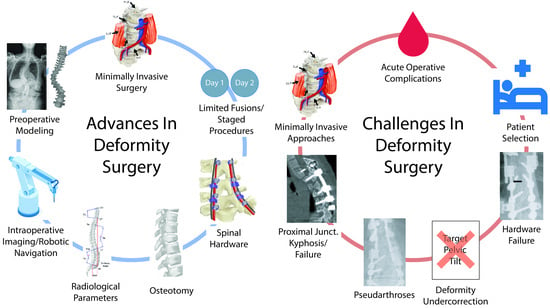
Advances and Evolving Challenges in Spinal Deformity Surgery
Abstract
1. Introduction
2. Advances in Surgical Approaches
2.1. Radiological Parameters and Scoring Systems
2.2. Pre-Operative Modeling
2.3. Minimally Invasive Surgery
2.4. Limited Fusions and Staged Procedures
2.5. Osteotomy
2.6. Intraoperative Imaging and Robotic Navigation
2.7. Hardware
3. Challenges in Spinal Deformity Surgery
3.1. Patient Selection
3.2. Acute Operative Complications
3.3. Undercorrection
3.4. Hardware Failure
3.5. Proximal Junctional Kyphosis and Failure
3.6. Pseudarthrosis
3.7. Minimally Invasive Approaches
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fehlings, M.G.; Tetreault, L.; Nater, A.; Choma, T.; Harrop, J.; Mroz, T.; Santaguida, C.; Smith, J.S. The Aging of the Global Population. Neurosurgery 2015, 77, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Dubey, A.; Gamez, L.; El Fegoun, A.B.; Hwang, K.; Pagala, M.; Farcy, J.-P. Adult Scoliosis: Prevalence, SF-36, and Nutritional Parameters in an Elderly Volunteer Population. Spine 2005, 30, 1082–1085. [Google Scholar] [CrossRef]
- Aebi, M. The Adult Scoliosis. Eur. Spine J. 2005, 14, 925–948. [Google Scholar] [CrossRef]
- Smith, J.S.; Shaffrey, C.I.; Bess, S.; Shamji, M.F.; Brodke, D.; Lenke, L.G.; Fehlings, M.G.; Lafage, V.; Schwab, F.; Vaccaro, A.R.; et al. Recent and Emerging Advances in Spinal Deformity. Neurosurgery 2017, 80, S70–S85. [Google Scholar] [CrossRef]
- Diebo, B.G.; Shah, N.V.; Boachie-Adjei, O.; Zhu, F.; Rothenfluh, D.A.; Paulino, C.B.; Schwab, F.J.; Lafage, V. Adult Spinal Deformity. Lancet 2019, 394, 160–172. [Google Scholar] [CrossRef]
- Zygourakis, C.C.; Liu, C.Y.; Keefe, M.; Moriates, C.; Ratliff, J.; Dudley, R.A.; Gonzales, R.; Mummaneni, P.V.; Ames, C.P. Analysis of National Rates, Cost, and Sources of Cost Variation in Adult Spinal Deformity. Neurosurgery 2018, 82, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Dubey, A.; Pagala, M.; Gamez, L.; Farcy, J.P. Adult Scoliosis: A Health Assessment Analysis by SF-36. Spine 2003, 28, 602–606. [Google Scholar] [CrossRef]
- Silva, F.E.; Lenke, L.G. Adult Degenerative Scoliosis: Evaluation and Management. Neurosurg. Focus 2010, 28, E1. [Google Scholar] [CrossRef] [PubMed]
- Mummaneni, P.V.; Tu, T.-H.; Ziewacz, J.E.; Akinbo, O.C.; Deviren, V.; Mundis, G.M. The Role of Minimally Invasive Techniques in the Treatment of Adult Spinal Deformity. Neurosurg. Clin. N. Am. 2013, 24, 231–248. [Google Scholar] [CrossRef]
- Acosta, F.L.; McClendon, J.; O’Shaughnessy, B.A.; Koller, H.; Neal, C.J.; Meier, O.; Ames, C.P.; Koski, T.R.; Ondra, S.L. Morbidity and Mortality after Spinal Deformity Surgery in Patients 75 Years and Older: Complications and Predictive Factors. J. Neurosurg. Spine 2011, 15, 667–674. [Google Scholar] [CrossRef]
- Mummaneni, P.V.; Shaffrey, C.I.; Lenke, L.G.; Park, P.; Wang, M.Y.; La Marca, F.; Smith, J.S.; Mundis, G.M.; Okonkwo, D.O.; Moal, B.; et al. The Minimally Invasive Spinal Deformity Surgery Algorithm: A Reproducible Rational Framework for Decision Making in Minimally Invasive Spinal Deformity Surgery. Neurosurg. Focus. 2014, 36, E6. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yang, J.H.; Chang, D.-G.; Suk, S.-I.; Suh, S.W.; Song, K.-S.; Park, J.-B.; Cho, W. Adult Spinal Deformity: Current Concepts and Decision-Making Strategies for Management. Asian Spine J. 2020, 14, 886–897. [Google Scholar] [CrossRef]
- Ailon, T.; Smith, J.S.; Shaffrey, C.I.; Lenke, L.G.; Brodke, D.; Harrop, J.S.; Fehlings, M.; Ames, C.P. Degenerative Spinal Deformity. Neurosurgery 2015, 77, S75–S91. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Ungar, B.; Blondel, B.; Buchowski, J.; Coe, J.; Deinlein, D.; DeWald, C.; Mehdian, H.; Shaffrey, C.; Tribus, C.; et al. Scoliosis Research Society—Schwab Adult Spinal Deformity Classification. Spine 2012, 37, 1077–1082. [Google Scholar] [CrossRef]
- Terran, J.; Schwab, F.; Shaffrey, C.I.; Smith, J.S.; Devos, P.; Ames, C.P.; Fu, K.-M.G.; Burton, D.; Hostin, R.; Klineberg, E.; et al. The SRS-Schwab Adult Spinal Deformity Classification. Neurosurgery 2013, 73, 559–568. [Google Scholar] [CrossRef]
- Smith, J.S.; Klineberg, E.; Schwab, F.; Shaffrey, C.I.; Moal, B.; Ames, C.P.; Hostin, R.; Fu, K.-M.G.; Burton, D.; Akbarnia, B.; et al. Change in Classification Grade by the SRS-Schwab Adult Spinal Deformity Classification Predicts Impact on Health-Related Quality of Life Measures. Spine 2013, 38, 1663–1671. [Google Scholar] [CrossRef]
- Yilgor, C.; Sogunmez, N.; Boissiere, L.; Yavuz, Y.; Obeid, I.; Kleinstück, F.; Pérez-Grueso, F.J.S.; Acaroglu, E.; Haddad, S.; Mannion, A.F.; et al. Global Alignment and Proportion (GAP) Score. J. Bone Jt. Surg. 2017, 99, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Jalai, C.M.; Cruz, D.L.; Diebo, B.G.; Poorman, G.; Lafage, R.; Bess, S.; Ramchandran, S.; Day, L.M.; Vira, S.; Liabaud, B.; et al. Full-Body Analysis of Age-Adjusted Alignment in Adult Spinal Deformity Patients and Lower-Limb Compensation. Spine 2017, 42, 653–661. [Google Scholar] [CrossRef]
- Lafage, R.; Schwab, F.; Challier, V.; Henry, J.K.; Gum, J.; Smith, J.; Hostin, R.; Shaffrey, C.; Kim, H.J.; Ames, C.; et al. Defining Spino-Pelvic Alignment Thresholds. Spine 2016, 41, 62–68. [Google Scholar] [CrossRef]
- Noh, S.H.; Ha, Y.; Obeid, I.; Park, J.Y.; Kuh, S.U.; Chin, D.K.; Kim, K.S.; Cho, Y.E.; Lee, H.S.; Kim, K.H. Modified Global Alignment and Proportion Scoring with Body Mass Index and Bone Mineral Density (GAPB) for Improving Predictions of Mechanical Complications after Adult Spinal Deformity Surgery. Spine J. 2020, 20, 776–784. [Google Scholar] [CrossRef]
- Bess, S.; Protopsaltis, T.S.; Lafage, V.; Lafage, R.; Ames, C.P.; Errico, T.; Smith, J.S. Clinical and Radiographic Evaluation of Adult Spinal Deformity. Clin. Spine Surg. Spine Publ. 2016, 29, 6–16. [Google Scholar] [CrossRef]
- Melhem, E.; Assi, A.; El Rachkidi, R.; Ghanem, I. EOS Biplanar X-Ray Imaging: Concept, Developments, Benefits, and Limitations. J. Child. Orthop. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Glaser, D.A.; Doan, J.; Newton, P.O. Comparison of 3-Dimensional Spinal Reconstruction Accuracy. Spine 2012, 37, 1391–1397. [Google Scholar] [CrossRef]
- Somoskeöy, S.; Tunyogi-Csapó, M.; Bogyó, C.; Illés, T. Accuracy and Reliability of Coronal and Sagittal Spinal Curvature Data Based on Patient-Specific Three-Dimensional Models Created by the EOS 2D/3D Imaging System. Spine J. 2012, 12, 1052–1059. [Google Scholar] [CrossRef]
- Al-Aubaidi, Z.; Lebel, D.; Oudjhane, K.; Zeller, R. Three-Dimensional Imaging of the Spine Using the EOS System. J. Pediatr. Orthop. B 2013, 22, 409–412. [Google Scholar] [CrossRef]
- Yeung, K.H.; Man, G.C.W.; Lam, T.P.; Ng, B.K.W.; Cheng, J.C.Y.; Chu, W.C.W. Accuracy on the Preoperative Assessment of Patients with Adolescent Idiopathic Scoliosis Using Biplanar Low-Dose Stereoradiography: A Comparison with Computed Tomography. BMC Musculoskelet. Disord. 2020, 21, 558. [Google Scholar] [CrossRef]
- Tan, L.A.; Yerneni, K.; Tuchman, A.; Li, X.J.; Cerpa, M.; Lehman Jr, R.A.; Lenke, L.G. Utilization of the 3D-Printed Spine Model for Freehand Pedicle Screw Placement in Complex Spinal Deformity Correction. J. Spine Surg. 2018, 4, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Gupta, M.; Singh, M.; Kalyanasundaram, D. Outcome and Safety Analysis of 3D-Printed Patient-Specific Pedicle Screw Jigs for Complex Spinal Deformities: A Comparative Study. Spine J. 2019, 19, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Cecchinato, R.; Berjano, P.; Zerbi, A.; Damilano, M.; Redaelli, A.; Lamartina, C. Pedicle Screw Insertion with Patient-Specific 3D-Printed Guides Based on Low-Dose CT Scan Is More Accurate than Free-Hand Technique in Spine Deformity Patients: A Prospective, Randomized Clinical Trial. Eur. Spine J. 2019, 28, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Durand, W.M.; DePasse, J.M.; Daniels, A.H. Predictive Modeling for Blood Transfusion After Adult Spinal Deformity Surgery. Spine 2018, 43, 1058–1066. [Google Scholar] [CrossRef]
- De la Garza Ramos, R.; Hamad, M.K.; Ryvlin, J.; Krol, O.; Passias, P.G.; Fourman, M.S.; Shin, J.H.; Yanamadala, V.; Gelfand, Y.; Murthy, S.; et al. An Artificial Neural Network Model for the Prediction of Perioperative Blood Transfusion in Adult Spinal Deformity Surgery. J. Clin. Med. 2022, 11, 4436. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.K.; Osorio, J.A.; Smith, J.S.; Schwab, F.; Lafage, V.; Hart, R.A.; Bess, S.; Line, B.; Diebo, B.G.; Protopsaltis, T.S.; et al. Development of Validated Computer-Based Preoperative Predictive Model for Proximal Junction Failure (PJF) or Clinically Significant PJK With 86% Accuracy Based on 510 ASD Patients With 2-Year Follow-Up. Spine 2016, 41, E1328–E1335. [Google Scholar] [CrossRef]
- Peng, L.; Lan, L.; Xiu, P.; Zhang, G.; Hu, B.; Yang, X.; Song, Y.; Yang, X.; Gu, Y.; Yang, R.; et al. Prediction of Proximal Junctional Kyphosis After Posterior Scoliosis Surgery with Machine Learning in the Lenke 5 Adolescent Idiopathic Scoliosis Patient. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.K.; Oh, T.; Smith, J.S.; Shaffrey, C.I.; Daniels, A.H.; Sciubba, D.M.; Hamilton, D.K.; Protopsaltis, T.S.; Passias, P.G.; Hart, R.A.; et al. Development of a Validated Computer-Based Preoperative Predictive Model for Pseudarthrosis with 91% Accuracy in 336 Adult Spinal Deformity Patients. Neurosurg. Focus 2018, 45, E11. [Google Scholar] [CrossRef]
- Smith, Z.A.; Fessler, R.G. Paradigm Changes in Spine Surgery—Evolution of Minimally Invasive Techniques. Nat. Rev. Neurol. 2012, 8, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Lak, A.M.; Lamba, N.; Pompilus, F.; Yunusa, I.; King, A.; Sultan, I.; Amamoo, J.; Al-Otaibi, N.M.; Alasmari, M.; Zaghloul, I.; et al. Minimally Invasive versus Open Surgery for the Correction of Adult Degenerative Scoliosis: A Systematic Review. Neurosurg. Rev. 2021, 44, 659–668. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar Interbody Fusion: Techniques, Indications and Comparison of Interbody Fusion Options Including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar] [CrossRef]
- Godzik, J.; Ohiorhenuan, I.E.; Xu, D.S.; de Andrada Pereira, B.; Walker, C.T.; Whiting, A.C.; Turner, J.D.; Uribe, J.S. Single-Position Prone Lateral Approach: Cadaveric Feasibility Study and Early Clinical Experience. Neurosurg. Focus 2020, 49, E15. [Google Scholar] [CrossRef]
- Akbarnia, B.A.; Mundis, G.M.; Moazzaz, P.; Kabirian, N.; Bagheri, R.; Eastlack, R.K.; Pawelek, J.B. Anterior Column Realignment (ACR) for Focal Kyphotic Spinal Deformity Using a Lateral Transpsoas Approach and ALL Release. J. Spinal Disord. Tech. 2014, 27, 29–39. [Google Scholar] [CrossRef]
- Wang, M.Y. Percutaneous Iliac Screws for Minimally Invasive Spinal Deformity Surgery. Minim. Invasive Surg. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Bae, J.; Lee, S.-H. Minimally Invasive Spinal Surgery for Adult Spinal Deformity. Neurospine 2018, 15, 18–24. [Google Scholar] [CrossRef]
- Kanter, A.S.; Tempel, Z.J.; Ozpinar, A.; Okonkwo, D.O. A Review of Minimally Invasive Procedures for the Treatment of Adult Spinal Deformity. Spine 2016, 1, S59–S65. [Google Scholar] [CrossRef]
- Costanzo, G.; Zoccali, C.; Maykowski, P.; Walter, C.M.; Skoch, J.; Baaj, A.A. The Role of Minimally Invasive Lateral Lumbar Interbody Fusion in Sagittal Balance Correction and Spinal Deformity. Eur. Spine J. 2014, 23, 699–704. [Google Scholar] [CrossRef]
- Park, S.W.; Ko, M.J.; Kim, Y.B.; Le Huec, J.C. Correction of Marked Sagittal Deformity with Circumferential Minimally Invasive Surgery Using Oblique Lateral Interbody Fusion in Adult Spinal Deformity. J. Orthop. Surg. Res. 2020, 15, 13. [Google Scholar] [CrossRef]
- Haque, R.M.; Mundis, G.M.; Ahmed, Y.; El Ahmadieh, T.Y.; Wang, M.Y.; Mummaneni, P.V.; Uribe, J.S.; Okonkwo, D.O.; Eastlack, R.K.; Anand, N.; et al. Comparison of Radiographic Results after Minimally Invasive, Hybrid, and Open Surgery for Adult Spinal Deformity: A Multicenter Study of 184 Patients. Neurosurg. Focus 2014, 36, E13. [Google Scholar] [CrossRef]
- Chou, D.; Lafage, V.; Chan, A.Y.; Passias, P.; Mundis, G.M.; Eastlack, R.K.; Fu, K.-M.; Fessler, R.G.; Gupta, M.C.; Than, K.D.; et al. Patient Outcomes after Circumferential Minimally Invasive Surgery Compared with Those of Open Correction for Adult Spinal Deformity: Initial Analysis of Prospectively Collected Data. J. Neurosurg. Spine 2021, 1–12. [Google Scholar] [CrossRef]
- Uribe, J.S.; Deukmedjian, A.R.; Mummaneni, P.V.; Fu, K.-M.G.; Mundis, G.M.; Okonkwo, D.O.; Kanter, A.S.; Eastlack, R.; Wang, M.Y.; Anand, N.; et al. Complications in Adult Spinal Deformity Surgery: An Analysis of Minimally Invasive, Hybrid, and Open Surgical Techniques. Neurosurg. Focus 2014, 36, E15. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Alayan, A.; Kong, C.; Kahwaty, S.; Khandehroo, B.; Gendelberg, D.; Chung, A. Management of Severe Adult Spinal Deformity with Circumferential Minimally Invasive Surgical Strategies without Posterior Column Osteotomies: A 13-Year Experience. Spine Deform. 2022, 10, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Mummaneni, P.V.; Park, P.; Shaffrey, C.I.; Wang, M.Y.; Uribe, J.S.; Fessler, R.G.; Chou, D.; Kanter, A.S.; Okonkwo, D.O.; Mundis, G.M.; et al. The MISDEF2 Algorithm: An Updated Algorithm for Patient Selection in Minimally Invasive Deformity Surgery. J. Neurosurg. Spine 2020, 32, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-J.; Suk, S.-I.; Park, S.-R.; Kim, J.-H.; Kim, S.-S.; Lee, T.-J.; Lee, J.-J.; Lee, J.-M. Short Fusion versus Long Fusion for Degenerative Lumbar Scoliosis. Eur. Spine J. 2008, 17, 650–656. [Google Scholar] [CrossRef]
- Nakajima, H.; Matsuo, H.; Naruse, H.; Watanabe, S.; Honjoh, K.; Shoji, K.; Kubota, A.; Matsumine, A. Clinical Impact of Short Limited Lumbar Fusion for Adult Spinal Deformity with Postural and Radiological Abnormalities. Sci. Rep. 2022, 12, 19439. [Google Scholar] [CrossRef]
- Yamin, S.; Li, L.; Xing, W.; Tianjun, G.; Yupeng, Z. Staged Surgical Treatment for Severe and Rigid Scoliosis. J. Orthop. Surg. Res. 2008, 3, 26. [Google Scholar] [CrossRef]
- Anand, N.; Kong, C.; Fessler, R.G. A Staged Protocol for Circumferential Minimally Invasive Surgical Correction of Adult Spinal Deformity. Neurosurgery 2017, 81, 733–739. [Google Scholar] [CrossRef]
- Kandwal, P.; Goswami, A.; Vijayaraghavan, G.; Subhash, K.R.; Jaryal, A.; Upendra, B.N.; Jayaswal, A. Staged Anterior Release and Posterior Instrumentation in Correction of Severe Rigid Scoliosis (Cobb Angle <100 Degrees). Spine Deform. 2016, 4, 296–303. [Google Scholar] [CrossRef]
- Xu, Z.; Li, F.; Chen, G.; Chen, Q. Reassessment System and Staged Surgical Strategy with Minimally Invasive Techniques for Treatment of Severe Adult Spinal Deformities. World Neurosurg. 2019, 126, e860–e868. [Google Scholar] [CrossRef] [PubMed]
- Than, K.D.; Park, P.; Tran, S.; Mundis, G.M.; Fu, K.-M.; Uribe, J.S.; Okonkwo, D.O.; Nunley, P.D.; Fessler, R.G.; Eastlack, R.K.; et al. Analysis of Complications with Staged Surgery for Less Invasive Treatment of Adult Spinal Deformity. World Neurosurg. 2019, 126, e1337–e1342. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Chung, A.; Kong, C.; Alayan, A.; Khandehroo, B.; Kahwaty, S.; Gendelberg, D. Prevalence and Modes of Posterior Hardware Failure with a Staged Protocol for Circumferential Minimally Invasive Surgical Correction of Adult Spinal Deformity: A 13-Year Experience. Int. J. Spine Surg. 2022, 16, 481–489. [Google Scholar] [CrossRef]
- Schwab, F.; Blondel, B.; Chay, E.; Demakakos, J.; Lenke, L.; Tropiano, P.; Ames, C.; Smith, J.S.; Shaffrey, C.I.; Glassman, S.; et al. The Comprehensive Anatomical Spinal Osteotomy Classification. Neurosurgery 2014, 74, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Smith-Petersen, M.N.; Larson, C.B.; Aufranc, O.E. Osteotomy of the Spine for Correction of Flexion Deformity in Rheumatoid Arthritis. Clin. Orthop. Relat. Res. 1969, 66, 6–9. [Google Scholar] [CrossRef]
- Geck, M.J.; Macagno, A.; Ponte, A.; Shufflebarger, H.L. The Ponte Procedure. J. Spinal Disord. Tech. 2007, 20, 586–593. [Google Scholar] [CrossRef]
- Thomasen, E. Vertebral Osteotomy for Correction of Kyphosis in Ankylosing Spondylitis. Clin. Orthop. Relat. Res. 1985, 142–152. [Google Scholar] [CrossRef]
- Bradford, D.S.; Tribus, C.B. Vertebral Column Resection for the Treatment of Rigid Coronal Decompensation. Spine 1997, 22, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Passias, P.G.; Krol, O.; Passfall, L.; Lafage, V.; Lafage, R.; Smith, J.S.; Line, B.; Vira, S.; Daniels, A.H.; Diebo, B.; et al. Three-Column Osteotomy in Adult Spinal Deformity. J. Bone Jt. Surg. 2022, 104, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Shaffrey, C.I.; Klineberg, E.; Lafage, V.; Schwab, F.; Lafage, R.; Kim, H.J.; Hostin, R.; Mundis, G.M.; Gupta, M.; et al. Complication Rates Associated with 3-Column Osteotomy in 82 Adult Spinal Deformity Patients: Retrospective Review of a Prospectively Collected Multicenter Consecutive Series with 2-Year Follow-Up. J. Neurosurg. Spine 2017, 27, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Dorward, I.G.; Lenke, L.G. Osteotomies in the Posterior-Only Treatment of Complex Adult Spinal Deformity: A Comparative Review. Neurosurg. Focus 2010, 28, E4. [Google Scholar] [CrossRef]
- Kosterhon, M.; Gutenberg, A.; Kantelhardt, S.R.; Archavlis, E.; Giese, A. Navigation and Image Injection for Control of Bone Removal and Osteotomy Planes in Spine Surgery. Oper. Neurosurg. 2017, 13, 297–304. [Google Scholar] [CrossRef]
- Takahashi, J.; Hashidate, H.; Hirabayashi, H.; Ogihara, N.; Mukaiyama, K.; Kato, H.; Ebara, S. Computer-Assisted Hemivertebral Resection for Congenital Spinal Deformity. J. Orthop. Sci. 2011, 16, 503–509. [Google Scholar] [CrossRef]
- Faundez, A.; Byrne, F.; Sylvestre, C.; Lafage, V.; Cogniet, A.; Le Huec, J.-C. Pedicle Subtraction Osteotomy in the Thoracic Spine and Thoracolumbar Junction: A Retrospective Series of 28 Cases. Eur. Spine J. 2015, 24, 42–48. [Google Scholar] [CrossRef]
- Shin, J.H.; Yanamadala, V.; Cha, T.D. Computer-Assisted Navigation for Real Time Planning of Pedicle Subtraction Osteotomy in Cervico-Thoracic Deformity Correction. Oper. Neurosurg. 2019, 16, 445–450. [Google Scholar] [CrossRef]
- Yoon, J.W.; Wang, M.Y. The Evolution of Minimally Invasive Spine Surgery. J. Neurosurg. Spine 2019, 30, 149–158. [Google Scholar] [CrossRef]
- Gadjradj, P.S.; Rubinstein, S.M.; Peul, W.C.; Depauw, P.R.; Vleggeert-Lankamp, C.L.; Seiger, A.; van Susante, J.L.; de Boer, M.R.; van Tulder, M.W.; Harhangi, B.S. Full Endoscopic versus Open Discectomy for Sciatica: Randomised Controlled Non-Inferiority Trial. BMJ 2022, 376, e065846. [Google Scholar] [CrossRef]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Full-Endoscopic Cervical Posterior Foraminotomy for the Operation of Lateral Disc Herniations Using 5.9-Mm Endoscopes. Spine 2008, 33, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Surgical Treatment for Lumbar Lateral Recess Stenosis with the Full-Endoscopic Interlaminar Approach versus Conventional Microsurgical Technique: A Prospective, Randomized, Controlled Study. J. Neurosurg. Spine 2009, 10, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Mittlmeier, T.; Gierer, P.; Harms, C.; Gradl, G. Benefit and Accuracy of Intraoperative 3D-Imaging after Pedicle Screw Placement: A Prospective Study in Stabilizing Thoracolumbar Fractures. Eur. Spine J. 2009, 18, 1469–1477. [Google Scholar] [CrossRef]
- Zhang, W.; Takigawa, T.; Wu, Y.; Sugimoto, Y.; Tanaka, M.; Ozaki, T. Accuracy of Pedicle Screw Insertion in Posterior Scoliosis Surgery: A Comparison between Intraoperative Navigation and Preoperative Navigation Techniques. Eur. Spine J. 2017, 26, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Elmi-Terander, A.; Burström, G.; Nachabe, R.; Skulason, H.; Pedersen, K.; Fagerlund, M.; Ståhl, F.; Charalampidis, A.; Söderman, M.; Holmin, S.; et al. Pedicle Screw Placement Using Augmented Reality Surgical Navigation with Intraoperative 3D Imaging. Spine 2019, 44, 517–525. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Kanemura, T.; Yoshida, G.; Matsumoto, A.; Ito, Z.; Tauchi, R.; Muramoto, A.; Ohno, S.; Nishimura, Y. Intraoperative, Full-Rotation, Three-Dimensional Image (O-Arm)–Based Navigation System for Cervical Pedicle Screw Insertion. J. Neurosurg. Spine 2011, 15, 472–478. [Google Scholar] [CrossRef]
- Kantelhardt, S.R.; Martinez, R.; Baerwinkel, S.; Burger, R.; Giese, A.; Rohde, V. Perioperative Course and Accuracy of Screw Positioning in Conventional, Open Robotic-Guided and Percutaneous Robotic-Guided, Pedicle Screw Placement. Eur. Spine J. 2011, 20, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, I.H.; Hardenbrook, M.A.; Wang, J.C.; Guyer, R.D. Assessment of Pedicle Screw Placement Accuracy, Procedure Time, and Radiation Exposure Using a Miniature Robotic Guidance System. J. Spinal Disord. Technol. 2012, 25, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ohnmeiss, D.D.; Lieberman, I.H. Robotic-Assisted Pedicle Screw Placement: Lessons Learned from the First 102 Patients. Eur. Spine J. 2013, 22, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Pappou, I.P.; Girardi, F.P.; Sandhu, H.S.; Parvataneni, H.K.; Cammisa, F.P.; Schneider, R.; Frelinghuysen, P.; Lane, J.M. Discordantly High Spinal Bone Mineral Density Values in Patients with Adult Lumbar Scoliosis. Spine 2006, 31, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Cha, T.; Schwab, J.; Fogel, H.; Tobert, D.; Razi, A.E.; Hecht, A.; Bono, C.M.; Hershman, S. Osteoporosis Increases the Likelihood of Revision Surgery Following a Long Spinal Fusion for Adult Spinal Deformity. Spine J. 2021, 21, 134–140. [Google Scholar] [CrossRef]
- Hu, S.S. Internal Fixation in the Osteoporotic Spine. Spine 1997, 22, 43S–48S. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, B.T.; Zarrabian, M.; Aleem, I.S.; Fogelson, J.L.; Currier, B.L.; Freedman, B.A.; Bydon, M.; Nassr, A. Incidence of Osteoporosis-Related Complications Following Posterior Lumbar Fusion. Glob. Spine J. 2018, 8, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gong, F.; Liu, L.; Ma, Z.; Zhang, Y.; Zhao, X.; Yang, M.; Lei, W.; Sang, H. A Comparative Study on Screw Loosening in Osteoporotic Lumbar Spine Fusion between Expandable and Conventional Pedicle Screws. Arch. Orthop. Trauma. Surg. 2012, 132, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sheng, J.; Luo, Y.; Huang, C.; Wu, H.-H.; Zhou, J.-J.; Zhang, X.-J.; Zheng, W. Biomechanical Comparative Study of the Stability of Injectable Pedicle Screws with Different Lateral Holes Augmented with Different Volumes of Polymethylmethacrylate in Osteoporotic Lumbar Vertebrae. Spine J. 2018, 18, 1637–1644. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Choi, W.-S.; Rhyu, K.-W. Assessment of Pedicle Screw Pullout Strength Based on Various Screw Designs and Bone Densities—An Ex Vivo Biomechanical Study. Spine J. 2012, 12, 164–168. [Google Scholar] [CrossRef]
- Gates, T.A.; Moldavsky, M.; Salloum, K.; Dunbar, G.L.; Park, J.; Bucklen, B. Biomechanical Analysis of a Novel Pedicle Screw Anchor Designed for the Osteoporotic Population. World Neurosurg. 2015, 83, 965–969. [Google Scholar] [CrossRef]
- Elder, B.D.; Ishida, W.; Lo, S.-F.L.; Holmes, C.; Goodwin, C.R.; Kosztowski, T.A.; Bydon, A.; Gokaslan, Z.L.; Wolinsky, J.-P.; Sciubba, D.M.; et al. Use of S2-Alar-Iliac Screws Associated with Less Complications Than Iliac Screws in Adult Lumbosacropelvic Fixation. Spine 2017, 42, E142–E149. [Google Scholar] [CrossRef]
- De la Garza Ramos, R.; Nakhla, J.; Sciubba, D.M.; Yassari, R. Iliac Screw versus S2 Alar-Iliac Screw Fixation in Adults: A Meta-Analysis. J. Neurosurg. Spine 2019, 30, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Nakahira, Y.; Watanabe, K.; Nakamura, M.; Matsumoto, M.; Iwamoto, M. The Effect of Posterior Tethers on the Biomechanics of Proximal Junctional Kyphosis: The Whole Human Finite Element Model Analysis. Sci. Rep. 2020, 10, 3433. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, E.P.; Buell, T.J.; Sardi, J.P.; Lazaro, B.C.R.; Shaffrey, C.I.; Smith, J.S. A Novel Weave Tether Technique for Proximal Junctional Kyphosis Prevention in 71 Adult Spinal Deformity Patients: A Preliminary Case Series Assessing Early Complications and Efficacy. Oper. Neurosurg. 2021, 21, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, E.P.; Snyder, M.H.; McClure, J.J.; Buell, T.J.; Smith, J.S.; Shaffrey, C.I.; Buchholz, A.L. Posterior Polyethylene Tethers Reduce Occurrence of Proximal Junctional Kyphosis After Multilevel Spinal Instrumentation for Adult Spinal Deformity: A Retrospective Analysis. Neurosurgery 2021, 89, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Buell, T.J.; Buchholz, A.L.; Quinn, J.C.; Bess, S.; Line, B.G.; Ames, C.P.; Schwab, F.J.; Lafage, V.; Shaffrey, C.I.; Smith, J.S. A Pilot Study on Posterior Polyethylene Tethers to Prevent Proximal Junctional Kyphosis After Multilevel Spinal Instrumentation for Adult Spinal Deformity. Oper. Neurosurg. 2019, 16, 256–266. [Google Scholar] [CrossRef]
- Guevara-Villazón, F.; Boissiere, L.; Hayashi, K.; Larrieu, D.; Ghailane, S.; Vital, J.-M.; Gille, O.; Pointillart, V.; Obeid, I.; Bourghli, A. Multiple-Rod Constructs in Adult Spinal Deformity Surgery for Pelvic-Fixated Long Instrumentations: An Integral Matched Cohort Analysis. Eur. Spine J. 2020, 29, 886–895. [Google Scholar] [CrossRef]
- Merrill, R.K.; Kim, J.S.; Leven, D.M.; Kim, J.H.; Cho, S.K. Multi-Rod Constructs Can Prevent Rod Breakage and Pseudarthrosis at the Lumbosacral Junction in Adult Spinal Deformity. Glob. Spine J. 2017, 7, 514–520. [Google Scholar] [CrossRef]
- Gupta, S.; Eksi, M.S.; Ames, C.P.; Deviren, V.; Durbin-Johnson, B.; Smith, J.S.; Gupta, M.C. A Novel 4-Rod Technique Offers Potential to Reduce Rod Breakage and Pseudarthrosis in Pedicle Subtraction Osteotomies for Adult Spinal Deformity Correction. Oper. Neurosurg. 2018, 14, 449–456. [Google Scholar] [CrossRef]
- Worley, N.; Marascalchi, B.; Jalai, C.M.; Yang, S.; Diebo, B.; Vira, S.; Boniello, A.; Lafage, V.; Passias, P.G. Predictors of Inpatient Morbidity and Mortality in Adult Spinal Deformity Surgery. Eur. Spine J. 2016, 25, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.B.C.; Daniels, A.H.; Ailon, T.; Miller, E.; Sciubba, D.M.; Smith, J.S.; Shaffrey, C.I.; Schwab, F.; Burton, D.; Hart, R.A.; et al. Frailty and Health-Related Quality of Life Improvement Following Adult Spinal Deformity Surgery. World Neurosurg. 2018, 112, e548–e554. [Google Scholar] [CrossRef] [PubMed]
- Pellisé, F.; Vila-Casademunt, A.; Núñez-Pereira, S.; Domingo-Sàbat, M.; Bagó, J.; Vidal, X.; Alanay, A.; Acaroglu, E.; Kleinstück, F.; Obeid, I.; et al. The Adult Deformity Surgery Complexity Index (ADSCI): A Valid Tool to Quantify the Complexity of Posterior Adult Spinal Deformity Surgery and Predict Postoperative Complications. Spine J. 2018, 18, 216–225. [Google Scholar] [CrossRef]
- Miller, E.K.; Lenke, L.G.; Neuman, B.J.; Sciubba, D.M.; Kebaish, K.M.; Smith, J.S.; Qiu, Y.; Dahl, B.T.; Pellisé, F.; Matsuyama, Y.; et al. External Validation of the Adult Spinal Deformity (ASD) Frailty Index (ASD-FI) in the Scoli-RISK-1 Patient Database. Spine 2018, 43, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Yearley, A.G.; Chalif, J.I.; Chalif, E.J.; Zaidi, H.A. The Relationship Among Surgeon Experience, Complications, and Radiographic Outcomes in Spine Deformity Surgery: The Experience of a Junior Surgeon. World Neurosurg. 2022, 168, e399–e407. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Klineberg, E.; Lafage, V.; Shaffrey, C.I.; Schwab, F.; Lafage, R.; Hostin, R.; Mundis, G.M.; Errico, T.J.; Kim, H.J.; et al. Prospective Multicenter Assessment of Perioperative and Minimum 2-Year Postoperative Complication Rates Associated with Adult Spinal Deformity Surgery. J. Neurosurg. Spine 2016, 25, 1–14. [Google Scholar] [CrossRef]
- Pull ter Gunne, A.F.; van Laarhoven, C.J.H.M.; Cohen, D.B. Incidence of Surgical Site Infection Following Adult Spinal Deformity Surgery: An Analysis of Patient Risk. Eur. Spine J. 2010, 19, 982–988. [Google Scholar] [CrossRef]
- Wang, M.; Xu, L.; Yang, B.; Du, C.; Zhu, Z.; Wang, B.; Qiu, Y.; Sun, X. Incidence, Management and Outcome of Delayed Deep Surgical Site Infection Following Spinal Deformity Surgery: 20-Year Experience at a Single Institution. Glob. Spine J. 2022, 12, 1141–1150. [Google Scholar] [CrossRef]
- Rihn, J.A.; Lee, J.Y.; Ward, W.T. Infection After the Surgical Treatment of Adolescent Idiopathic Scoliosis. Spine 2008, 33, 289–294. [Google Scholar] [CrossRef]
- Ramo, B.A.; Roberts, D.W.; Tuason, D.; McClung, A.; Paraison, L.E.; Moore, H.G.; Sucato, D.J. Surgical Site Infections After Posterior Spinal Fusion for Neuromuscular Scoliosis. J. Bone Jt. Surg. Am. Vol. 2014, 96, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.A.; Savage, J.W.; Vaccaro, A.R.; Radcliff, K.; Arnold, P.M.; Lawrence, B.D.; Shamji, M.F. Prevention of Surgical Site Infection in Spine Surgery. Neurosurgery 2017, 80, S114–S123. [Google Scholar] [CrossRef]
- Hu, S.S. Blood Loss in Adult Spinal Surgery. Eur. Spine J. 2004, 13, S3–S5. [Google Scholar] [CrossRef]
- Pernik, M.N.; Dosselman, L.J.; Aoun, S.G.; Walker, A.D.; Hall, K.; Peinado Reyes, V.; McDonagh, D.L.; Bagley, C.A. The Effectiveness of Tranexamic Acid on Operative and Perioperative Blood Loss in Long-Segment Spinal Fusions: A Consecutive Series of 119 Primary Procedures. J. Neurosurg. Spine 2020, 32, 768–774. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, H.; He, S. Efficacy and Safety of Using Antifibrinolytic Agents in Spine Surgery: A Meta-Analysis. PLoS ONE 2013, 8, e82063. [Google Scholar] [CrossRef][Green Version]
- Lu, V.M.; Ho, Y.-T.; Nambiar, M.; Mobbs, R.J.; Phan, K. The Perioperative Efficacy and Safety of Antifibrinolytics in Adult Spinal Fusion Surgery. Spine 2018, 43, E949–E958. [Google Scholar] [CrossRef]
- Mikhail, C.; Pennington, Z.; Arnold, P.M.; Brodke, D.S.; Chapman, J.R.; Chutkan, N.; Daubs, M.D.; DeVine, J.G.; Fehlings, M.G.; Gelb, D.E.; et al. Minimizing Blood Loss in Spine Surgery. Glob. Spine J. 2020, 10, 71S–83S. [Google Scholar] [CrossRef]
- Park, C.K. The Effect of Patient Positioning on Intraabdominal Pressure and Blood Loss in Spinal Surgery. Anesth. Analg. 2000, 552–557. [Google Scholar] [CrossRef]
- Akinci, I.O.; Tunali, U.; Kyzy, A.A.; Guresti, E.; Sencer, A.; Karasu, A.; Telci, L. Effects of Prone and Jackknife Positioning on Lumbar Disc Herniation Surgery. J. Neurosurg. Anesthesiol. 2011, 23, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Sun, R.; Ba, C.; Gong, X.; Liu, W.; Zhai, R. Bipolar Sealer Device Reduces Blood Loss and Transfusion Requirements in Posterior Spinal Fusion for Degenerative Lumbar Scoliosis. Clin. Spine Surg. Spine Publ. 2016, 29, E107–E111. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Dai, L.; Yang, Y.; Liu, H. Comparison the Efficacy of Hemorrhage Control of Surgiflo Haemostatic Matrix and Absorbable Gelatin Sponge in Posterior Lumbar Surgery. Medicine 2018, 97, e13511. [Google Scholar] [CrossRef]
- Guan, J.; Cole, C.D.; Schmidt, M.H.; Dailey, A.T. Utility of Intraoperative Rotational Thromboelastometry in Thoracolumbar Deformity Surgery. J. Neurosurg. Spine 2017, 27, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Naik, B.I.; Pajewski, T.N.; Bogdonoff, D.I.; Zuo, Z.; Clark, P.; Terkawi, A.S.; Durieux, M.E.; Shaffrey, C.I.; Nemergut, E.C. Rotational Thromboelastometry–Guided Blood Product Management in Major Spine Surgery. J. Neurosurg. Spine 2015, 23, 239–249. [Google Scholar] [CrossRef]
- Ellis, R.; Hardie, J.A.; Summerton, D.J.; Brennan, P.A. Dual Surgeon Operating to Improve Patient Safety. Br. J. Oral. Maxillofac. Surg. 2021, 59, 752–756. [Google Scholar] [CrossRef]
- Ames, C.P.; Barry, J.J.; Keshavarzi, S.; Dede, O.; Weber, M.H.; Deviren, V. Perioperative Outcomes and Complications of Pedicle Subtraction Osteotomy in Cases with Single Versus Two Attending Surgeons. Spine Deform. 2013, 1, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Stienen, M.N.; Medress, Z.A.; Varshneya, K.; Ho, A.L.; Ratliff, J.K.; Veeravagu, A. Single- versus Dual-Attending Strategy for Spinal Deformity Surgery: 2-Year Experience and Systematic Review of the Literature. J. Neurosurg. Spine 2020, 33, 560–571. [Google Scholar] [CrossRef]
- Bauer, J.M.; Yanamadala, V.; Shah, S.A.; Sethi, R.K. Two Surgeon Approach for Complex Spine Surgery. J. Am. Acad. Orthop. Surg. 2019, 27, e408–e413. [Google Scholar] [CrossRef] [PubMed]
- Lak, A.M.; Abunimer, A.M.; Goedmakers, C.M.W.; Aglio, L.S.; Smith, T.R.; Makhni, M.; Mekary, R.A.; Zaidi, H.A. Single- versus Dual-Attending Surgeon Approach for Spine Deformity: A Systematic Review and Meta-Analysis. Oper. Neurosurg. 2021, 20, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.K.; Lafage, R.; Schwab, F.J.; Liabaud, B.; Smith, J.S.; Mundis, G.M.; Hostin, R.; Shaffrey, C.I.; Burton, D.C.; Hart, R.A.; et al. Under Correction of Sagittal Deformities Based on Age-Adjusted Alignment Thresholds Leads to Worse Health-Related Quality of Life Whereas Over Correction Provides No Additional Benefit. Spine 2018, 43, 388–393. [Google Scholar] [CrossRef]
- Akıntürk, N.; Zileli, M.; Yaman, O. Complications of Adult Spinal Deformity Surgery: A Literature Review. J. Craniovertebral Junction Spine 2022, 13, 17. [Google Scholar] [CrossRef]
- Smith, J.S.; Shaffrey, E.; Klineberg, E.; Shaffrey, C.I.; Lafage, V.; Schwab, F.J.; Protopsaltis, T.; Scheer, J.K.; Mundis, G.M.; Fu, K.-M.G.; et al. Prospective Multicenter Assessment of Risk Factors for Rod Fracture Following Surgery for Adult Spinal Deformity. J. Neurosurg. Spine 2014, 21, 994–1003. [Google Scholar] [CrossRef]
- Passias, P.G.; Soroceanu, A.; Yang, S.; Schwab, F.; Ames, C.; Boniello, A.; Smith, J.; Shaffrey, C.; Boachie-Adjei, O.; Mundis, G.; et al. Predictors of Revision Surgical Procedure Excluding Wound Complications in Adult Spinal Deformity and Impact on Patient-Reported Outcomes and Satisfaction. J. Bone Jt. Surg. 2016, 98, 536–543. [Google Scholar] [CrossRef]
- Lertudomphonwanit, T.; Kelly, M.P.; Bridwell, K.H.; Lenke, L.G.; McAnany, S.J.; Punyarat, P.; Bryan, T.P.; Buchowski, J.M.; Zebala, L.P.; Sides, B.A.; et al. Rod Fracture in Adult Spinal Deformity Surgery Fused to the Sacrum: Prevalence, Risk Factors, and Impact on Health-Related Quality of Life in 526 Patients. Spine J. 2018, 18, 1612–1624. [Google Scholar] [CrossRef]
- Tang, J.A.; Leasure, J.M.; Smith, J.S.; Buckley, J.M.; Kondrashov, D.; Ames, C.P. Effect of Severity of Rod Contour on Posterior Rod Failure in the Setting of Lumbar Pedicle Subtraction Osteotomy (PSO). Neurosurgery 2013, 72, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Clark, A.J.; Scheer, J.K.; Daubs, M.D.; Coe, J.D.; Paonessa, K.J.; LaGrone, M.O.; Kasten, M.D.; Amaral, R.A.; Trobisch, P.D.; et al. Proximal Junctional Kyphosis and Failure After Spinal Deformity Surgery. Spine 2014, 39, 2093–2102. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Shen, B.; Wang, C.; Li, M. Risk Factor Analysis of Proximal Junctional Kyphosis after Posterior Fusion in Patients with Idiopathic Scoliosis. Injury 2010, 41, 415–420. [Google Scholar] [CrossRef]
- Kim, H.J.; Bridwell, K.H.; Lenke, L.G.; Park, M.S.; Ahmad, A.; Song, K.-S.; Piyaskulkaew, C.; Hershman, S.; Fogelson, J.; Mesfin, A. Proximal Junctional Kyphosis Results in Inferior SRS Pain Subscores in Adult Deformity Patients. Spine 2013, 38, 896–901. [Google Scholar] [CrossRef]
- Yagi, M.; King, A.B.; Boachie-Adjei, O. Incidence, Risk Factors, and Natural Course of Proximal Junctional Kyphosis. Spine 2012, 37, 1479–1489. [Google Scholar] [CrossRef]
- Bridwell, K.H.; Lenke, L.G.; Cho, S.K.; Pahys, J.M.; Zebala, L.P.; Dorward, I.G.; Cho, W.; Baldus, C.; Hill, B.W.; Kang, M.M. Proximal Junctional Kyphosis in Primary Adult Deformity Surgery. Neurosurgery 2013, 72, 899–906. [Google Scholar] [CrossRef]
- Park, S.-J.; Lee, C.-S.; Chung, S.-S.; Lee, J.-Y.; Kang, S.-S.; Park, S.-H. Different Risk Factors of Proximal Junctional Kyphosis and Proximal Junctional Failure Following Long Instrumented Fusion to the Sacrum for Adult Spinal Deformity: Survivorship Analysis of 160 Patients. Neurosurgery 2017, 80, 279–286. [Google Scholar] [CrossRef]
- Yagi, M.; Akilah, K.B.; Boachie-Adjei, O. Incidence, Risk Factors and Classification of Proximal Junctional Kyphosis. Spine 2011, 36, E60–E68. [Google Scholar] [CrossRef]
- Yagi, M.; Rahm, M.; Gaines, R.; Maziad, A.; Ross, T.; Kim, H.J.; Kebaish, K.; Boachie-Adjei, O. Characterization and Surgical Outcomes of Proximal Junctional Failure in Surgically Treated Patients with Adult Spinal Deformity. Spine 2014, 39, E607–E614. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.; McCarthy, I.; O’Brien, M.; Bess, S.; Line, B.; Adjei, O.B.; Burton, D.; Gupta, M.; Ames, C.; Deviren, V.; et al. Identification of Decision Criteria for Revision Surgery Among Patients with Proximal Junctional Failure After Surgical Treatment of Spinal Deformity. Spine 2013, 38, E1223–E1227. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Funao, H.; Clark, A.J.; Nicholls, F.; Smith, J.; Bess, S.; Shaffrey, C.; Schwab, F.J.; Lafage, V.; Deviren, V.; et al. The Clinical Correlation of the Hart-ISSG Proximal Junctional Kyphosis Severity Scale with Health-Related Quality-of-Life Outcomes and Need for Revision Surgery. Spine 2016, 41, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Lee, C.-S.; Park, J.-S.; Kang, B.-J.; Shin, T.S.; Park, S.-J. Characteristics of Patients Undergoing Revision Surgery for Proximal Junctional Failure after Adult Spinal Deformity Surgery: Revalidation of the Hart–International Spine Study Group Proximal Junctional Kyphosis Severity Scale. J. Neurosurg. Spine 2022, 37, 402–409. [Google Scholar] [CrossRef]
- Yagi, M.; Ohne, H.; Konomi, T.; Fujiyoshi, K.; Kaneko, S.; Komiyama, T.; Takemitsu, M.; Yato, Y.; Machida, M.; Asazuma, T. Teriparatide Improves Volumetric Bone Mineral Density and Fine Bone Structure in the UIV+1 Vertebra, and Reduces Bone Failure Type PJK after Surgery for Adult Spinal Deformity. Osteoporos. Int. 2016, 27, 3495–3502. [Google Scholar] [CrossRef]
- Ghobrial, G.M.; Eichberg, D.G.; Kolcun, J.P.G.; Madhavan, K.; Lebwohl, N.H.; Green, B.A.; Gjolaj, J.P. Prophylactic Vertebral Cement Augmentation at the Uppermost Instrumented Vertebra and Rostral Adjacent Vertebra for the Prevention of Proximal Junctional Kyphosis and Failure Following Long-Segment Fusion for Adult Spinal Deformity. Spine J. 2017, 17, 1499–1505. [Google Scholar] [CrossRef]
- Hart, R.A.; Prendergast, M.A.; Roberts, W.G.; Nesbit, G.M.; Barnwell, S.L. Proximal Junctional Acute Collapse Cranial to Multi-Level Lumbar Fusion: A Cost Analysis of Prophylactic Vertebral Augmentation. Spine J. 2008, 8, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Kebaish, K.M.; Martin, C.T.; O’Brien, J.R.; LaMotta, I.E.; Voros, G.D.; Belkoff, S.M. Use of Vertebroplasty to Prevent Proximal Junctional Fractures in Adult Deformity Surgery: A Biomechanical Cadaveric Study. Spine J. 2013, 13, 1897–1903. [Google Scholar] [CrossRef]
- Hassanzadeh, H.; Gupta, S.; Jain, A.; El Dafrawy, M.H.; Skolasky, R.L.; Kebaish, K.M. Type of Anchor at the Proximal Fusion Level Has a Significant Effect on the Incidence of Proximal Junctional Kyphosis and Outcome in Adults After Long Posterior Spinal Fusion. Spine Deform. 2013, 1, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Glassman, S.D.; Sucato, D.; Hresko, M.T.; Carreon, L.Y. Incidence of Proximal Junctional Kyphosis with Pedicle Screws at Upper Instrumented Vertebrae in Posterior Spinal Fusion for Adolescent Idiopathic Scoliosis. Glob. Spine J. 2021, 11, 1019–1024. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lenke, L.G.; Bridwell, K.H.; Kim, J.; Cho, S.K.; Cheh, G.; Yoon, J. Proximal Junctional Kyphosis in Adolescent Idiopathic Scoliosis After 3 Different Types of Posterior Segmental Spinal Instrumentation and Fusions. Spine 2007, 32, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, M.D.; Shah, S.A.; Newton, P.O.; Clements, D.H.; Betz, R.R.; Marks, M.C.; Bastrom, T. Evaluation of Proximal Junctional Kyphosis in Adolescent Idiopathic Scoliosis Following Pedicle Screw, Hook, or Hybrid Instrumentation. Spine 2010, 35, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Lafage, R.; Schwab, F.; Glassman, S.; Bess, S.; Harris, B.; Sheer, J.; Hart, R.; Line, B.; Henry, J.; Burton, D.; et al. Age-Adjusted Alignment Goals Have the Potential to Reduce PJK. Spine 2017, 42, 1275–1282. [Google Scholar] [CrossRef]
- How, N.E.; Street, J.T.; Dvorak, M.F.; Fisher, C.G.; Kwon, B.K.; Paquette, S.; Smith, J.S.; Shaffrey, C.I.; Ailon, T. Pseudarthrosis in Adult and Pediatric Spinal Deformity Surgery: A Systematic Review of the Literature and Meta-Analysis of Incidence, Characteristics, and Risk Factors. Neurosurg. Rev. 2019, 42, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.I.; Mirza, S.K.; Comstock, B.A.; Gray, D.T.; Kreuter, W.; Deyo, R.A. Reoperation Rates Following Lumbar Spine Surgery and the Influence of Spinal Fusion Procedures. Spine 2007, 32, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Bridwell, K.H.; Lenke, L.G.; Rhim, S.; Cheh, G. Pseudarthrosis in Long Adult Spinal Deformity Instrumentation and Fusion to the Sacrum: Prevalence and Risk Factor Analysis of 144 Cases. Spine 2006, 31, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Albert, T.J.; Pinto, M.; Denis, F. Management of Symptomatic Lumbar Pseudarthrosis with Anteroposterior Fusion. Spine 2000, 25, 123. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.; Macdonald, N.A.; Gendreau, J.L.; Duddleston, P.J.; Feng, A.Y.; Ho, A.L. Graft Materials and Biologics for Spinal Interbody Fusion. Biomedicines 2019, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Dakwar, E.; Cardona, R.F.; Smith, D.A.; Uribe, J.S. Early Outcomes and Safety of the Minimally Invasive, Lateral Retroperitoneal Transpsoas Approach for Adult Degenerative Scoliosis. Neurosurg. Focus. 2010, 28, E8. [Google Scholar] [CrossRef]
- Januszewski, J.; Vivas, A.C.; Uribe, J.S. Limitations and Complications of Minimally Invasive Spinal Surgery in Adult Deformity. Ann. Transl. Med. 2018, 6, 109. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Härtl, R.; Elowitz, E. Challenges Hindering Widespread Adoption of Minimally Invasive Spinal Surgery. World Neurosurg. 2022, 163, 228–232. [Google Scholar] [CrossRef]
- Mekhael, E.; El Rachkidi, R.; Saliby, R.M.; Nassim, N.; Semaan, K.; Massaad, A.; Karam, M.; Saade, M.; Ayoub, E.; Rteil, A.; et al. Functional Assessment Using 3D Movement Analysis Can Better Predict Health-Related Quality of Life Outcomes in Patients with Adult Spinal Deformity: A Machine Learning Approach. Front. Surg. 2023, 10, 1166734. [Google Scholar] [CrossRef]
- Prost, S.; Pesenti, S.; Farah, K.; Tropiano, P.; Fuentes, S.; Blondel, B. Adult Spinal Deformities: Can Patient-Specific Rods Change the Preoperative Planning into Clinical Reality? Feasibility Study and Preliminary Results about 77 Cases. Adv. Orthop. 2020, 2020, 6120580. [Google Scholar] [CrossRef]
- Felix, B.; Kalatar, S.B.; Moatz, B.; Hofstetter, C.; Karsy, M.; Parr, R.; Gibby, W. Augmented Reality Spine Surgery Navigation. Spine 2022, 47, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Burström, G.; Persson, O.; Edström, E.; Elmi-Terander, A. Augmented Reality Navigation in Spine Surgery: A Systematic Review. Acta Neurochir. 2021, 163, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Pellisé, F.; Serra-Burriel, M.; Smith, J.S.; Haddad, S.; Kelly, M.P.; Vila-Casademunt, A.; Sánchez Pérez-Grueso, F.J.; Bess, S.; Gum, J.L.; Burton, D.C.; et al. Development and Validation of Risk Stratification Models for Adult Spinal Deformity Surgery. J. Neurosurg. Spine 2019, 31, 587–599. [Google Scholar] [CrossRef]
- Ames, C.P.; Smith, J.S.; Pellisé, F.; Kelly, M.; Alanay, A.; Acaroğlu, E.; Pérez-Grueso, F.J.S.; Kleinstück, F.; Obeid, I.; Vila-Casademunt, A.; et al. Artificial Intelligence Based Hierarchical Clustering of Patient Types and Intervention Categories in Adult Spinal Deformity Surgery. Spine 2019, 44, 915–926. [Google Scholar] [CrossRef] [PubMed]






| Anatomic Regions Resected | |
|---|---|
| Grade 1 | Partial facet joint (inferior facet and joint capsule) |
| Grade 2 | Complete facet joint (superior and inferior facets with ligamentum flavum removal) |
| Grade 3 | Pedicle and partial body (posterior vertebral body partial wedge resection and posterior vertebral elements) |
| Grade 4 | Pedicle, partial body, and disc (posterior vertebral body wider wedge resection, posterior vertebral elements, and portion of >1 endplate and intervertebral disc) |
| Grade 5 | Complete vertebra and both adjacent discs |
| Grade 6 | Multiple vertebrae and discs |
| Category | Description | |
|---|---|---|
| Type | Type 1 | Disc and ligamentous failure |
| Type 2 | Bone failure | |
| Type 3 | Implant/bone failure | |
| Grade | Grade A | Proximal junction increase of 10°–19° |
| Grade B | Proximal junction increase of 20°–29° | |
| Grade C | Proximal junction increase ≥30° | |
| Spondylo- listhesis | PJF-N | No spondylolisthesis present above the uppermost instrumented vertebra |
| PJF-S | Spondylolisthesis present above the uppermost instrumented vertebra |
| Characteristic | Severity Score | |
|---|---|---|
| Neurological Deficit | None | 0 |
| Radicular Pain | 2 | |
| Myelopathy or Motor Deficit | 4 | |
| Focal Pain | None | 0 |
| VAS ≤ 4 | 1 | |
| VAS ≥ 5 | 3 | |
| Instrumentation Problem | None | 0 |
| Partial Fixation Loss | 1 | |
| Prominence | 1 | |
| Complete Fixation Loss | 2 | |
| Change In Kyphosis | 0–10° | 0 |
| 10°–20° | 1 | |
| >20° | 2 | |
| PLC Failure | 2 | |
| UIV Changes | None | 0 |
| Compression Fracture | 1 | |
| Burst/Chance Fracture | 2 | |
| Translation | 3 | |
| Level of UV | Thoracolumbar Junction | 0 |
| Upper Thoracic | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, R.V.; Yearley, A.G.; Isaac, H.; Chalif, E.J.; Chalif, J.I.; Zaidi, H.A. Advances and Evolving Challenges in Spinal Deformity Surgery. J. Clin. Med. 2023, 12, 6386. https://doi.org/10.3390/jcm12196386
Patel RV, Yearley AG, Isaac H, Chalif EJ, Chalif JI, Zaidi HA. Advances and Evolving Challenges in Spinal Deformity Surgery. Journal of Clinical Medicine. 2023; 12(19):6386. https://doi.org/10.3390/jcm12196386
Chicago/Turabian StylePatel, Ruchit V., Alexander G. Yearley, Hannah Isaac, Eric J. Chalif, Joshua I. Chalif, and Hasan A. Zaidi. 2023. "Advances and Evolving Challenges in Spinal Deformity Surgery" Journal of Clinical Medicine 12, no. 19: 6386. https://doi.org/10.3390/jcm12196386
APA StylePatel, R. V., Yearley, A. G., Isaac, H., Chalif, E. J., Chalif, J. I., & Zaidi, H. A. (2023). Advances and Evolving Challenges in Spinal Deformity Surgery. Journal of Clinical Medicine, 12(19), 6386. https://doi.org/10.3390/jcm12196386