Mesenchymal Stem Cell Therapy in Multiple Sclerosis: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Review Protocol
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Data Extraction
2.5. Quality Assessment
2.6. Determination of Safety and Efficacy
2.7. Subgroup and Sensitivity Analysis
3. Results
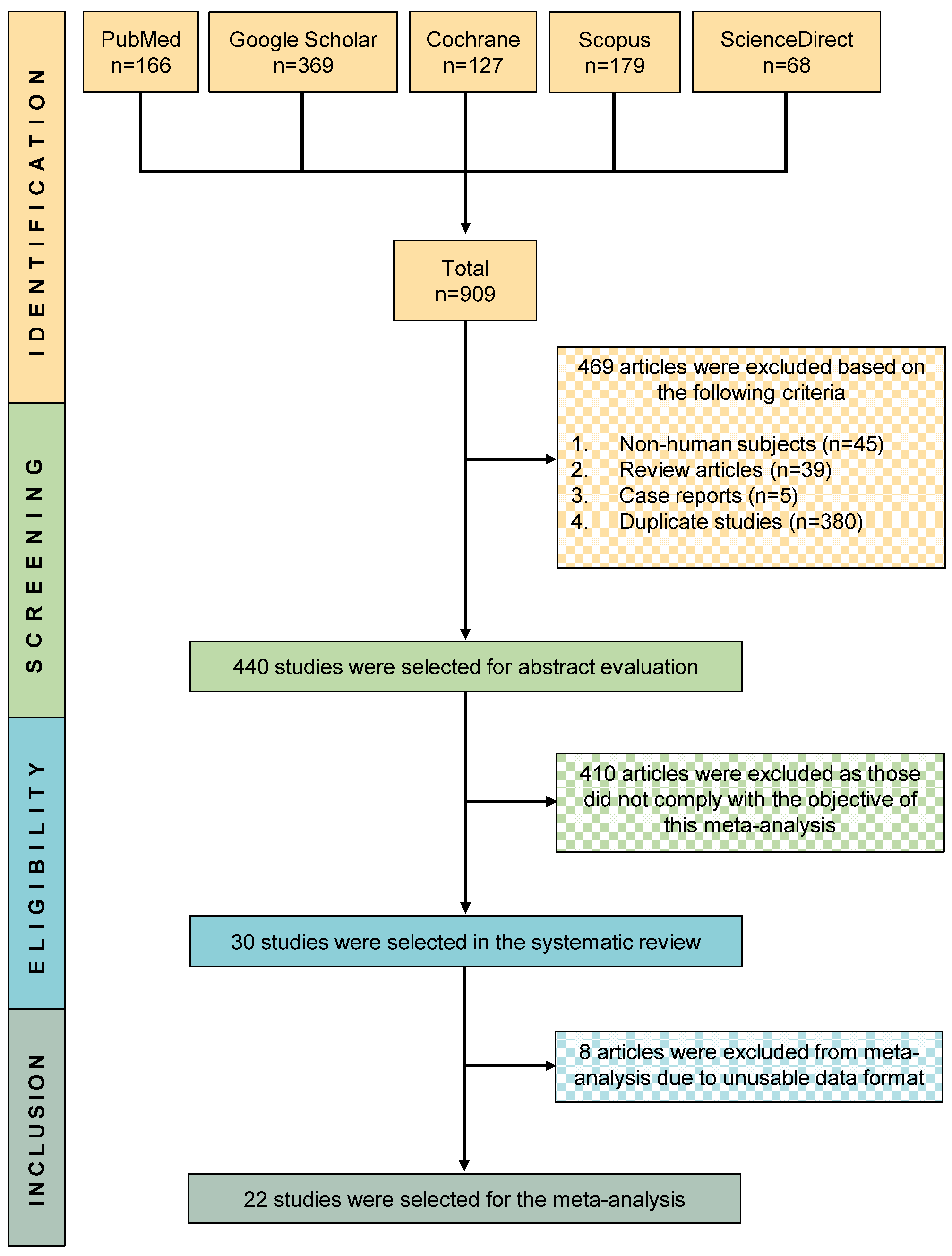
3.1. Study Selection and Characteristics
3.2. Safety and Efficacy
3.3. Publication Bias Assessment
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mansoor, S.R.; Zabihi, E.; Ghasemi-Kasman, M. The potential use of mesenchymal stem cells for the treatment of multiple sclerosis. Life Sci. 2019, 235, 116830. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Morel, A.; Redlicka, J.; Miller, I.; Saluk, J. Pharmacological and non-pharmacological therapies of cognitive impairment in multiple sclerosis. Curr. Neuropharmacol. 2018, 16, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A. Mesenchymal stem cell transplantation in multiple sclerosis. J. Neurol. Sci. 2013, 333, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Bonab, M.M.; Yazdanbakhsh, S.; Alimoghaddom, K.; Ghavamzadeh, A.; Hooshmand, F.; Lotfi, J.; Talebian, F.; Nikbin, B. Mesenchymal stem cell therapy for multiple sclerosis. Int. J. Hematol. Oncol. Stem Cell Res. 2005, 2, 10–15. [Google Scholar]
- Islam, M.A.; Kundu, S.; Hassan, R. Gene Therapy Approaches in an Autoimmune Demyelinating Disease: Multiple Sclerosis. Curr. Gene Ther. 2019, 19, 376–385. [Google Scholar] [CrossRef]
- Lattanzi, S.; Cagnetti, C.; Danni, M.; Provinciali, L.; Silvestrini, M. Oral and intravenous steroids for multiple sclerosis relapse: A systematic review and meta-analysis. J. Neurol. 2017, 264, 1697–1704. [Google Scholar] [CrossRef]
- Jacob, S.; Mazibrada, G.; Irani, S.R.; Jacob, A.; Yudina, A. The Role of Plasma Exchange in the Treatment of Refractory Autoimmune Neurological Diseases: A Narrative Review. J. Neuroimmune Pharmacol. 2021, 16, 806–817. [Google Scholar] [CrossRef]
- Pezeshki Naraghi, S.; Hashemi, S.M. Immunomodulatory effect of mesenchymal stem cells in multiple sclerosis and experimental autoimmune encephalomyelitis: A review study. Immunoregulation 2019, 1, 67–80. [Google Scholar] [CrossRef]
- Fernández, O.; Izquierdo, G.; Fernández, V.; Leyva, L.; Reyes, V.; Guerrero, M.; León, A.; Arnaiz, C.; Navarro, G.; Páramo, M.D. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE 2018, 13, e0195891. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhang, D.-J.; Geng, T.; Chen, L.; Huang, H.; Yin, H.-L.; Zhang, Y.-z.; Lou, J.-Y.; Cao, B.; Wang, Y.-L. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. 2014, 23, 113–122. [Google Scholar] [CrossRef]
- Meng, M.; Liu, Y.; Wang, W.; Wei, C.; Liu, F.; Du, Z.; Xie, Y.; Tang, W.; Hou, Z.; Li, Q. Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Am. J. Transl. Res. 2018, 10, 212–223. [Google Scholar] [PubMed]
- Connick, P.; Kolappan, M.; Crawley, C.; Webber, D.J.; Patani, R.; Michell, A.W.; Du, M.-Q.; Luan, S.-L.; Altmann, D.R.; Thompson, A.J. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012, 11, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.S.; Bar-Or, A.; Atkins, H.L.; Karussis, D.; Frassoni, F.; Lazarus, H.; Scolding, N.; Slavin, S.; Le Blanc, K.; Uccelli, A. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: Consensus report of the International MSCT Study Group. Mult. Scler. J. 2010, 16, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Amir Ali, S. The Use of Mesenchymal Stem Cells in the Treatment of Multiple Sclerosis: An Overview of Open Labels and Ongoing Studies. J. Neurol. Neurophysiol. 2014, 5, 2. [Google Scholar] [CrossRef]
- Uccelli, A.; Laroni, A.; Freedman, M.S. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011, 10, 649–656. [Google Scholar] [CrossRef]
- Siatskas, C.; Payne, N.L.; Short, M.A.; Bernard, C.C. A consensus statement addressing mesenchymal stem cell transplantation for multiple sclerosis: It’s time! Stem Cell Rev. Rep. 2010, 6, 500–506. [Google Scholar] [CrossRef]
- Markov, A.; Thangavelu, L.; Aravindhan, S.; Zekiy, A.O.; Jarahian, M.; Chartrand, M.S.; Pathak, Y.; Marofi, F.; Shamlou, S.; Hassanzadeh, A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res. Ther. 2021, 12, 192. [Google Scholar] [CrossRef]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef]
- O’Connor, P.; Wolinsky, J.S.; Confavreux, C.; Comi, G.; Kappos, L.; Olsson, T.P.; Benzerdjeb, H.; Truffinet, P.; Wang, L.; Miller, A.; et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N. Engl. J. Med. 2011, 365, 1293–1303. [Google Scholar] [CrossRef]
- Kappos, L.; Radue, E.W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef]
- Cohen, J.A.; Imrey, P.B.; Planchon, S.M.; Bermel, R.A.; Fisher, E.; Fox, R.J.; Bar-Or, A.; Sharp, S.L.; Skaramagas, T.T.; Jagodnik, P. Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Mult. Scler. J. 2018, 24, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Karussis, D.; Karageorgiou, C.; Vaknin-Dembinsky, A.; Gowda-Kurkalli, B.; Gomori, J.M.; Kassis, I.; Bulte, J.W.; Petrou, P.; Ben-Hur, T.; Abramsky, O. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010, 67, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Ozakbas, S.; Ormeci, B.; Idiman, E. Utilization of the multiple sclerosis functional composite in follow-up: Relationship to disease phenotype, disability and treatment strategies. J. Neurol. Sci. 2005, 232, 65–69. [Google Scholar] [CrossRef]
- Demir, S. Expanded Disability Status Scale (EDSS) in Multiple Sclerosis. Cam Sakura Med. J. 2022, 2, 82–89. [Google Scholar] [CrossRef]
- Ahmed, S.; Chowdhury, M.I.H.; Sultana, S.; Alam, S.S.; Marzan, M.; Islam, M.A. Prevalence of Antibiotic-Resistant Shigella spp. in Bangladesh: A Systematic Review and Meta-Analysis of 44,519 Samples. Antibiotics 2023, 12, 817. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Karimi, S.; Arab, L.; Aghdami, N.; Joghtaei, N.; Maroufizadeh, S.; Jarooghi, N.; Bolurieh, T.; Abbasi, F.; Mardpour, S. Intravenous Transplantation of Bone Marrow-Derived Mesenchymal stromal cells in Patients with Multiple Sclerosis, A Phase I/IIa, Double blind, Randomized Controlled study. Mult. Scler. Relat. Disord. 2023, 78, 104895. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Lublin, F.D.; Lock, C.; Pelletier, D.; Chitnis, T.; Mehra, M.; Gothelf, Y.; Aricha, R.; Lindborg, S.; Lebovits, C.; et al. Evaluation of neurotrophic factor secreting mesenchymal stem cells in progressive multiple sclerosis. Mult. Scler. 2023, 29, 92–106. [Google Scholar] [CrossRef]
- Tremblay, F.; Ansari, Y.; Remaud, A.; Freedman, M.S. Neurophysiological outcomes following mesenchymal stem cell therapy in multiple sclerosis. Clin. Neurophysiol. 2022, 136, 69–81. [Google Scholar] [CrossRef]
- Harris, V.K.; Stark, J.W.; Yang, S.; Zanker, S.; Tuddenham, J.; Sadiq, S.A. Mesenchymal stem cell-derived neural progenitors in progressive MS: Two-year follow-up of a phase I study. Neurol Neuroimmunol. Neuroinflamm. 2021, 8, e928. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Laroni, A.; Ali, R.; Battaglia, M.A.; Blinkenberg, M.; Brundin, L.; Clanet, M.; Fernandez, O.; Marriot, J.; Muraro, P.; et al. Safety, tolerability, and activity of mesenchymal stem cells versus placebo in multiple sclerosis (MESEMS): A phase 2, randomised, double-blind crossover trial. Lancet Neurol. 2021, 20, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Petrou, P.; Kassis, I.; Ginzberg, A.; Halimi, M.; Yaghmour, N.; Abramsky, O.; Karussis, D. Long-Term Clinical and Immunological Effects of Repeated Mesenchymal Stem Cell Injections in Patients with Progressive Forms of Multiple Sclerosis. Front. Neurol. 2021, 12, 639315. [Google Scholar] [CrossRef] [PubMed]
- Petrou, P.; Kassis, I.; Levin, N.; Paul, F.; Backner, Y.; Benoliel, T.; Oertel, F.C.; Scheel, M.; Hallimi, M.; Yaghmour, N.; et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 2020, 143, 3574–3588. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, L.E.; Planchon, S.M.; Bermel, R.A.; Nakamura, K.; Fisher, E.; Feng, J.; Sakaie, K.E.; Ontaneda, D.; Cohen, J.A. Serum neurofilament light chain concentration in a phase 1/2 trial of autologous mesenchymal stem cell transplantation. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bonab, M.M.; Alimoghaddom, K.; Ghavamzadeh, A.; Yazdanbakhsh, S.; Lotfi, J.; Talebian, F.; Nikbin, B.; Hooshmand, F. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran. J. Immunol. 2007, 4, 50–57. [Google Scholar]
- Bonab, M.M.; Sahraian, M.A.; Aghsaie, A.; Ahmadi, S.K.; Massoud, S.H.; Nikbin, B.; Lotfi, J.; Khorramnia, S.; Reza, M.M.; Togha, M. Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: An open label study. Curr. Stem Cell Res. Ther. 2012, 7, 407–414. [Google Scholar] [CrossRef]
- Llufriu, S.; Sepulveda, M.; Blanco, Y.; Marin, P.; Moreno, B.; Berenguer, J.; Gabilondo, I.; Martinez-Heras, E.; Sola-Valls, N.; Arnaiz, J.A.; et al. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS ONE 2014, 9, e113936. [Google Scholar] [CrossRef]
- Dahbour, S.; Jamali, F.; Alhattab, D.; Al-Radaideh, A.; Ababneh, O.; Al-Ryalat, N.; Al-Bdour, M.; Hourani, B.; Msallam, M.; Rasheed, M. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 2017, 23, 866–874. [Google Scholar] [CrossRef]
- De Oliveira, G.L.; De Lima, K.W.; Colombini, A.M.; Pinheiro, D.G.; Panepucci, R.A.; Palma, P.V.; Brum, D.G.; Covas, D.T.; Simões, B.P.; De Oliveira, M.C. Bone marrow mesenchymal stromal cells isolated from multiple sclerosis patients have distinct gene expression profile and decreased suppressive function compared with healthy counterparts. Cell Transplant. 2015, 24, 151–165. [Google Scholar] [CrossRef]
- Harris, V.K.; Vyshkina, T.; Sadiq, S.A. Clinical safety of intrathecal administration of mesenchymal stromal cell–derived neural progenitors in multiple sclerosis. Cytotherapy 2016, 18, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.K.; Stark, J.; Vyshkina, T.; Blackshear, L.; Joo, G.; Stefanova, V.; Sara, G.; Sadiq, S.A. Phase I trial of intrathecal mesenchymal stem cell-derived neural progenitors in progressive multiple sclerosis. EBioMedicine 2018, 29, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Iacobaeus, E.; Kadri, N.; Lefsihane, K.; Boberg, E.; Gavin, C.; Törnqvist Andrén, A.; Lilja, A.; Brundin, L.; Blanc, K.L. Short and Long Term Clinical and Immunologic Follow up after Bone Marrow Mesenchymal Stromal Cell Therapy in Progressive Multiple Sclerosis—A Phase I Study. J Clin. Med. 2019, 8, 2102. [Google Scholar] [CrossRef]
- Lu, Z.; Zhao, H.; Xu, J.; Zhang, Z.; Zhang, X. Human umbilical cord mesenchymal stem cells in the treatment of secondary progressive multiple sclerosis. J. Stem Cell Res. Ther. 2013, 6, 1–7. [Google Scholar]
- Lublin, F.D.; Bowen, J.D.; Huddlestone, J.; Kremenchutzky, M.; Carpenter, A.; Corboy, J.R.; Freedman, M.S.; Krupp, L.; Paulo, C.; Hariri, R.J. Human placenta-derived cells (PDA-001) for the treatment of adults with multiple sclerosis: A randomized, placebo-controlled, multiple-dose study. Mult. Scler. Relat. Disord. 2014, 3, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Odinak, M.; Bisaga, G.; Novitskii, A.; Tyrenko, V.; Fominykh, M.; Bilibina, A.; Kruglyakov, P.; Polyntsev, D. Transplantation of mesenchymal stem cells in multiple sclerosis. Neurosci. Behav. Physiol. 2012, 42, 516–520. [Google Scholar] [CrossRef]
- Riordan, N.H.; Morales, I.; Fernández, G.; Allen, N.; Fearnot, N.E.; Leckrone, M.E.; Markovich, D.J.; Mansfield, D.; Avila, D.; Patel, A.N. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J. Transl. Med. 2018, 16, 1–12. [Google Scholar] [CrossRef]
- Sahraian, M.A.; Bonab, M.M.; Karvigh, S.A.; Yazdanbakhsh, S.; Nikbin, B.; Lotfi, J. Intrathecal mesenchymal stem cell therapy in multiple sclerosis: A follow-up study for five years after injection. Arch. Neurosci. 2014, 1, 71–75. [Google Scholar] [CrossRef]
- Sahraian, M.A.; Mohyeddin Bonab, M.; Baghbanian, S.M.; Owji, M.; Naser Moghadasi, A. Therapeutic use of intrathecal mesenchymal stem cells in patients with multiple sclerosis: A pilot study with booster injection. Immunol. Investig. 2019, 48, 160–168. [Google Scholar] [CrossRef]
- Yamout, B.; Hourani, R.; Salti, H.; Barada, W.; El-Hajj, T.; Al-Kutoubi, A.; Herlopian, A.; Baz, E.K.; Mahfouz, R.; Khalil-Hamdan, R. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J. Neuroimmunol. 2010, 227, 185–189. [Google Scholar] [CrossRef]
- Lunn, J.S.; Sakowski, S.A.; Hur, J.; Feldman, E.L. Stem cell technology for neurodegenerative diseases. Ann. Neurol. 2011, 70, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-G.; Zhang, Y.-Z.; Wu, H.-N.; Cao, X.-L.; Guo, C.-J.; Li, Y.-Q.; Zheng, M.-H.; Han, H. Stem cells: A promising candidate to treat neurological disorders. Neural. Regen. Res. 2018, 13, 1294–1304. [Google Scholar] [PubMed]
- Aktas, O.; Ullrich, O.; Infante-Duarte, C.; Nitsch, R.; Zipp, F. Neuronal damage in brain inflammation. Arch. Neurol. 2007, 64, 185–189. [Google Scholar] [CrossRef]
- Rolak, L.A. Multiple sclerosis: It’s not the disease you thought it was. Clin. Med. Res. 2003, 1, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lim, J.Y.; Lim, T.; Im, K.I.; Kim, N.; Nam, Y.S.; Jeon, Y.W.; Shin, J.C.; Ko, H.S.; Park, I.Y.; et al. Human mesenchymal stem cells derived from umbilical cord and bone marrow exert immunomodulatory effects in different mechanisms. World J. Stem Cells 2020, 12, 1032–1049. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Z.; Lu, Z.; Borlongan, C.; Pan, J.; Chen, J.; Qian, L.; Liu, Z.; Zhu, L.; Zhang, J.; et al. Human umbilical cord stem cells ameliorate experimental autoimmune encephalomyelitis by regulating immunoinflammation and remyelination. Stem Cells Dev. 2013, 22, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Kunkl, M.; Frascolla, S.; Amormino, C.; Volpe, E.; Tuosto, L. T Helper Cells: The Modulators of Inflammation in Multiple Sclerosis. Cells 2020, 9, 482. [Google Scholar] [CrossRef]
- Al Jumah, M.A.; Abumaree, M.H. The immunomodulatory and neuroprotective effects of mesenchymal stem cells (MSCs) in experimental autoimmune encephalomyelitis (EAE): A model of multiple sclerosis (MS). Int. J. Mol. Sci. 2012, 13, 9298–9331. [Google Scholar] [CrossRef]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922. [Google Scholar] [CrossRef]
- Urrutia, D.N.; Caviedes, P.; Mardones, R.; Minguell, J.J.; Vega-Letter, A.M.; Jofre, C.M. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLoS ONE 2019, 14, e0213032. [Google Scholar] [CrossRef]
- Mukhamedshina, Y.O.; Gracheva, O.A.; Mukhutdinova, D.M.; Chelyshev, Y.A.; Rizvanov, A.A. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural. Regen. Res. 2019, 14, 227–237. [Google Scholar] [CrossRef] [PubMed]






| Study ID [References] | Study Design | Country | Total Participants (Female) | Age (Mean ± SD/Range) (Years) | Patient Enrolment Time | Disease Duration (Mean ± SD/Range) (Years) | Types of MS with Corresponding Number of Participants | Source of MSCs | Amount of Cell Infusion | Method of Cell Suspension Administration | Follow–Up Period | Summary of Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nabavi 2023 [28] | Randomised controlled trial | Iran | 21 (16) | 35.29 ± 8.44 | December 2011– May 2014 | 9.71 ± 3.18 | RRMS: 14 SPMS: 5 PPMS: 2 | Bone marrow | 2 × 106 cells/kg | Intravenous | 18 months | Although efficacy findings were not notable based on EDSS score changes, no major adverse events were reported. |
| Cohen 2023 [29] | Clinical trial | USA | 18 (10) | 47.4 ± 9.6 | March 2019–March 2021 | 17.7 ± 7.9 | SPMS: 14 PPMS: 4 | Bone marrow | 5 mL, 100–125 million | Intrathecal | 28 weeks | Based on changes in EDSS score, MSCs therapy increased efficacy with some minor adverse events in patients. |
| Tremblay 2022 [30] | Randomised controlled trial | Canada | 20 (7) | 37.6 ± 6.9 for early and 37.6 ± 5.1 for delayed group | NR | 5.7 ± 2.9 for early and 6.6 ± 2.7 for delayed group | PPMS: 6 RRMS: 8 SPMS: 6 | Bone marrow | 1–2 × 106 MSCs/Kg | Intravenous | 48 weeks | MSCs therapy did not cause significant changes in the EDSS score, hence it does not improve neurophysiological and clinical outcomes in patients with MS. |
| Harris 2021 [31] | Clinical trial | 20 (14) | 49 (27–65) | 2014–2016 | 19 (10–32) | PPMS: 16 SPMS: 4 | Bone marrow | 9.4 × 106 cells | Intrathecal | 2 years | 39% of MS patients improved after MSCs therapy based on EDSS without serious adverse events. | |
| Uccelli 2021 [32] | Randomised controlled trial | Austria, Canada, Denmark, France, Italy, Iran, Spain, Sweden, and the UK | 144 (87) | 39.9 ± 6.70 | July 16, 2012–July 31, 2019 | 2–15 | PPMS: 6 RRMS: 8 SPMS: 6 | Bone marrow | 1–2 × 106 MSCs/Kg | Intravenous | 48 weeks | No significant changes in EDSS score occurred between the early and delayed group of MS patients. However, several adverse events were observed in the patients. |
| Petrou 2021 [33] | Clinical trial | Israel | 24 (12) | 47.0 ± 9.22 | NR | 13.4 ± 6.6 | SPMS: 22 PPMS: 2 | Bone marrow | 1 × 106 MSCs/Kg | Intravenous and Intrathecal | 4 years | EDSS score was shown to decline in the majority of the patients (71%), and rest of them were stable. Also, no serious adverse events were observed. |
| Petrou 2020 [34] | Randomised controlled trial | Israel | 48 (20) | 47.63 ± 9.72 | Feb 2015–June 2018 | 12.70 ± 7.51 | SPMS: 41 PPMS: 7 | Bone marrow | 1 × 106 MSCs/Kg | Intravenous and Intrathecal | 14 months | Following MSCs therapy, 53% and 38% of the MS patients were shown to be improved and stable, evidenced by the declining EDSS score. |
| Baldassari 2019 [35] | Clinical trial | USA | 22 (16) | 46.4 ± 5.2 | Mar 2011–Apr 2013 | 12.4 ± 9.4 | SPMS: 13 RRMS: 9 | Bone marrow and adipose tissue | NR | Intravenous | 6 months | Treatment with MSCs did not exhibit any significant alteration in the EDSS score among patients with MS. |
| Bonab 2005 [4] | Clinical trial | Iran | 5 (3) | 31.0 ± NR | NR | 6.0–15.0 | NR | Bone marrow | 5.5 mL; 6.0 × 106 cells | Intrathecal | 7 months | Although most of the patients did not improve according to EDSS score, the treatment procedure was considered to be safe. |
| Bonab 2007 [36] | Clinical trial | Iran | 10 (7) | 33.0 ± 5.9 | NR | 3.0–21.0 | SPMS: 8 PPMS: 2 | Bone marrow | 5.5 mL; 8.7 × 106 cells | Intrathecal | 13–26 months | Treatment with MSCs could not be demonstrated as an effective strategy as 50% of the patients exhibited an increased EDSS score when compared to baseline. |
| Bonab 2012 [37] | Clinical trial | Iran | 22 (18) | 18.0–50.0 | Jan 2008–Aug 2010 | ≤2– ≥ 15 | SPMS: 20 PRMS: 2 | Bone marrow | 10.0 mL; 29.5 × 106 cells | Intrathecal | 12 months | Administration was reported to be safe; however, almost all the patients exhibited fever. Most of the patients remained stable at the end of follow–up. |
| Llufriu 2014 [38] | Randomised controlled trial | Spain | 9 (7) | 36.8 ± 8.4 | Nov 2010–June 2012 | 8.1 ± 2.15 | All RRMS | Bone marrow | 1.03 × 106–2.16 × 106 (mean = 1.87 × 106) cells/kg | Intravenous | 12 months | No significant changes occurred in EDSS score after MSCs therapy, but it was considered to be safe. |
| Cohen 2017 [21] | Clinical trial | USA | 25 (17) | 46.4 ± 5.2 | NR | 15.4 ± 9.0 | SPMS: 14 RRMS: 10 | Bone marrow | 1.9 × 106 cells/kg | Intravenous | 6 months | Administration of MSCs showed a noteworthy efficacy (decline of EDSS in 71% of patients). Although 40% of the patients experienced some minor adverse events though the treatment procedure, it was overall well-tolerated. |
| Cornick 2012 [12] | Clinical trial | UK | 10 (3) | 48.8 ± 4.1 | Nov 2007–Aug 2010 | 14.4 ± 7.9 | All SPMS | Bone marrow | 1·6 × 10⁶ cells/kg | Intravenous | 10 months | Significant improvements were observed (p = 0.028) based on the EDSS score, and the treatment was safe except for some minor adverse events associated with infections. |
| Dahbour 2017 [39] | Clinical trial | Jordan | 10 (4) | 34.9 ± 9.5 | NR | 9.6 ± 2.9 | NR | Bone marrow | 18.3 mL; 110 × 106 cells | Intrathecal | 12 months | Treatment with MSCs did not lower the EDSS score of most of the patients; however, it was reported to be safe, and some minor adverse events were observed. |
| De Oliveira 2015 [40] | Clinical trial | Brazil | 44 (30) | 37.3 ± 9.4 | NR | 4.0–20.0 | SPMS: 34 PPMS: 3 RRMS: 7 | Bone marrow | NR | NR | 6 months | EDSS score declines in one-fourth of the patients, and 60% remained stable. |
| Fernandez 2018 [9] | Randomised controlled trial | Spain | 30 (21) | 46.3 ± 8.9 | NR | 17.7 ± 7.4 | All SPMS | Adipose tissue | Low dose: 1.0 × 106 cells/kg high dose: 4.0 x 106 cells/kg | Intravenous | 12 months | No significant change was noticed in the mean EDSS level upon completion of the trial. |
| Harris 2016 [41] | Clinical trial | USA | 6 (4) | 28.0–64.0 | 2005–2007 | 7.0–27.0 | SPMS: 4 PPMS: 2 | Bone marrow | 0.06 × 106 cells–16.0 × 106 cells | Intrathecal | 7.4 years | Treatment with MSCs depicted an effective outcome, as 66.6% were improved and the rest were stable. |
| Harris 2018 [42] | Clinical trial | USA | 20 (6) | 27.0–65.0 | NR | 10.0–32.0 | SPMS: 16 PPMS: 4 | Bone marrow | 9.4 × 106 cells | Intrathecal | 12 months | 40% of the patients showed a declined EDSS score. Although overall the treatment was safe and well-tolerated, headache occurred in 85% of the patients. |
| Iacobaeus 2019 [43] | Clinical trial | Sweden | 7 (6) | 18.0–50.0 | Oct 2012–Jan 2015 | 2.0–20.0 | SPMS: 5 PPMS: 2 | Bone marrow | 1.0–2.0 × 106 cells/kg | Intrathecal | 48 weeks | 60% of the patients improved, and the rest remained stable. |
| Karussis 2010 [22] | Clinical trial | Israel | 15 (8) | 35.3 ± 8.6 | NR | 10.7 ± 2.9 | NR | Bone marrow | 63.2 ± 2.5 × 106 cells | Intrathecal | 6 months | EDSS score declined significantly; however, 66.6% of the participants suffered from fever and headache. |
| Li 2014 [10] | Randomised controlled trial | China | 13 (9) | 41.7 ± 5.6 | Jan 2010–Dec 2012 | 2.9 ± 0.9 | NR | Umbilical cord | 4.0 × 106 cells/kg | Intravenous | 12 months | Marginal decrease of EDSS score was observed, indicating it as an efficacious strategy. |
| Lu 2013 [44] | Clinical trial | China | 8 (6) | 18.0–59.0 | May 2010–Dec 2010 | >4.0 | All SPMS | Umbilical cord | Day 0: 40 mL, day 7, 14 and 21: 20 mL; 2.0 × 107 cells | Intravenous | 18 months | The treatment with MSCs was highly efficacious, and the EDSS scores of 75% of the patients decreased. |
| Lublin 2014 [45] | Randomised controlled trial | USA and Canada | 16 (11) | 18.0–65.0 | NR | ≥2.0 | SPMS: 6 RRMS: 10 | Placenta | 240 mL; Low dose: 150.0 × 106 cells, high dose: 600.0 × 106 cells | Intravenous | 12 months | This study exhibited a mixed outcome in terms of the EDSS score, although the rate of improvement was slightly satisfactory. |
| Meng 2018 [11] | Clinical trial | China | 3 (1) | 30.0–33.0 | NR | 5.0–9.0 | SPMS: 2 RRMS: 1 | Umbilical cord | 1.0–2.0 × 106 cells/kg. | Intravenous | 10 years | With a prolonged follow-up period, 50% of the participants improved in case of EDSS score. |
| Odinak 2012 [46] | Clinical trial | Russia | 8 (3) | 24.0–47.0 | NR | 4.0–14.0 | SPMS: 3 RRMS: 3 PPMS: 2 | Bone marrow | 2.0 × 106 cells/kg. | Intravenous | 12 months | Treatment with MSCs was highly efficacious, with 75% improvements and no notable adverse events. |
| Riordan 2018 [47] | Clinical trial | Panama | 20 (12) | 41.1 ± 9.2 | Oct 2014–Feb 2015 | 7.7 ± NR | SPMS: 1 RRMS: 15 PPMS: 4 | Umbilical cord | 20.0 × 106 cells/day | Intravenous | 12 months | A mean decrease of 0.68 ± 1.49 was observed in the overall population. |
| Sahraian 2013 [48] | Clinical trial | Iran | 10 (3) | 28.0 ± 4.3 | NR | 3.0–16.0 | All SPMS | Bone marrow | 5.5 mL; 7.5 × 106 cells | Intrathecal | 5 years | Treatment with MSCs was not highly efficacious, as there was a mixture of improvement and worsening of the disease condition. |
| Sahraian 2019 [49] | Clinical trial | Iran | 4 (1) | 26.0–31.0 | NR | 5.0–10.0 | SPMS: 3 RRMS:1 | Bone marrow | 57.0 × 106 cells | Intrathecal | 2 years | 75% of the participants improved or remained stable following the MSC therapy, with no major adverse events. |
| Yamout 2010 [50] | Clinical trial | Lebanon | 10 (6) | 34.0–56.0 | NR | 11.0–31.0 | SPMS: 9 RRMS: 1 | Bone marrow | 10.0 mL × 106 cells | Intrathecal | 12 months | Treatment with MSCs was efficacious, and the EDSS score declined for half of the patients. The treatment procedure was also reported to be safe. |
| Adverse Events | Adverse Events [95% CIs] (%) | Number of Studies Analysed | Total Number of Multiple Sclerosis Patients | Heterogeneity | |
|---|---|---|---|---|---|
| I2 | p–Value | ||||
| Headache | 57.6 [37.9–77.3] | 15 | 236 | 94% | <0.01 |
| Fever | 53.1 [20.7–85.4] | 10 | 146 | 98% | <0.01 |
| Urinary tract infection | 23.9 [9.5–38.3] | 7 | 132 | 81% | <0.01 |
| Respiratory tract infection | 7.9 [0.7–15.1] | 5 | 94 | 41% | 0.15 |
| Dizziness | 28.8 [5.6–51.9] | 4 | 64 | 84% | <0.01 |
| Fatigue | 26.5 [0.0–54.3] | 4 | 91 | 94% | <0.01 |
| Skin disorder | 23.7 [1.0–46.3] | 4 | 55 | 85% | <0.01 |
| Back pain | 26.5 [1.5–51.5] | 5 | 104 | 93% | <0.01 |
| Balance disorder | 22.8 [9.7–36.0] | 2 | 39 | 0% | 0.68 |
| Depression | 7.6 [0.0–15.3] | 2 | 44 | 0% | 0.37 |
| Fall | 18.0 [6.7–29.2] | 4 | 79 | 38% | 0.18 |
| Rash | 4.2 [0.0–9.9] | 3 | 47 | 0% | 0.53 |
| Musculoskeletal stiffness | 12.6 [3.9–21.4] | 3 | 55 | 0% | 0.97 |
| Sinusitis | 16.3 [0.0–46.5] | 2 | 37 | 56% | 0.13 |
| Cervical pain | 3.7 [0.0–9.6] | 2 | 38 | 0% | 0.38 |
| Injection site pain | 40.3 [3.3–77.2] | 2 | 33 | 82% | 0.02 |
| Outcomes | Prevalence [95% CIs] (%) | Number of Studies Analysed | Total Number of Multiple Sclerosis Patients | Heterogeneity | |
|---|---|---|---|---|---|
| I2 | p–Value | ||||
| Follow-up: ≤6 months | |||||
| Improved | 45.8 [20.2–71.5] | 4 | 84 | 82% | <0.01 |
| Stable | 35.6 [11.2–60.0] | 82% | <0.01 | ||
| Worsened | 15.4 [3.9–26.8] | 48% | 0.12 | ||
| Follow-up: >6 to 12 months | |||||
| Improved | 31.5 [17.8–45.2] | 9 | 108 | 65% | <0.01 |
| Stable | 34.9 [22.0–47.9] | 8 | 92 | 47% | 0.07 |
| Worsened | 22.8 [13.2–32.4] | 8 | 92 | 23% | 0.25 |
| Follow-up: >12 months | |||||
| Improved | 48.0 [31.3–64.7] | 9 | 93 | 65% | <0.01 |
| Stable | 29.9 [20.8–39.0] | 0% | 0.79 | ||
| Worsened | 15.3 [4.5–26.0] | 50% | 0.04 | ||
| Bone-marrow-derived stem cells | |||||
| Improved | 38.5 [28.2–48.9] | 19 | 263 | 71% | <0.01 |
| Stable | 34.1 [26.0–42.3] | 18 | 247 | 47% | 0.01 |
| Worsened | 18.4 [11.7–25.0] | 18 | 247 | 48% | 0.01 |
| Umbilical cord or placenta-derived stem cells | |||||
| Improved | 56.7 [33.3–80.1] | 3 | 22 | 22% | 0.28 |
| Stable | 23.0 [6.2–39.9] | 0% | 0.37 | ||
| Worsened | 15.8 [0.3–31.4] | 0% | 0.56 | ||
| Intravenous administration | |||||
| Improved | 57.6 [44.1–71.0] | 7 | 79 | 35% | 0.16 |
| Stable | 18.6 [9.2–28.0] | 6 | 63 | 0% | 0.76 |
| Worsened | 15.9 [7.9–23.9] | 7 | 79 | 0% | 0.83 |
| Intrathecal administration | |||||
| Improved | 32.8 [21.6–44.0] | 14 | 159 | 63% | <0.01 |
| Stable | 37.4 [29.3–45.5] | 12 | 127 | 0% | 0.47 |
| Worsened | 22.3 [10.7–33.9] | 12 | 125 | 70% | <0.01 |
| Strategies of Sensitivity Analyses | Efficacy [95% Cis] (%) | Difference of Pooled Prevalence Compared to the Main Result | Number of Studies Analysed | Total Number of Multiple Sclerosis Patients |
|---|---|---|---|---|
| Excluding low-quality studies | ||||
| Improved | 40.4 [30.6–50.2] | Unchanged | 22 | 285 |
| Stable | 32.8 [25.5–40.1] | Unchanged | 21 | 269 |
| Worsened | 18.1 [12.0–24.2] | Unchanged | 21 | 269 |
| Excluding small studies | ||||
| Improved | 33.5 [22.2–44.7] | 6.9% lower | 11 | 219 |
| Stable | 39.2 [29.2–49.3] | 6.4% higher | 10 | 203 |
| Worsened | 20.3 [13.0–27.6] | 2.2% higher | 10 | 203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.A.; Alam, S.S.; Kundu, S.; Ahmed, S.; Sultana, S.; Patar, A.; Hossan, T. Mesenchymal Stem Cell Therapy in Multiple Sclerosis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 6311. https://doi.org/10.3390/jcm12196311
Islam MA, Alam SS, Kundu S, Ahmed S, Sultana S, Patar A, Hossan T. Mesenchymal Stem Cell Therapy in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(19):6311. https://doi.org/10.3390/jcm12196311
Chicago/Turabian StyleIslam, Md Asiful, Sayeda Sadia Alam, Shoumik Kundu, Saleh Ahmed, Shabiha Sultana, Azim Patar, and Tareq Hossan. 2023. "Mesenchymal Stem Cell Therapy in Multiple Sclerosis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 19: 6311. https://doi.org/10.3390/jcm12196311
APA StyleIslam, M. A., Alam, S. S., Kundu, S., Ahmed, S., Sultana, S., Patar, A., & Hossan, T. (2023). Mesenchymal Stem Cell Therapy in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(19), 6311. https://doi.org/10.3390/jcm12196311







