Agreement and Reproducibility of Anterior Chamber Angle Measurements between CASIA2 Built-In Software and Human Graders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sample Size Calculation
2.3. AS-OCT Image Acquisition
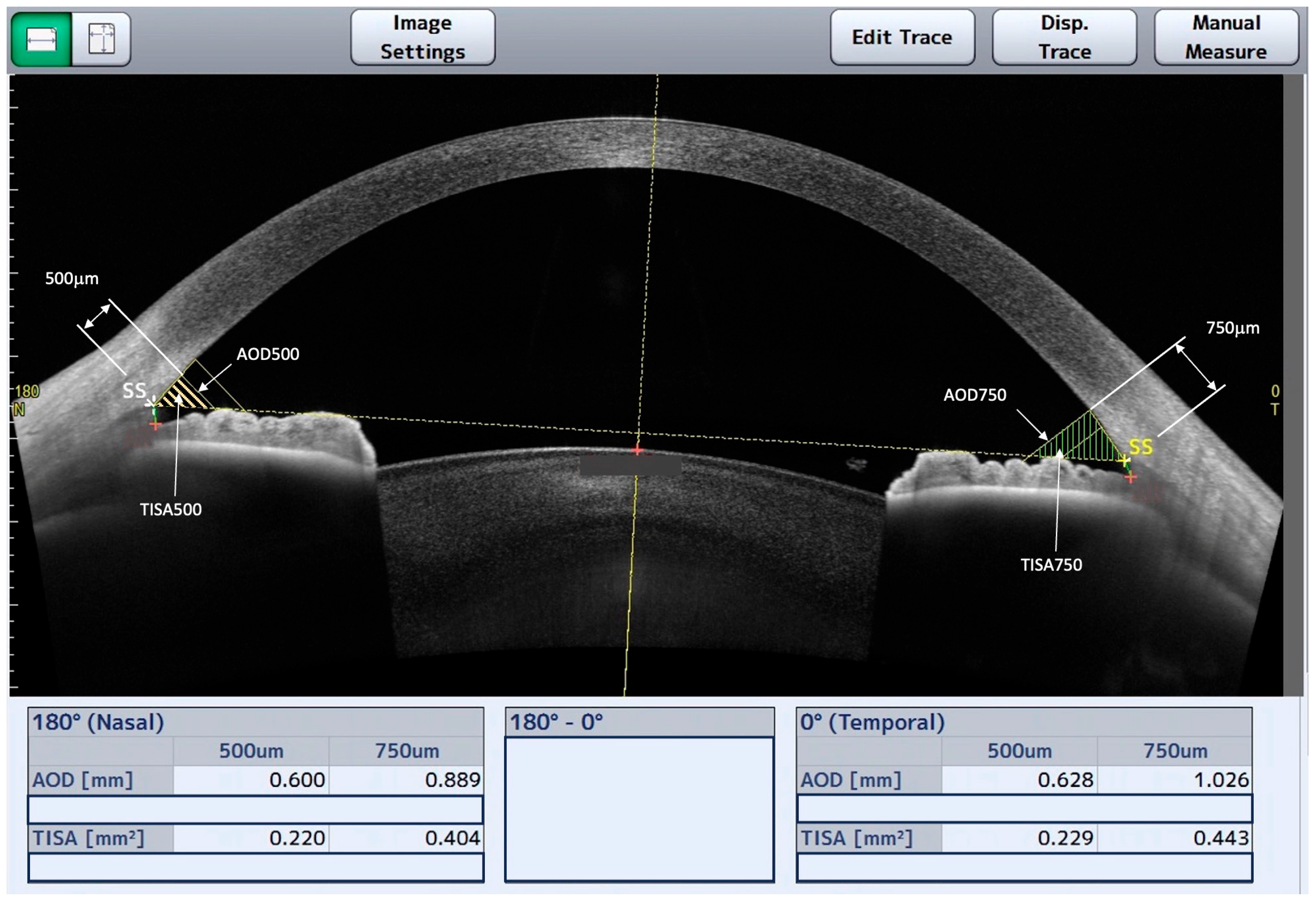
2.4. AS-OCT Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Repeatability of CASIA2 Automated Software
3.2. Intraobserver ACA Measurements’ Reproducibility
3.3. Interobserver ACA Measurements’ Agreement
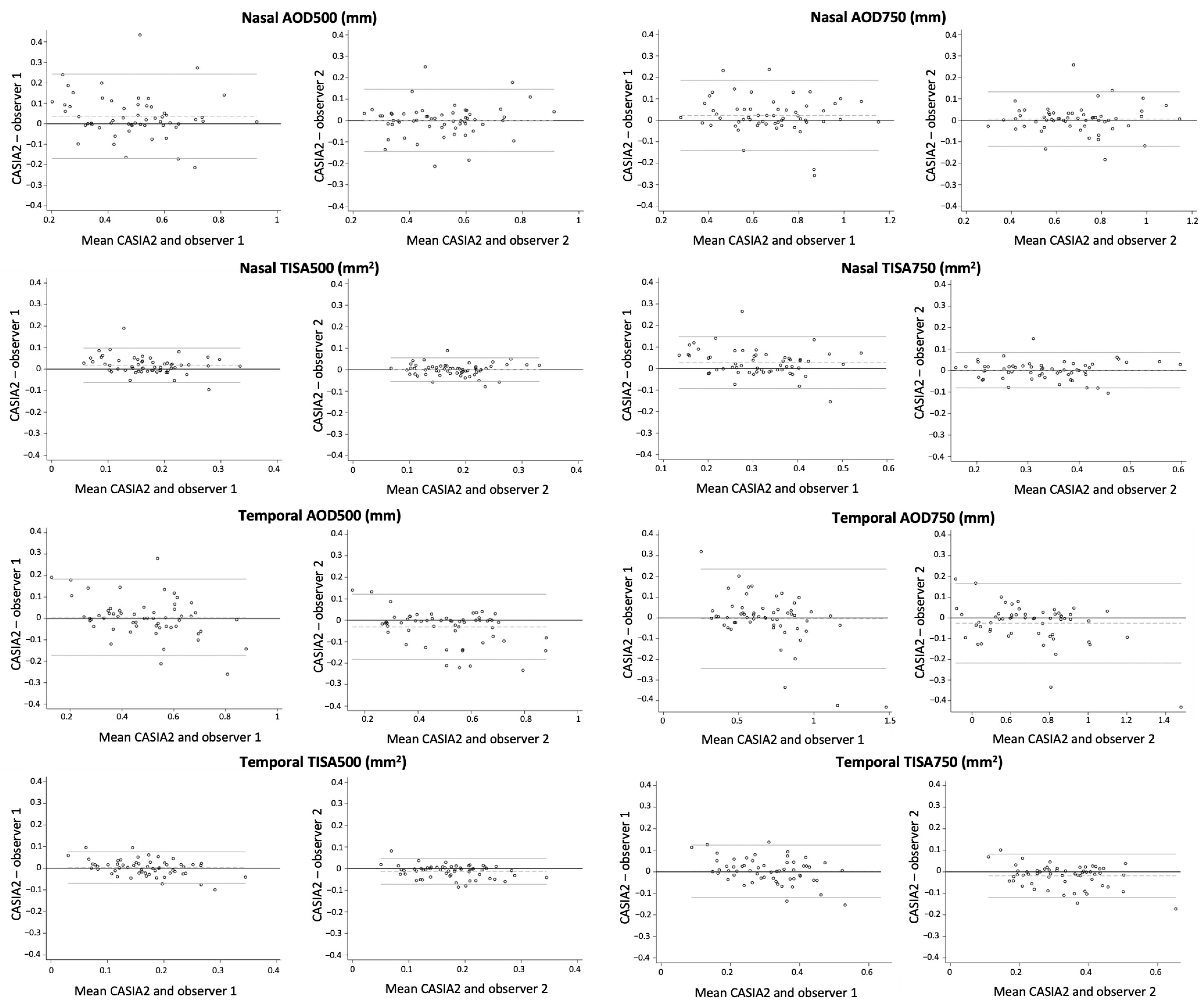
3.4. Agreement of ACA Measurements between CASIA2 and Both Observers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.L.; Yu, F.; Evans, S.J. Use of gonioscopy in Medicare beneficiaries before glaucoma surgery. J. Glaucoma 2006, 15, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Friedman, D.S.; Hahn, S.R. Evaluation of Practice Patterns for the Care of Open-angle Glaucoma Compared with Claims Data. Ophthalmology 2007, 114, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Rollins, A.M.; Roth, J.E.; Yazdanfar, S.; Westphal, V.; Bardenstein, D.S.; Izatt, J.A. Real-time optical coherence tomography of the anterior segment at 1310 nm. Arch. Ophthalmol. 2001, 119, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, A.; Sakata, L.M.; He, M.G.; Friedman, D.S.; Chan, Y.H.; Lavanya, R.; Baskaran, M.; Foster, P.J.; Aung, T. Diagnostic performance of anterior chamber angle measurements for detecting eyes with narrow angles: An anterior segment OCT study. Arch. Ophthalmol. 2010, 128, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.Y.; Burkemper, B.; Lewinger, J.P.; Jiang, X.; Pardeshi, A.A.; Richter, G.; Torres, M.; McKean-Cowdin, R.; Varma, R. Correlation between Intraocular Pressure and Angle Configuration Measured by OCT. Ophthalmol. Glaucoma 2018, 1, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.K.; Xu, B.Y.; Friedman, D.S.; Cho, A.; Foster, P.J.; Jiang, Y.; Porporato, N.; Pardeshi, A.A.; Jiang, Y.; Munoz, B.; et al. Biometric Risk Factors for Angle Closure Progression after Laser Peripheral Iridotomy. JAMA Ophthalmol. 2023, 141, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, C.J.; Harasiewicz, K.; Foster, F.S. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am. J. Ophthalmol. 1992, 113, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Huang, D.; Smith, S.D. Optical coherence tomography imaging of the anterior chamber angle. Ophthalmol. Clin. North Am. 2005, 18, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Seager, F.E.; Wang, J.; Arora, K.S.; Quigley, H.A. The effect of scleral spur identification methods on structural measurements by anterior segment optical coherence tomography. J. Glaucoma 2014, 23, e10–e15. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.N.; Sauren, L.D.; de Brabander, J.; Berendschot, T.T.; Passos, V.L.; Webers, C.A.; Nuijts, R.M.; Beckers, H.J. Reproducibility of anterior chamber angle measurements with anterior segment optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.Y.; Mai, D.D.; Penteado, R.C.; Saunders, L.; Weinreb, R.N. Reproducibility and Agreement of Anterior Segment Parameter Measurements Obtained Using the CASIA2 and Spectralis OCT2 Optical Coherence Tomography Devices. J. Glaucoma 2017, 26, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lai, G.; Chiu, V.; Chong, A.; Yu, M.; Leung, C.K. Anterior chamber angle imaging with swept-source optical coherence tomography: Comparison between CASIAII and ANTERION. Sci. Rep. 2020, 10, 18771. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Higashita, R.; Guo, P.Y.; Okamoto, K.; Li, F.; Nguyen, A.; Sakata, R.; Duan, L.; Aihara, M.; Lin, S.; et al. Reproducibility of deep learning based scleral spur localisation and anterior chamber angle measurements from anterior segment optical coherence tomography images. Br. J. Ophthalmol. 2023, 107, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.Y.; Chiang, M.; Pardeshi, A.A.; Moghimi, S.; Varma, R. Deep Neural Network for Scleral Spur Detection in Anterior Segment OCT Images: The Chinese American Eye Study. Transl. Vis. Sci. Technol. 2020, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Devalla, S.K.; Ang, A.; Soh, Z.-D.; Thiery, A.H.; Boote, C.; Cheng, C.-Y.; A Girard, M.J.; Koh, V. Deep learning algorithms to isolate and quantify the structures of the anterior segment in optical coherence tomography images. Br. J. Ophthalmol. 2021, 105, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, A.A.; Song, A.E.; Lazkani, N.; Xie, X.; Huang, A.; Xu, B.Y. Intradevice Repeatability and Interdevice Agreement of Ocular Biometric Measurements: A Comparison of Two Swept-Source Anterior Segment OCT Devices. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Chansangpetch, S.; Nguyen, A.; Mora, M.; Badr, M.; He, M.; Porco, T.C.; Lin, S.C. Agreement of Anterior Segment Parameters Obtained from Swept-Source Fourier-Domain and Time-Domain Anterior Segment Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1554–1561. [Google Scholar] [CrossRef] [PubMed]

| Mean Capture 1 | Mean Capture 2 | p-Value | Mean Diff | ICC | ICC 95% CI | |
|---|---|---|---|---|---|---|
| Temporal parameters | ||||||
| AOD500 (mm) | 0.490 ± 0.152 | 0.492 ± 0.152 | 0.94 | −0.001 | 0.90 | 0.83–0.94 |
| AOD750 (mm) | 0.684 ± 0.212 | 0.690 ± 0.211 | 0.88 | −0.005 | 0.88 | 0.80–0.93 |
| TISA500 (mm2) | 0.168 ± 0.057 | 0.166 ± 0.050 | 0.89 | 0.001 | 0.84 | 0.73–0.90 |
| TISA750 (mm2) | 0.316 ± 0.100 | 0.316 ± 0.093 | 0.99 | 0.000 | 0.87 | 0.78–0.92 |
| Nasal parameters | ||||||
| AOD500 (mm) | 0.510 ± 0.156 | 0.497 ± 0.166 | 0.68 | 0.012 | 0.92 | 0.87–0.95 |
| AOD750 (mm) | 0.684 ± 0.186 | 0.679 ± 0.212 | 0.89 | 0.005 | 0.93 | 0.89–0.96 |
| TISA500 (mm2) | 0.180 ± 0.057 | 0.178 ± 0.060 | 0.88 | 0.001 | 0.91 | 0.85–0.95 |
| TISA750 (mm2) | 0.332 ± 0.097 | 0.328 ± 0.109 | 0.81 | 0.004 | 0.92 | 0.87–0.95 |
| Mean Evaluation 1 | Mean Evaluation 2 | p-Value | Mean Diff | ICC | ICC 95% CI | |
|---|---|---|---|---|---|---|
| Observer 1 Temporal parameters | ||||||
| AOD500 (mm) | 0.487 ± 0.190 | 0.478 ± 0.185 | 0.71 | 0.008 | 0.97 | 0.96–0.98 |
| AOD750 (mm) | 0.685 ± 0.267 | 0.671 ± 0.271 | 0.70 | 0.013 | 0.96 | 0.95–0.97 |
| TISA500 (mm2) | 0.166 ± 0.073 | 0.162 ± 0.071 | 0.67 | 0.004 | 0.96 | 0.95–0.98 |
| TISA750 (mm2) | 0.314 ± 0.129 | 0.308 ± 0.125 | 0.68 | 0.006 | 0.97 | 0.95–0.97 |
| Nasal parameters | ||||||
| AOD500 (mm) | 0.469 ± 0.176 | 0.459 ± 0.171 | 0.66 | 0.009 | 0.97 | 0.96–0.98 |
| AOD750 (mm) | 0.656 ± 0.209 | 0.643 ± 0.206 | 0.61 | 0.013 | 0.97 | 0.96–0.98 |
| TISA500 (mm2) | 0.161 ± 0.067 | 0.157 ± 0.065 | 0.61 | 0.004 | 0.98 | 0.97–0.98 |
| TISA750 (mm2) | 0.303 ± 0.113 | 0.296 ± 0.110 | 0.61 | 0.007 | 0.98 | 0.97–0.98 |
| Observer 2 Temporal parameters | ||||||
| AOD500 (mm) | 0.522 ± 0.180 | 0.537 ± 0.188 | 0.54 | −0.014 | 0.98 | 0.97–0.98 |
| AOD750 (mm) | 0.713 ± 0.242 | 0.742 ± 0.261 | 0.38 | −0.028 | 0.97 | 0.96–0.98 |
| TISA500 (mm2) | 0.182 ± 0.064 | 0.187 ± 0.068 | 0.51 | −0.005 | 0.97 | 0.96–0.98 |
| TISA750 (mm2) | 0.337 ± 0.113 | 0.349 ± 0.123 | 0.42 | −0.012 | 0.97 | 0.96–0.98 |
| Nasal parameters | ||||||
| AOD500 (mm) | 0.505 ± 0.151 | 0.515 ± 0.160 | 0.61 | −0.010 | 0.97 | 0.96–0.98 |
| AOD750 (mm) | 0.681 ± 0.189 | 0.698 ± 0.202 | 0.50 | −0.017 | 0.98 | 0.97–0.99 |
| TISA500 (mm2) | 0.179 ± 0.057 | 0.183 ± 0.061 | 0.57 | −0.004 | 0.97 | 0.96–0.98 |
| TISA750 (mm2) | 0.330 ± 0.097 | 0.338 ± 0.103 | 0.53 | −0.008 | 0.98 | 0.97–0.98 |
| Mean Evaluation 1 Observer 1 | Mean Evaluation 1 Observer 2 | p-Value | Mean Diff | ICC | ICC 95% CI | |
|---|---|---|---|---|---|---|
| Temporal parameters | ||||||
| AOD500 (mm) | 0.487 ± 0.190 | 0.522 ± 0.180 | 0.14 | −0.035 | 0.95 | 0.93–0.96 |
| AOD750 (mm) | 0.685 ± 0.267 | 0.713 ± 0.242 | 0.40 | −0.028 | 0.96 | 0.95–0.97 |
| TISA500 (mm2) | 0.166 ± 0.073 | 0.182 ± 0.064 | 0.08 | −0.015 | 0.95 | 0.93–0.96 |
| TISA750 (mm2) | 0.314 ± 0.129 | 0.337 ± 0.113 | 0.16 | −0.022 | 0.95 | 0.93–0.97 |
| Nasal parameters | ||||||
| AOD500 (mm) | 0.469 ± 0.176 | 0.505 ± 0.151 | 0.09 | −0.035 | 0.91 | 0.87–0.93 |
| AOD750 (mm) | 0.656 ± 0.209 | 0.681 ± 0.189 | 0.34 | −0.024 | 0.94 | 0.92–0.96 |
| TISA500 (mm2) | 0.161 ± 0.067 | 0.179 ± 0.057 | <0.05 * | −0.017 | 0.90 | 0.85–0.93 |
| TISA750 (mm2) | 0.303 ± 0.113 | 0.330 ± 0.097 | 0.06 | −0.026 | 0.91 | 0.88–0.94 |
| Mean Observer Measurements | Mean CASIA2 Measurements | p-Value | Mean Diff | ICC | ICC 95% CI | |
|---|---|---|---|---|---|---|
| Observer 1 Temporal parameters | ||||||
| AOD500 (mm) | 0.487 ± 0.190 | 0.491 ± 0.151 | 0.86 | 0.003 | 0.90 | 0.85–0.93 |
| AOD750 (mm) | 0.685 ± 0.267 | 0.687 ± 0.211 | 0.95 | 0.001 | 0.90 | 0.86–0.93 |
| TISA500 (mm2) | 0.166 ± 0.073 | 0.167 ± 0.054 | 0.08 | 0.001 | 0.88 | 0.82–0.91 |
| TISA750 (mm2) | 0.314 ± 0.129 | 0.316 ± 0.096 | 0.92 | 0.001 | 0.89 | 0.84–0.92 |
| Nasal parameters | ||||||
| AOD500 (mm) | 0.469 ± 0.176 | 0.504 ± 0.160 | 0.11 | 0.034 | 0.88 | 0.81–0.92 |
| AOD750 (mm) | 0.656 ± 0.209 | 0.681 ± 0.199 | 0.35 | 0.025 | 0.93 | 0.90–0.95 |
| TISA500 (mm2) | 0.161 ± 0.067 | 0.179 ± 0.060 | <0.05 * | 0.017 | 0.87 | 0.77–0.92 |
| TISA750 (mm2) | 0.303 ± 0.113 | 0.330 ± 0.009 | 0.06 | 0.026 | 0.89 | 0.81–0.93 |
| Observer 2 Temporal parameters | ||||||
| AOD500 (mm) | 0.522 ± 0.180 | 0.491 ± 0.151 | 0.15 | −0.031 | 0.92 | 0.88–0.95 |
| AOD750 (mm) | 0.713 ± 0.242 | 0.687 ± 0.211 | 0.37 | −0.026 | 0.93 | 0.90–0.95 |
| TISA500 (mm2) | 0.182 ± 0.064 | 0.167 ± 0.054 | 0.05 | −0.014 | 0.90 | 0.83–0.94 |
| TISA750 (mm2) | 0.337 ± 0.113 | 0.316 ± 0.096 | 0.13 | −0.021 | 0.92 | 0.87–0.95 |
| Nasal parameters | ||||||
| AOD500 (mm) | 0.505 ± 0.151 | 0.504 ± 0.160 | 0.96 | −0.001 | 0.93 | 0.91–0.95 |
| AOD750 (mm) | 0.681 ± 0.189 | 0.681 ± 0.199 | 0.98 | 0.000 | 0.96 | 0.94–0.97 |
| TISA500 (mm2) | 0.179 ± 0.057 | 0.179 ± 0.060 | 0.99 | 0.000 | 0.92 | 0.89–0.94 |
| TISA750 (mm2) | 0.330 ± 0.097 | 0.330 ± 0.009 | 0.96 | 0.000 | 0.94 | 0.91–0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, G.; Iglesias, K.; Parra, J.C.; Rodriguez-Una, I.; Serrano-Gomez, S.; Prada, A.M.; Galvis, V. Agreement and Reproducibility of Anterior Chamber Angle Measurements between CASIA2 Built-In Software and Human Graders. J. Clin. Med. 2023, 12, 6381. https://doi.org/10.3390/jcm12196381
Espinoza G, Iglesias K, Parra JC, Rodriguez-Una I, Serrano-Gomez S, Prada AM, Galvis V. Agreement and Reproducibility of Anterior Chamber Angle Measurements between CASIA2 Built-In Software and Human Graders. Journal of Clinical Medicine. 2023; 12(19):6381. https://doi.org/10.3390/jcm12196381
Chicago/Turabian StyleEspinoza, Gustavo, Katheriene Iglesias, Juan C. Parra, Ignacio Rodriguez-Una, Sergio Serrano-Gomez, Angelica M. Prada, and Virgilio Galvis. 2023. "Agreement and Reproducibility of Anterior Chamber Angle Measurements between CASIA2 Built-In Software and Human Graders" Journal of Clinical Medicine 12, no. 19: 6381. https://doi.org/10.3390/jcm12196381
APA StyleEspinoza, G., Iglesias, K., Parra, J. C., Rodriguez-Una, I., Serrano-Gomez, S., Prada, A. M., & Galvis, V. (2023). Agreement and Reproducibility of Anterior Chamber Angle Measurements between CASIA2 Built-In Software and Human Graders. Journal of Clinical Medicine, 12(19), 6381. https://doi.org/10.3390/jcm12196381






