Mitral Regurgitation Severity Assessment after Transcutaneous Edge-to-Edge Mitral Valve Repair: Recommended Integration versus Volumetric Assessment Guidelines
Abstract
:1. Introduction
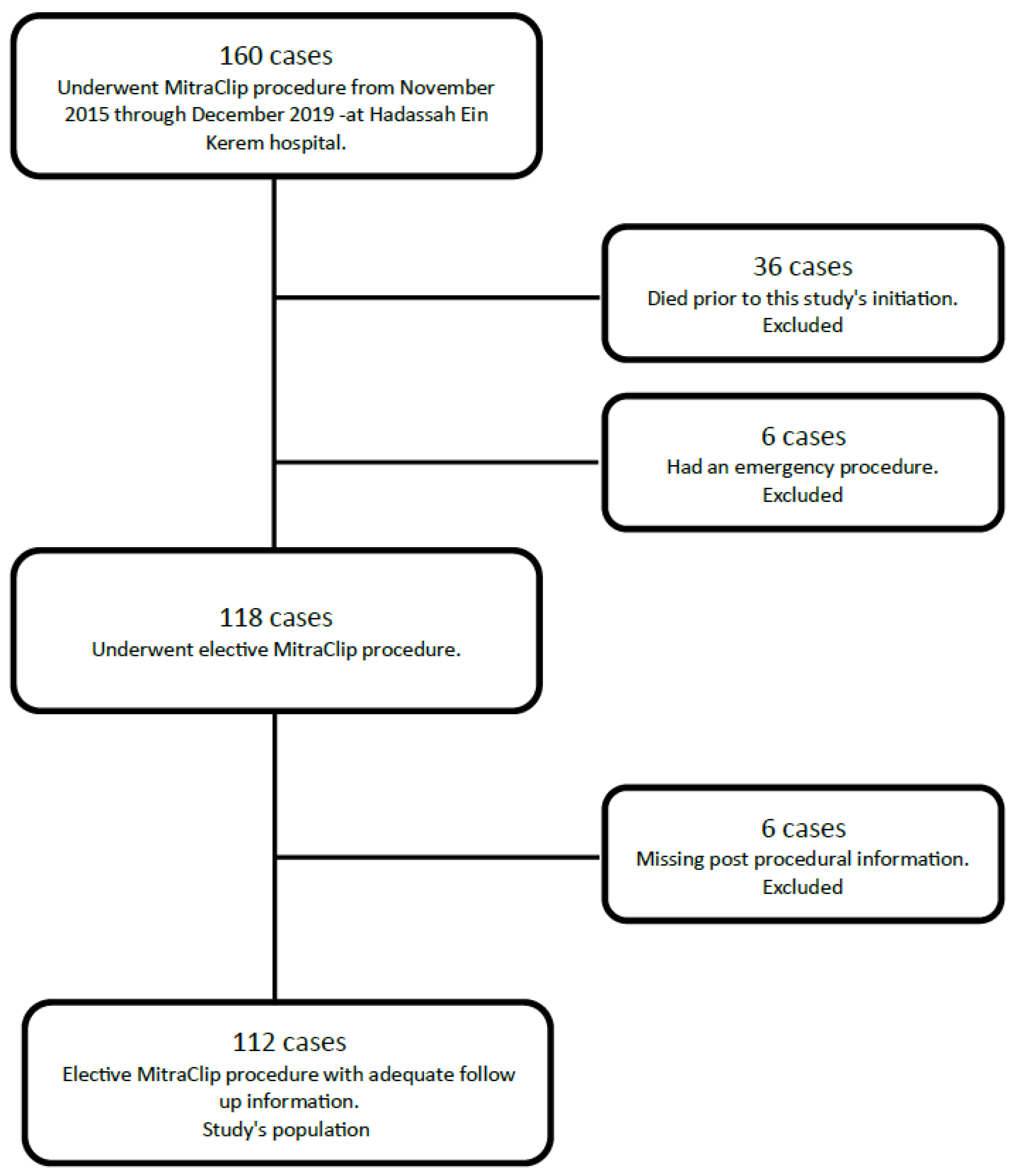
2. Methods
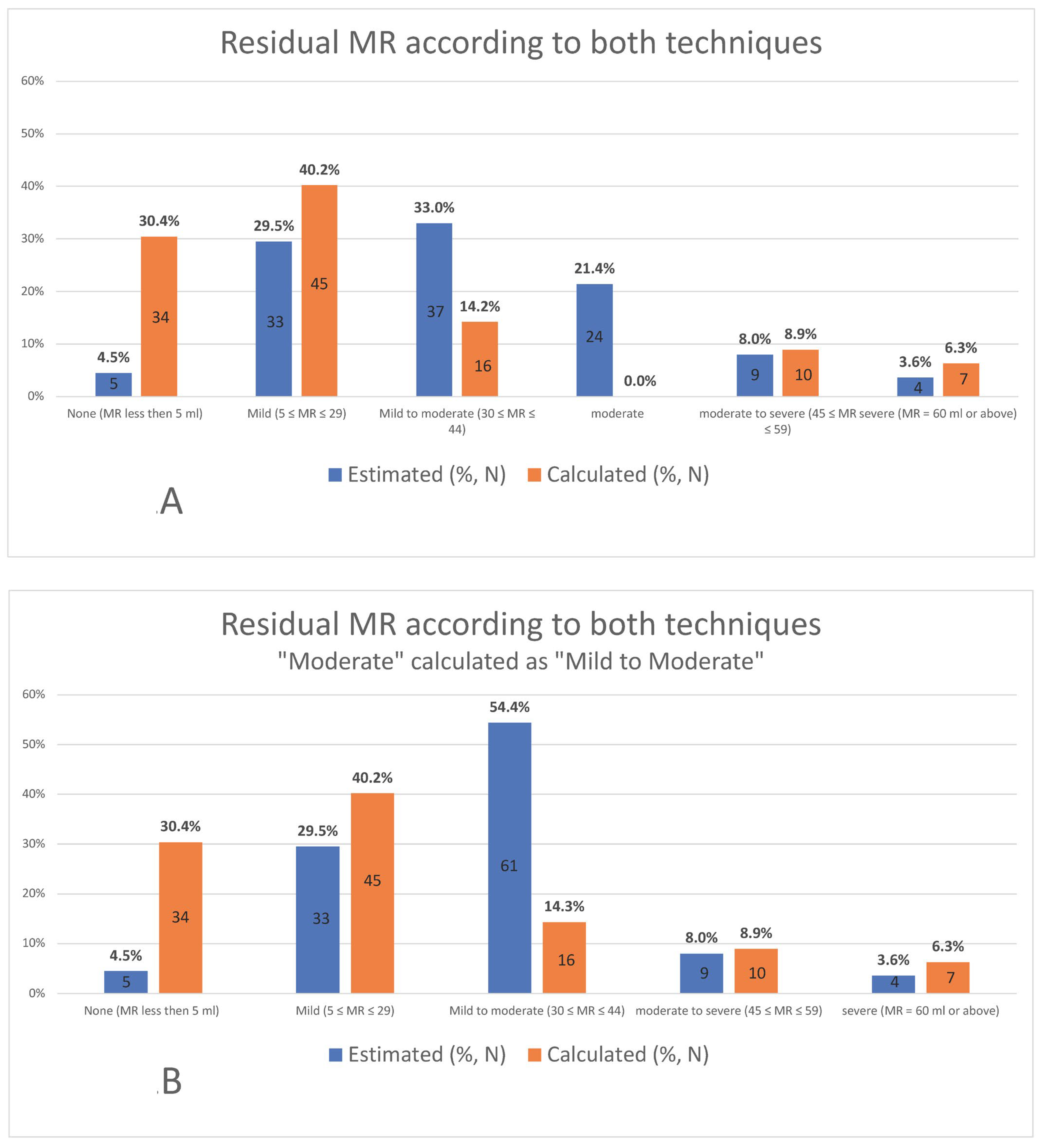
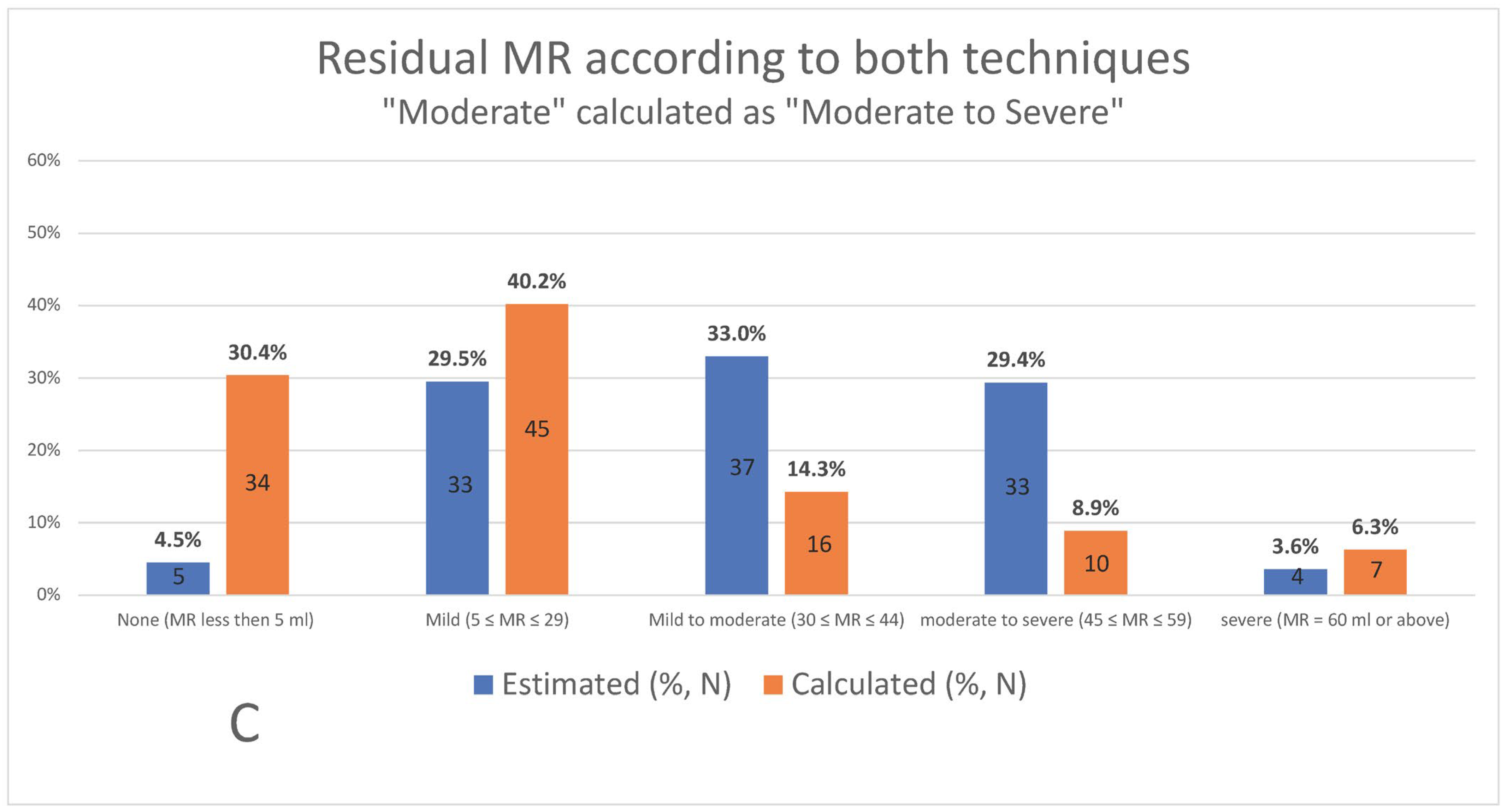
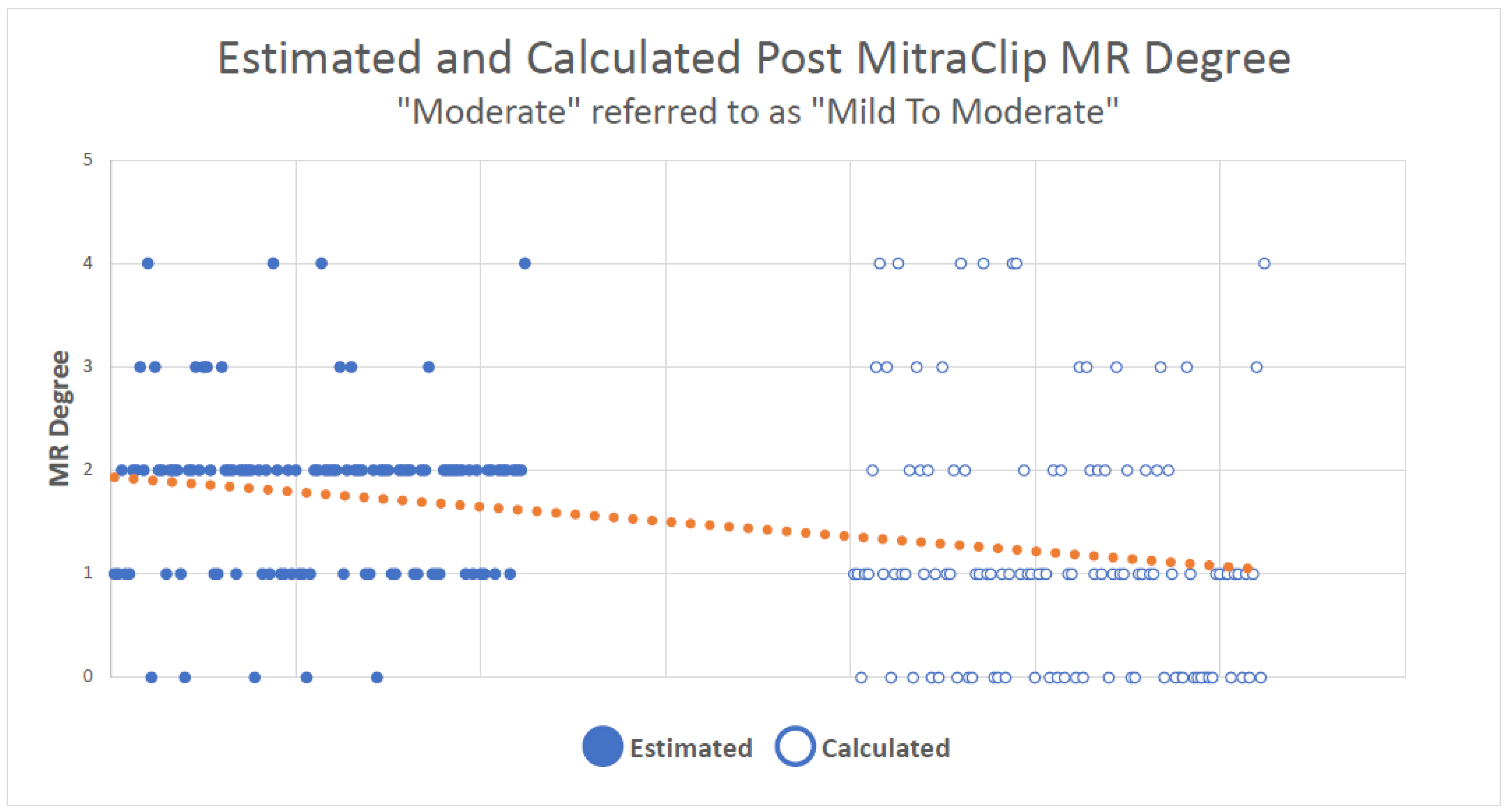
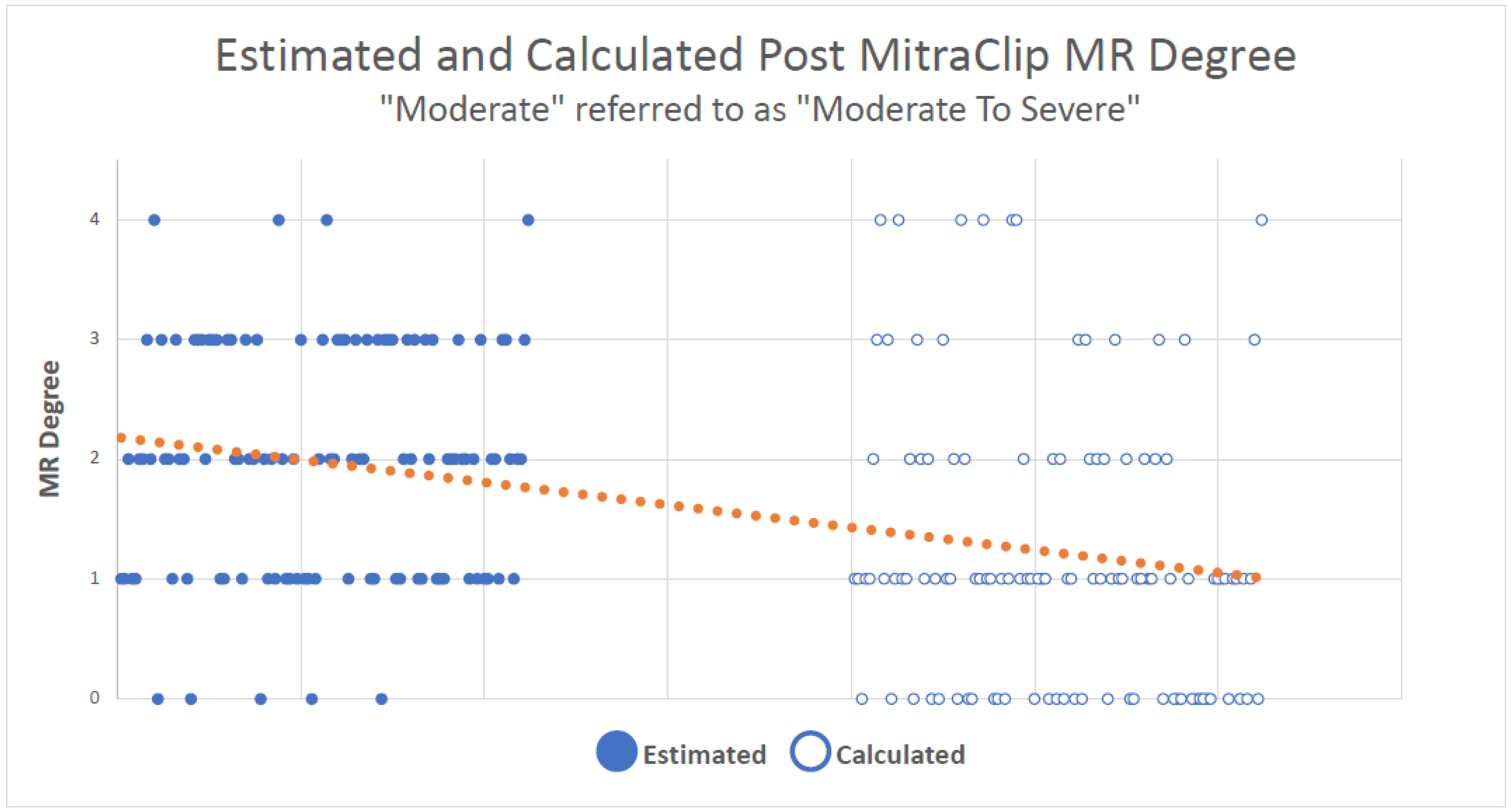
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| Non. | Standard Abbreviations and Acronyms |
| MR | Mitral regurgitation |
| TEER | Trans cutaneous edge-to-edge repair |
| TTE | Transthoracic echocardiography |
| CFD | Color flow Doppler |
| RVol | Residual volume |
| LV | Left ventricle |
| EDS | End-diastolic volume |
| ESV | End-systolic volume |
| SV | Stroke volume |
| LVOT | Left ventricle outflow tract |
References
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease; Oxford Academic: Oxford, UK, 2017; Volume 38, ISBN 9780198784906. [Google Scholar]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous Repair or Surgery for Mitral Regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.; Wasserman, H.S.; Gray, W.; Homma, S.; Di Tullio, M.R.; Rodriguez, L.; Stewart, W.J.; Whitlow, P.; Block, P.; Martin, R.; et al. Quantitative Assessment of Severity of Mitral Regurgitation by Serial Echocardiography in a Multicenter Clinical Trial of Percutaneous Mitral Valve Repair. Am. J. Cardiol. 2007, 100, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Kraft, C.D.; Levine, R.A.; Nihoyannopoulos, P.; Otto, C.M.; Quinones, M.A.; Rakowski, H.; et al. Recommendations for Evaluation of the Severity of Native Valvular Regurgitation with Two-dimensional and Doppler Echocardiography. J. Am. Soc. Echocardiogr. 2003, 16, 777–802. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Asch, F.M.; Bruce, C.; Gillam, L.D.; Grayburn, P.A.; Hahn, R.T.; Inglessis, I.; Islam, A.M.; Lerakis, S.; Little, S.H.; et al. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Ja. J. Am. Soc. Echocardiogr. 2019, 32, 431–475. [Google Scholar] [CrossRef] [PubMed]
- Maisano, F.; La Canna, G.; Colombo, A.; Alfieri, O. The evolution from surgery to percutaneous mitral valve interventions: The role of the edge-to-edge technique. J. Am. Coll. Cardiol. 2011, 58, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.A.; Forouhar, A.S.; Pahlevan, N.M.; Anastassiou, C.A.; Grayburn, P.A.; Thomas, J.D.; Gharib, M. Color Doppler jet area overestimates regurgitant volume when multiple jets are present. J. Am. Soc. Echocardiogr. 2010, 23, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Mack, M.J.; Lindenfeld, J.A.; Abraham, W.T.; Asch, F.M.; Weissman, N.J.; Enriquez-Sarano, M.; Lim, D.S.; Mishell, J.M.; Whisenant, B.K.; et al. Relationship between Residual Mitral Regurgitation and Clinical and Quality-of-Life Outcomes after Transcatheter and Medical Treatments in Heart Failure: COAPT Trial. Circulation 2021, 144, 426–437. [Google Scholar] [CrossRef] [PubMed]
- El Shaer, A.; Thaden, J.; Eleid, M.; Simard, T.; Guerrero, M.; Rihal, C.S.; Alkhouli, M. Hemodynamic Success Is an Independent Predictor of Mid-Term Survival after Transcatheter Edge-to-Edge Mitral Valve Repair. Circ. Cardiovasc. Interv. 2022, 15, E011542. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; Hagendorff, A.; Monin, J.L.; Badano, L.; Zamorano, J.L.; et al. European association of echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Tajik, A.J. Quantitative hemodynamics by Doppler echocardiography: A noninvasive alternative to cardiac catheterization. Prog. Cardiovasc. Dis. 1994, 36, 309–342. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocard. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Zamorano, J.L.; Vannan, M.A. Imaging challenges in secondary mitral regurgitation unsolved issues and perspectives. Circ. Cardiovasc. Imaging 2014, 7, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Gavazzoni, M.; Kuwata, S.; Meier, P.; Maisano, F. From Color to Hemodynamic Assessment: Is it Time to Change the Paradigm in Judging MitraClip Outcomes? JACC Cardiovasc. Interv. 2019, 12, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Glower, D.D.; Kar, S.; Trento, A.; Lim, D.S.; Bajwa, T.; Quesada, R.; Whitlow, P.L.; Rinaldi, M.J.; Grayburn, P.; Mack, M.J.; et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: Results of the EVEREST II study. J. Am. Coll. Cardiol. 2014, 64, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Orban, M.; Stolz, L.; Karam, N.; Praz, F.; Kalbacher, D.; Ludwig, S.; Braun, D.; Näbauer, M.; Wild, M.G.; et al. Impact of Residual Mitral Regurgitation on Survival After Transcatheter Edge-to-Edge Repair for Secondary Mitral Regurgitation. JACC Cardiovasc. Interv. 2021, 14, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, H.; Yoshida, J.; Hayashi, A.; Nagaura, T.; Yamaguchi, S.; Rader, F.; Siegel, R.J.; Kar, S.; Shiota, T. Usefulness of Intraprocedural Pulmonary Venous Flow for Predicting Recurrent Mitral Regurgitation and Clinical Outcomes After Percutaneous Mitral Valve Repair With the MitraClip. JACC Cardiovasc. Interv. 2019, 12, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) (Age range) | 74 ± 11 (44–93) |
| Gender (%) | Male: 73 (65.2%) |
| Female: 39 (34.8%) | |
| Diabetes (%) | 30 (26.8%) |
| Hypertension (%) | 101 (90.2%) |
| Hyperlipidemia (%) | 91 (81.3%) |
| Smoking (%) Tables: | None: 63 (56.3%) |
| Current: 17 (15.2%) | |
| Past: 27 (24.1%) | |
| No information: 5 (4.4%) | |
| Coronary artery disease (%) | 62 (55.4%) |
| MR etiology (%) | Functional: 66 (58.9%) |
| Degenerative: 39 (34.8%) | |
| Mixed: 4 (3.6%) | |
| No information: 3 (2.7%) | |
| Number of clips placed in the procedure (%) | 1: 34 (30.4%) |
| 2: 61 (54.5%) | |
| 3: 15 (13.4%) | |
| No information: 2 (1.7%) | |
| Estimated LV dysfunction according to Echocardiography (%) | Normal: 43 (38.4%) |
| Mild: 11 (9.8%) | |
| Moderate: 17 (15.2%) | |
| Severe: 37 (33%) | |
| No information: 4 (3.6%) | |
| Mean mitral valve gradient (%) | <5 mmHg: 81 (72.3%) |
| ≧5 mmHg: 29 (25.9%) | |
| No information: 2 (1.8%) | |
| Degree of improvement on NYHA scale (%) | 0 4 (3.6%) |
| 1: 47 (42%) | |
| 2: 48 (42.9%) | |
| 3: 6 (5.4%) | |
| No information: 7 (6.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shekel, E.; Shuvy, M.; Danenberg, H.; Planer, D.; Gilon, D.; Leibowitz, D.; Beeri, R. Mitral Regurgitation Severity Assessment after Transcutaneous Edge-to-Edge Mitral Valve Repair: Recommended Integration versus Volumetric Assessment Guidelines. J. Clin. Med. 2023, 12, 6347. https://doi.org/10.3390/jcm12196347
Shekel E, Shuvy M, Danenberg H, Planer D, Gilon D, Leibowitz D, Beeri R. Mitral Regurgitation Severity Assessment after Transcutaneous Edge-to-Edge Mitral Valve Repair: Recommended Integration versus Volumetric Assessment Guidelines. Journal of Clinical Medicine. 2023; 12(19):6347. https://doi.org/10.3390/jcm12196347
Chicago/Turabian StyleShekel, Efrat, Mony Shuvy, Haim Danenberg, David Planer, Dan Gilon, David Leibowitz, and Ronen Beeri. 2023. "Mitral Regurgitation Severity Assessment after Transcutaneous Edge-to-Edge Mitral Valve Repair: Recommended Integration versus Volumetric Assessment Guidelines" Journal of Clinical Medicine 12, no. 19: 6347. https://doi.org/10.3390/jcm12196347
APA StyleShekel, E., Shuvy, M., Danenberg, H., Planer, D., Gilon, D., Leibowitz, D., & Beeri, R. (2023). Mitral Regurgitation Severity Assessment after Transcutaneous Edge-to-Edge Mitral Valve Repair: Recommended Integration versus Volumetric Assessment Guidelines. Journal of Clinical Medicine, 12(19), 6347. https://doi.org/10.3390/jcm12196347






