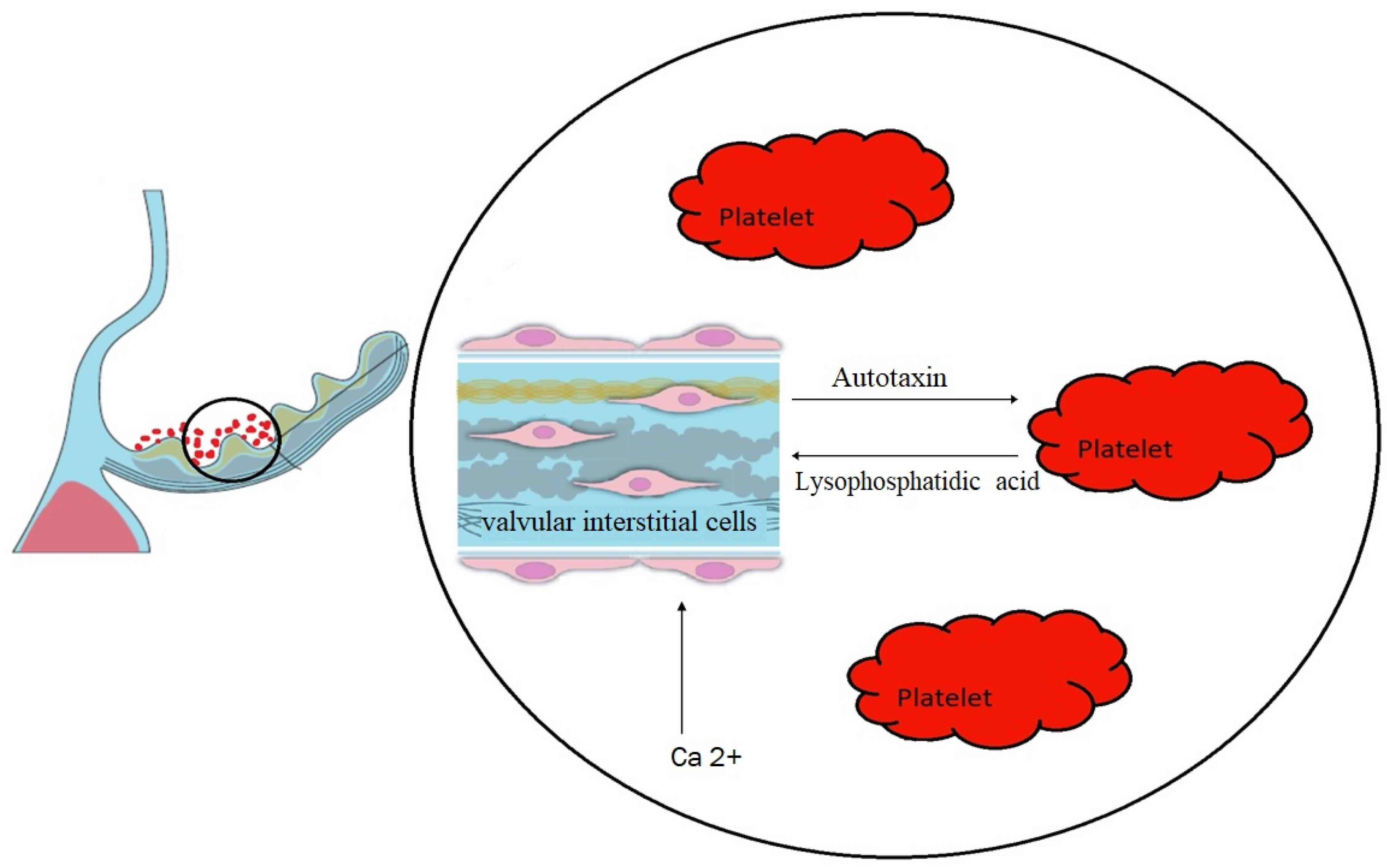
| Bouchareb, 2019 [5] | Autotaxin and lysophosphatidic acid | The release of autotaxin by VICs was induced by adenosine diphosphate derived from platelets. Autotaxin, in turn, bound to GPIIb/IIIa receptors on platelets, resulting in the generation of lysophosphatidic acid, which possesses pro-osteogenic properties. |
| Parra-Izquierdo, 2019 [51] | HIF-1α | HIF-1α activation via STAT1 in valve cells results in the proangiogenic, proinflammatory, and pro-osteogenic effects of IFN-γ |
| Wang, 2022 [52] | PALMD (Palmdelphin) | PALMD, a protein involved in myoblast differentiation, enhancing VIC osteogenic differentiation and inflammation through the activation of NF-κB. |
| Voicu, 2022 [53] | V-LPP/shRunx2 lipopolyplexes | VCAM-1 targeted lipopolyplexes, which downregulate the Runx2 gene and decrease the expression of osteogenic molecules OSP, BSP, and BMP-2 in VICs |
| Liu, 2022 [54] | MMP9 | MMP9 expression was distinctly increased in AS, and its inhibition attenuated the calcification of valve interstitial cells by suppressing mitochondrial damage and oxidative stress. |
| Iqbal, 2023 [55] | Sortilin (SORT1) | Sortilin enhances fibrosis and calcification in aortic valve disease via the transformation of valvular interstitial cells into pathological phenotypes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bańka, P.; Wybraniec, M.; Bochenek, T.; Gruchlik, B.; Burchacka, A.; Swinarew, A.; Mizia-Stec, K. Influence of Aortic Valve Stenosis and Wall Shear Stress on Platelets Function. J. Clin. Med. 2023, 12, 6301. https://doi.org/10.3390/jcm12196301
Bańka P, Wybraniec M, Bochenek T, Gruchlik B, Burchacka A, Swinarew A, Mizia-Stec K. Influence of Aortic Valve Stenosis and Wall Shear Stress on Platelets Function. Journal of Clinical Medicine. 2023; 12(19):6301. https://doi.org/10.3390/jcm12196301
Chicago/Turabian StyleBańka, Paweł, Maciej Wybraniec, Tomasz Bochenek, Bartosz Gruchlik, Aleksandra Burchacka, Andrzej Swinarew, and Katarzyna Mizia-Stec. 2023. "Influence of Aortic Valve Stenosis and Wall Shear Stress on Platelets Function" Journal of Clinical Medicine 12, no. 19: 6301. https://doi.org/10.3390/jcm12196301
APA StyleBańka, P., Wybraniec, M., Bochenek, T., Gruchlik, B., Burchacka, A., Swinarew, A., & Mizia-Stec, K. (2023). Influence of Aortic Valve Stenosis and Wall Shear Stress on Platelets Function. Journal of Clinical Medicine, 12(19), 6301. https://doi.org/10.3390/jcm12196301








