Examination of Myofascial Stiffness and Elasticity in the Upper Trapezius Region in Patients with Unilateral Neck Pain: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Ethics
2.2. Participants
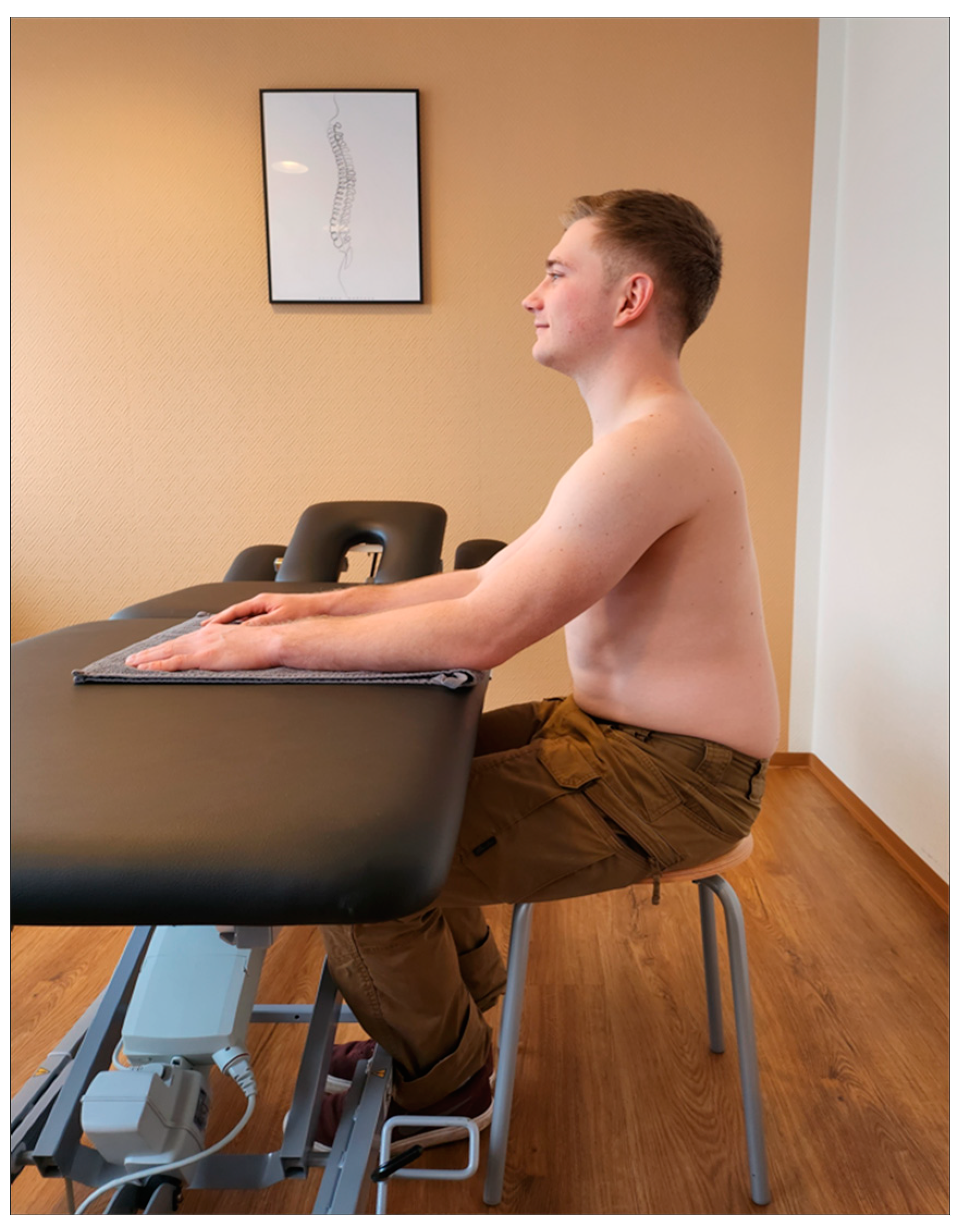
2.3. Measurements
2.4. Data Analysis and Statistics
3. Results
3.1. Study Group Characteristics
3.2. General Correlations between Age, Gender, Pain, and Mobility
3.3. Comparison of Symptomatic and Non-Symptomatic Side
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haldeman, S.; Caroll, L.; Cassidy, D. The Bone and Joint Decade 2000–2010. Task Force on Neck Pain and Its Associated Disorders. Spine 2008, 33, S5–S7. [Google Scholar] [CrossRef]
- Petersen, S.M.; Wyatt, S.N. Lower trapezius muscle strength in individuals with unilateral neck pain. J. Orthop. Sport. Phys. Ther. 2011, 41, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, R.; Anap, D.; Rao, K.; Iyer, C. Comparison of upper, middle, and lower trapezius strength in individuals with unilateral neck pain. J. Spine 2012, 1, 3. [Google Scholar] [CrossRef]
- Park, K.N.; Jung, D.Y.; Kim, S.H. Trapezius and serratus anterior muscle strength in violinists with unilateral neck pain. J. Back Musculoskelet. Rehabil. 2020, 33, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Uthaikhup, S.; Pensri, C.; Kawsoiy, K. Decreased thickness of the lower trapezius muscle in patients with unilateral neck pain. Muscle Nerve 2016, 54, 439–443. [Google Scholar] [CrossRef]
- Kuo, W.H.; Jian, D.W.; Wang, T.G.; Wang, Y.C. Neck muscle stiffness quantified by sonoelastography is correlated with body mass index and chronic neck pain symptoms. Ultrasound Med. Biol. 2013, 39, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Kisilewicz, A.; Madeleine, P.; Ignasiak, Z.; Ciszek, B.; Kawczynski, A.; Larsen, R.G. Eccentric exercise reduces upper trapezius muscle stiffness assessed by shear wave elastography and myotonometry. Front. Bioeng. Biotechnol. 2020, 8, 928. [Google Scholar] [CrossRef]
- Taş, S.; Korkusuz, F.; Erden, Z. Neck muscle stiffness in participants with and without chronic neck pain: A shear-wave elastography study. J. Manip. Physiol. Ther. 2018, 41, 580–588. [Google Scholar] [CrossRef]
- Kocur, P.; Tomczak, M.; Wiernicka, M.; Goliwąs, M.; Lewandowski, J.; Łochyński, D. Relationship between age, BMI, head posture and superficial neck muscle stiffness and elasticity in adult women. Sci. Rep. 2019, 9, 8515. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- MacDermid, J.C.; Walton, D.M.; Avery, S.; Blanchard, A.; Etruw, E.; Mcalpine, C.; Goldsmith, C.H. Measurement properties of the neck disability index: A systematic review. J. Orthop. Sport. Phys. Ther. 2009, 39, 400–417. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Okawara, H.; Nakashima, D.; Iwabuchi, S.; Matsumoto, M.; Nakamura, M.; Nagura, T. Reliability of trapezius muscle hardness measurement: A comparison between portable muscle hardness meter and ultrasound strain elastography. Sensors 2020, 20, 7200. [Google Scholar] [CrossRef] [PubMed]
- Hochschild, J. Strukturen und Funktionen Begreifen. Grundlagen zur Wirbelsäule, HWS, Schädel, BWS und Brustkorb, Obere Extremität; Thieme-Verlag: Leipzig, Germany, 2015; Volume 4. [Google Scholar]
- Masi, A.T.; Andrade, C.K. Das Konzept Ruhemuskeltonus: Integrativ „übersetzt “. Manuelletherapie 2014, 18, 69–73. [Google Scholar] [CrossRef]
- Dieterich, A. Gefühlt steif heißt nicht objektiv steif–Myth-Busters: Die verspannte Nackenmuskulatur. Physiopraxis 2020, 18, 20–24. [Google Scholar] [CrossRef]
- Bartsch, K.; Brandl, A.; Weber, P.; Wilke, J.; Bensamoun, S.F.; Bauermeister, W.; Klingler, W.; Schleip, R. Assessing reliability and validity of different stiffness measurement tools on a multi-layered phantom tissue model. Sci. Rep. 2023, 13, 815. [Google Scholar] [CrossRef]
- Dellalana, L.E.; Chen, F.; Vain, A.; Gandelman, J.S.; Põldemaa, M.; Chen, H.; Tkaczyk, E.R. Reproducibility of the durometer and myoton devices for skin stiffness measurement in healthy subjects. Ski. Res. Technol. 2019, 25, 289–293. [Google Scholar] [CrossRef]
- Park, S.K.; Yang, D.J.; Kim, J.H.; Heo, J.W.; Uhm, Y.H.; Yoon, J.H. Analysis of mechanical properties of cervical muscles in patients with cervicogenic headache. J. Phys. Ther. Sci. 2017, 29, 332–335. [Google Scholar] [CrossRef]
- Akhbari, B.; Salavati, M.; Ebarahimi, I.; Ezzati, K.; Haghighat Khah, H. Association of ultrasonography findings with pain, range of motion, disability, and pressure pain threshold in subjects with upper trapezius myofascial pain syndrome. Phys. Treat.-Specif. Phys. Ther. J. 2015, 4, 221–227. [Google Scholar]
- Koch, V.; Wilke, J. Reliability of a new indentometer device for measuring myofascial tissue stiffness. J. Clin. Med. 2022, 11, 5194. [Google Scholar] [CrossRef]
- Bartsch, K.; Brandl, A.; Weber, P.; Wilke, J.; Bensamoun, S.F.; Bauermeister, W.; Klingler, W.; Schleip, R. The princess and the pea-Comparison of different stiffness assessment tools on a multi-layered phantom tissue model. 2022. Available online: https://doi.org/10.21203/rs.3.rs-2039032/v1 (accessed on 27 September 2023). [CrossRef]
- Von Pickartz, H.; Andreotti, D.; Arendt-Nielsen, L. Kiefer, Gesichts- und Zervikalregion; Thieme-Verlag: Stuttgart, Germany, 2015; Volume 2. [Google Scholar]
- Grossi, D.B.; Chaves, T.C.; Gonçalves, M.C.; Moreira, V.C.; Canonica, A.C.; Florencio, L.L.; Bordini, C.A.; Speciali, J.G. Pressure pain threshold in the craniocervical muscles of women with episodic and chronic migraine: A controlled study. Arq. Neuro-Psiquiatr. 2011, 69, 607–612. [Google Scholar] [CrossRef]
- Rolke, R.; Magerl, W.; Campbell, K.A.; Schalber, C.; Caspari, S.; Birklein, F.; Treede, R.D. Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur. J. Pain 2006, 10, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, K.; Schoettker-Königer, T.; Hall, T.; Reimer, C.; Grassold, M.; Hasselhoff-Styhler, P.; Neulinger, C.; Obrocki, M.; Przyhoda, P. Concurrent validity and reliability of measuring range of motion during the cervical flexion rotation test with a novel digital goniometer. BMC Musculoskelet. Disord. 2020, 21, 535. [Google Scholar] [CrossRef]
- Stone, E.R. T Test—Paired Samples. Encyclopedia of Research Design; Stalkind, N.J., Ed.; SAGE: Los Angeles, CA, USA, 2010; pp. 1560–1565. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 115–119. [Google Scholar] [CrossRef]
- Kim, C.Y.; Kang, J.H.; Park, T.S. Intra-Rater Reliability of the Shore Durometer in the Assessment of Upper Trapezius Muscle Hardness. Res. J. Pharm. Technol. 2019, 12, 2461–2464. [Google Scholar] [CrossRef]
- Brandl, A.; Egner, C.; Schwarze, M.; Reer, R.; Schmidt, T.; Schleip, R. Immediate Effects of Instrument-Assisted Soft Tissue Mobilization on Hydration Content in Lumbar Myofascial Tissues: A Quasi-Experiment. J. Clin. Med. 2023, 12, 1009. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Immediate Effects of Myofascial Release Treatment on Lumbar Microcirculation: A Randomized, Placebo-Controlled Trial. J. Clin. Med. 2023, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Pruyn, E.C.; Watsford, M.L.; Murphy, A.J.; Pine, M.J.; Spurrs, R.W.; Cameron, M.L.; Johnston, R.J. Relationship between leg stiffness and lower body injuries in professional Australian football. J. Sports Sci. 2012, 30, 71–78. [Google Scholar] [CrossRef]
- Von Piekartz, H.J.M. Untersuchung und Behandlung des kranialen Nervengewebes am Beispiel des N. accessorius. Manuelletherapie 2005, 9, 237–241. [Google Scholar] [CrossRef]
- Hockenholz, F.; Roos, K.; Emmert, A. Physiotherapie bei Schmerzen; Thieme-Verlag: Stuttgart, Germany, 2016. [Google Scholar]
- Schleip, R.; Jäger, H.; Klingler, W. What is ‘fascia’? A review of different nomenclatures. J. Bodyw. Mov. Ther. 2012, 16, 496–502. [Google Scholar] [CrossRef]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study. Life 2022, 12, 1735. [Google Scholar] [CrossRef]
- Ng, C.P.; Hinz, B.; Swartz, M.A. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J. Cell Sci 2005, 118, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Pavan, P.G.; Stecco, A.; Stern, R.; Stecco, C. Painful Connections: Densification Versus Fibrosis of Fascia. Curr. Pain Headache Rep. 2014, 18, 441. [Google Scholar] [CrossRef]
- Böhni, U.; Lauper, M.; Lochner, H. Manuelle Medizin 2; Thieme-Verlag: Leipzig, Germany, 2012; pp. 298–302. [Google Scholar] [CrossRef]
- Heizelmann, A.; Tasdemir, S.; Schmidberger, J.; Gräter, T.; Kratzer, W.; Grüner, B. Measurements of the trapezius and erector spinae muscles using virtual touch imaging quantification ultrasound-Elastography: A cross section study. BMC Musculoskelet. Disord. 2017, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Rizo, A.M.; Petersen, K.K.; Arendt-Nielsen, L.; Madeleine, P. Eccentric training changes the pressure pain and stiffness maps of the upper trapezius in females with chronic neck-shoulder pain: A preliminary study. Pain Med. 2020, 21, 1936–1946. [Google Scholar] [CrossRef]
- Geri, T.; Botticchio, A.; Rossettini, G.; Pournajaf, S.; Pellicciari, L.; Di Antonio, S.; Castaldo, M. Pressure Pain Threshold of the Upper Trapezius Trigger Point: A Systematic Review with Meta-Analysis of Baseline Values and Their Modification after Physical Therapy. J. Clin. Med. 2022, 11, 7243. [Google Scholar] [CrossRef] [PubMed]
- Arokoski, J.P.; Surakka, J.; Ojala, T.; Kolari, P.; Jurvelin, J.S. Feasibility of the use of a novel soft tissue stiffness meter. Physiol. Meas. 2005, 26, 215. [Google Scholar] [CrossRef]
- Viir, R.; Virkus, A.; Laiho, K.; Rajaleid, K.; Selart, A.; Mikkelsson, M. Trapezius muscle tone and viscoelastic properties in sitting and supine positions. Scand. J. Work. Environ. Health 2007, 33, 76. [Google Scholar]






| Epidemiological Data | Participants (n = 40) | |
|---|---|---|
| Gender (men/women) | 7/33 | |
| Age (years) | mean ± SD | 42.2 ± 14.7 |
| Size (m) | mean ± SD | 1.66 ± 0.1 |
| Weight (kg) | mean ± SD | 72.9 ± 17.5 |
| BMI (kg/m2) | mean ± SD | 26.5 ± 5.9 |
| Handedness (right/left) | 37/3 | |
| Painful side (right/left) | 25/15 | |
| Pain intensity (VAS) | mean ± SD | 5.7 ± 1.5 |
| Headache frequency (0/1/2) 1 | 18/16/6 | |
| Headache intensity (0/1/2) 2 | 18/5/15/2 |
| Variable 1 | Variable 2 | Correlation | p-Value * |
|---|---|---|---|
| Durometer | IndentoPro | 0.29 1 | 0.010 |
| VAS | Headache | 0.16 2 | 0.474 |
| VAS | Headache intensity | 0.17 2 | 0.416 |
| Gender | Headache | 0.06 2 | 1 |
| Gender | Headache intensity | 0.08 2 | 0.700 |
| Handedness | Painful side | 0.09 2 | 0.311 |
| Age | Lateral flexion SS | −0.47 1 | 0.008 |
| Age | Lateral flexion HS | −0.31 1 | 0.203 |
| Age | Rotation SS | −0.30 1 | 0.233 |
| Age | Rotation HS | −0.25 1 | 0.491 |
| Age | Elasticity SS | −0.361 | 0.048 |
| Age | Elasticity HS | −0.10 1 | 1 |
| Outcome | MW ± SD | 95%-CI | t-Test (T) | df | p-Value * | |
|---|---|---|---|---|---|---|
| Algometry (g) | SS | 36.41 ± 17.53 | 30.80–42.02 | −0.447 | 39 | 0.657 |
| HS | 37.22 ± 17.00 | 31.78–42.65 | ||||
| Lateral flexion (°) | SS | 37.08 ± 8.15 | 34.47–39.68 | −0.590 | 39 | 0.559 |
| HS | 37.73 ± 7.61 | 35.29–40.15 | ||||
| Rotation (°) | SS | 73.55 ± 12.37 | 69.59–77.51 | 0.443 | 39 | 0.660 |
| HS | 72.85 ± 11.10 | 69.30–76.40 | ||||
| Durometer (kPa) | SS | 33.76 ± 7.78 | 31.27–36.25 | 4.995 | 39 | <0.001 |
| HS | 29.75 ± 7.45 | 27.37–32.13 | ||||
| IndentoPro (kPa) | SS | 59.73 ± 33.93 | 48.88–70.59 | 2.352 | 39 | 0.024 |
| HS | 49.18 ± 12.69 | 45.12–53.23 | ||||
| Elasticity (N/mm) | SS | 0.101 ± 1.09 | −0.246–0.448 | −0.822 | 39 | 0.416 |
| HS | -0.006 ± 0.29 | −0.098–0.087 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidel, A.; Brandl, A.; Egner, C.; Schleip, R. Examination of Myofascial Stiffness and Elasticity in the Upper Trapezius Region in Patients with Unilateral Neck Pain: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 6339. https://doi.org/10.3390/jcm12196339
Seidel A, Brandl A, Egner C, Schleip R. Examination of Myofascial Stiffness and Elasticity in the Upper Trapezius Region in Patients with Unilateral Neck Pain: A Cross-Sectional Study. Journal of Clinical Medicine. 2023; 12(19):6339. https://doi.org/10.3390/jcm12196339
Chicago/Turabian StyleSeidel, Anika, Andreas Brandl, Christoph Egner, and Robert Schleip. 2023. "Examination of Myofascial Stiffness and Elasticity in the Upper Trapezius Region in Patients with Unilateral Neck Pain: A Cross-Sectional Study" Journal of Clinical Medicine 12, no. 19: 6339. https://doi.org/10.3390/jcm12196339





