Robotized Knee-Ankle-Foot Orthosis-Assisted Gait Training on Genu Recurvatum during Gait in Patients with Chronic Stroke: A Feasibility Study and Case Report
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Healthy Individuals
2.1.2. Stroke Patients
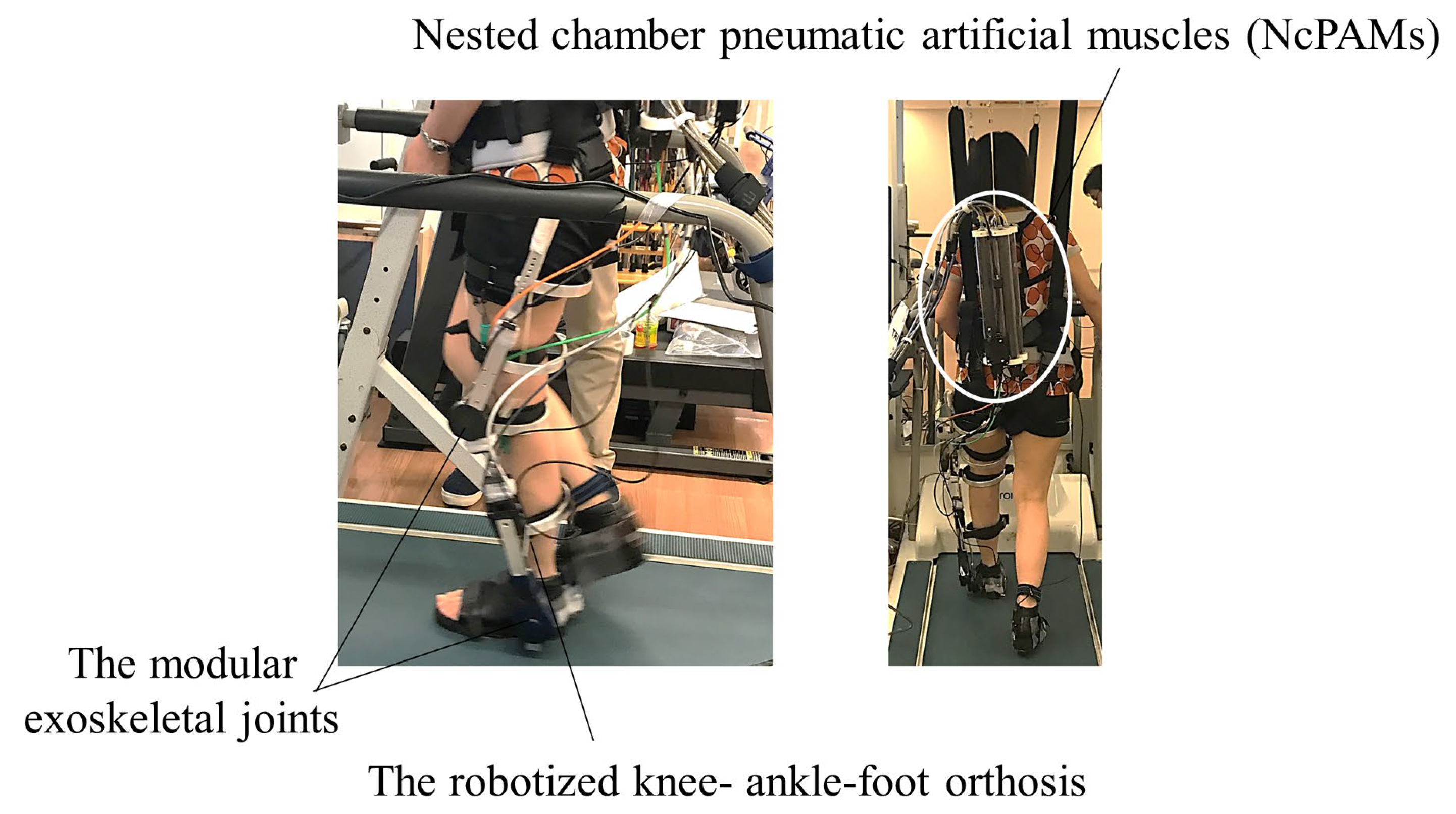
2.2. Study Device
2.2.1. Robotized Knee-Ankle-Foot Orthosis
2.2.2. Assist Setting
2.3. Experimental Procedure
2.3.1. Experiment 1
2.3.2. Experiment 2
2.4. Assessments
2.4.1. Kinematic Data during Overground Gait
2.4.2. Clinical Assessments
2.4.3. Data Analysis of Kinematic Data
3. Results
3.1. Safety of Robot-Assisted Gait Training
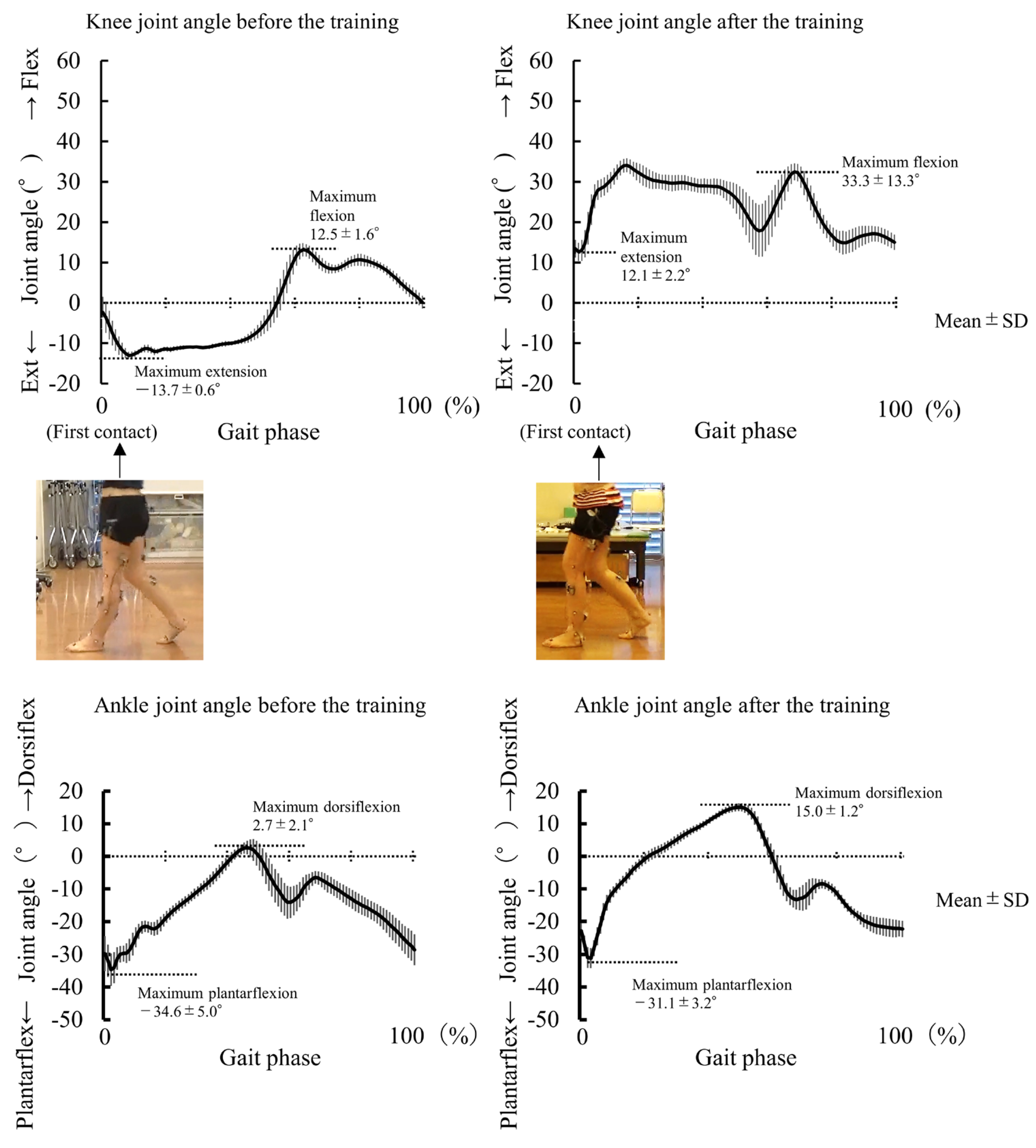
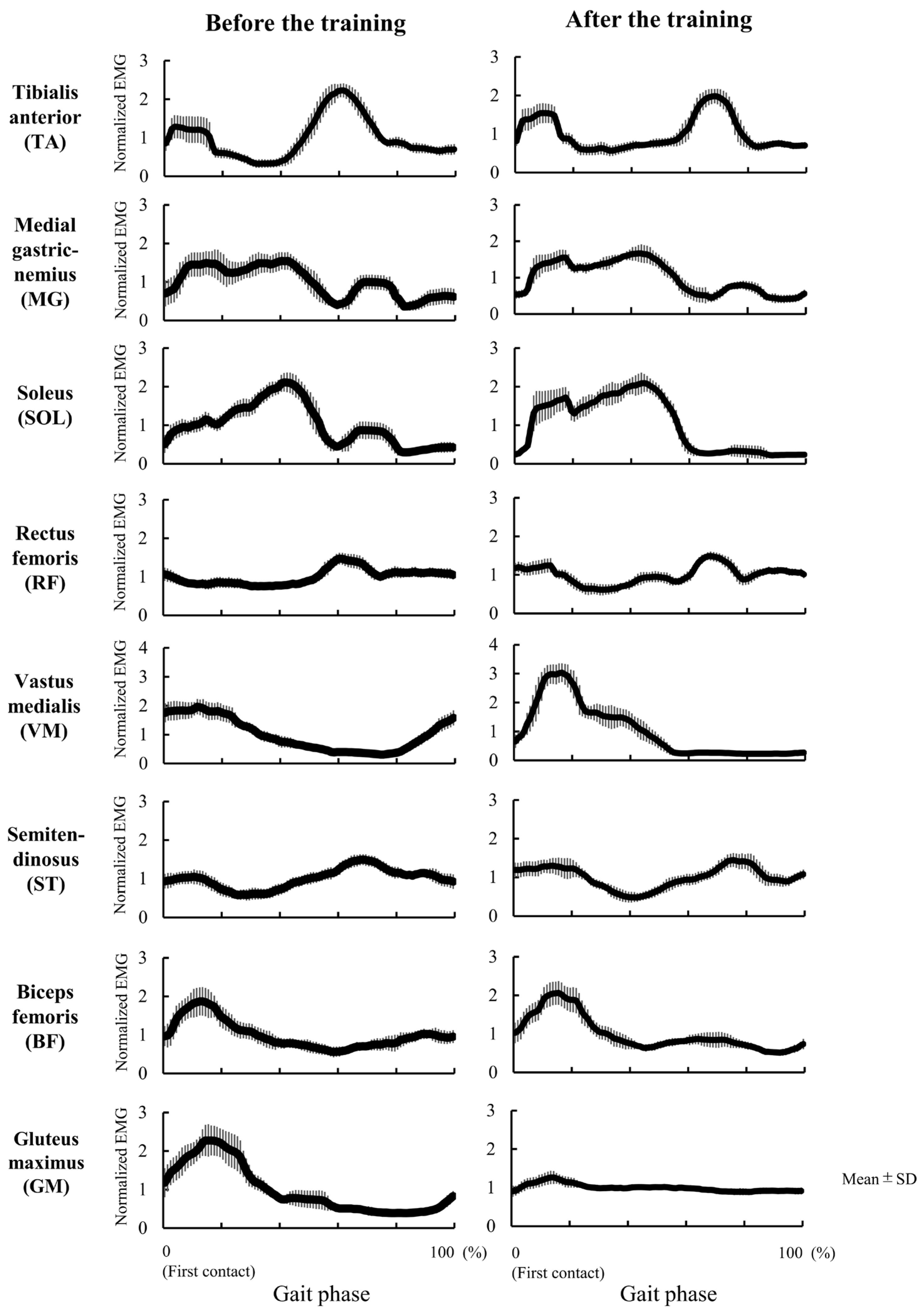
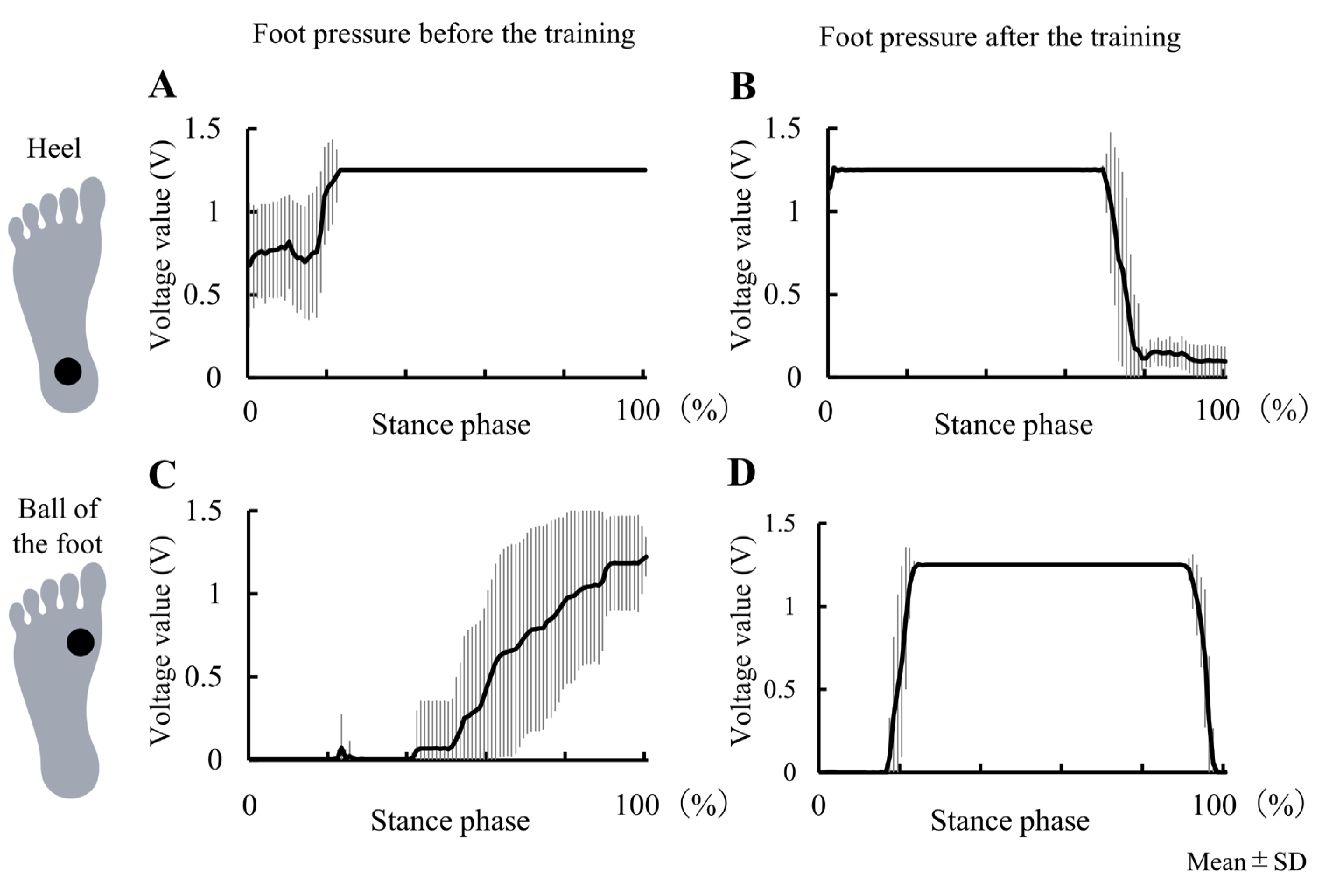
3.2. Experiment 2
4. Discussion
4.1. Safety of Robotized KAFO-Assisted Gait Training
4.2. Case Study of 9 Days Intervention on Genu Recurvatum (Experiment 2)
4.3. Clinical Implication
4.4. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Moseley, A.; Wales, A.; Herbert, R.; Schurr, K.; Moore, S. Observation and analysis of hemiplegic gait: Stance phase. Aust. J. Physiother. 1993, 39, 259–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.E.; Matyas, T.A.; Bach, T.M.; Goldie, P.A. Electrogoniometric feedback: Its effect on genu recurvatum in stroke. Arch. Phys. Med. Rehabil. 1992, 73, 1147–1154. [Google Scholar] [PubMed]
- Knutsson, E.; Richards, C. Different types of disturbed motor control in gait of hemiparetic patients. Brain 1979, 102, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Tani, Y.; Otaka, Y.; Kudo, M.; Kurayama, T.; Kondo, K. Prevalence of genu recurvatum during walking and associated knee pain in chronic hemiplegic stroke patients: A preliminary survey. J. Stroke Cerebrovasc. Dis. 2016, 25, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Bleyenheuft, C.; Bleyenheuft, Y.; Hanson, P.; Deltombe, T. Treatment of genu recurvatum in hemiparetic adult patients: A systematic literature review. Ann. Phys. Rehabil. Med. 2010, 53, 189–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Lo, W.L.; Xu, G.; Li, L.S.; Li, L.; Huang, D. Reduced knee hyperextension after wearing a robotic knee orthosis during gait training—A case study. Bio-Med. Mater. Eng. 2015, 26 (Suppl. 1), S381–S388. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.; Alghamdi, G.A.; Alghamdi, M.A.; Altowaijri, A.; Richardson, S. The relationship of lower limb muscle strength and knee joint hyperextension during the stance phase of gait in hemiparetic stroke patients. Physiother. Res. Int. 2012, 17, 150–156. [Google Scholar] [CrossRef]
- Hipps, H.E. A back-knee brace. J. Bone Jt. Surg. 1954, 36–A, 1080–1081. [Google Scholar] [CrossRef]
- Boudarham, J.; Zory, R.; Genet, F.; Vigné, G.; Bensmail, D.; Roche, N. Effects of a knee-ankle-foot orthosis on gait biomechanical characteristics of paretic and non-paretic limbs in hemiplegic patients with genu recurvatum. Clin. Biomech. 2013, 28, 73–78. [Google Scholar] [CrossRef]
- Portnoy, S.; Frechtel, A.; Raveh, E.; Schwartz, I. Prevention of genu recurvatum in poststroke patients using a hinged soft knee orthosis. PM&R 2015, 7, 1042–1051. [Google Scholar]
- Chantraine, F.; Schreiber, C.; Kolanowski, E.; Moissenet, F. Control of stroke-related genu recurvatum with prolonged timing of dorsiflexor functional electrical stimulation: A case study. J. Neurol. Phys. Ther. 2016, 40, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.Y.; Shin, J.H.; Kim, J.S. Effects of dorsiflexor functional electrical stimulation compared to an ankle/foot orthosis on stroke-related genu recurvatum gait. J. Phys. Ther. Sci. 2019, 31, 865–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, R.; Delporte, L.; Arsenault, L.; Revol, P.; Lefevre, M.; Clevenot, D. Does the rectus femoris nerve block improve knee recurvatum in adult stroke patients? A kinematic and electromyographic study. Gait Posture 2014, 39, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Daral, K.K.; Joshua, A.M.; Nayak, A.; Mithra, P.; Misri, Z.; Unnikrishnan, B. Effectiveness of prowling with proprioceptive training on knee hyperextension among stroke subjects using videographic observation-a randomised controlled trial. Gait Posture 2018, 6, 232–237. [Google Scholar]
- Moucheboeuf, G.; Griffier, R.; Gasq, D.; Glize, B.; Bouyer, L.; Dehail, P.; Cassoudesalle, H. Effects of robotic gait training after stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2020, 639, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Baronchelli, F.; Zucchella, C.; Serrao, M.; Intiso, D.; Bartolo, M. The effect of robotic assisted gait training with Lokomat® on balance control after stroke: Systematic review and meta-analysis. Front. Neurol. 2021, 12, 661815. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, CD006185. [Google Scholar]
- Nedergård, H.; Arumugam, A.; Sandlund, M.; Bråndal, A.; Häger, C.K. Effect of robotic-assisted gait training on objective biomechanical measures of gait in persons post-stroke: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2021, 18, 64. [Google Scholar] [CrossRef]
- Schröder, J.; Truijen, S.; Van Criekinge, T.V.; Saeys, W. Feasibility and effectiveness of repetitive gait training early after stroke: A systematic review and meta-analysis. J. Rehabil. Med. 2019, 51, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Maranesi, E.; Riccardi, G.R.; Di Donna, V.; Di Rosa, M.; Fabbietti, P.; Luzi, R.; Pranno, L.; Lattanzio, F.; Bevilacqua, R. Effectiveness of intervention based on end-effector gait trainer in older patients with stroke: A systematic review. J. Am. Med. Dir. Assoc. 2020, 21, 1036–1044. [Google Scholar] [CrossRef]
- Lewek, M.D.; Cruz, T.H.; Moore, J.L.; Roth, H.R.; Dhaher, Y.Y.; Hornby, T.G. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: A subgroup analysis from a randomized. Phys. Ther. 2009, 89, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Takai, A.; Teramae, T.; Hirookai, E.; Hase, K.; Morimoto, J. Robotizing double–bar ankle-foot orthosis. In Proceedings of the 2018 IEEE International Conference on Robotics and Automation (ICRA), Brisbane, Australia, 21–25 May 2018; pp. 2782–2787. [Google Scholar]
- Fugl-Meyer, A.R.; Jaasko, L.; Leymen, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. I. A. Method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Daly, J.; Nethery, J.; McCabe, J.P.; Brenner, I.; Rogers, J.; Gansen, J.; Butler, K.; Burdsall, K.; Roenigk, R.; Holcomb, J. Development and testing of the Gait Assessment and Intervention Tool (G.A.I.T.): A measure of coordinated gait components. J. Neurosci. Methods 2009, 178, 334–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Gandolfi, M.; Geroin, C.; Tomelleri, C.; Maddalena, I.; Kirilova, D.E.; Picelli, A.; Smania, N.; Waldner, A. Feasibility and safety of early lower limb robot-assisted training in sub-acute stroke patients: A pilot study. Eur. J. Phys. Rehabil. Med. 2017, 53, 870–882. [Google Scholar] [CrossRef]
- McIntosh, K.; Charbonneau, R.; Bensaada, Y.; Bhatiya, U.; Ho, C. The Safety and feasibility of exoskeletal-assisted walking in acute rehabilitation after spinal cord injury. Arch. Phys. Med. Rehabil. 2020, 101, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, A.; Vreede, K.S.; Häglund, V.; Kawamoto, H.; Sankai, Y.; Borg, J. Gait training early after stroke with a new exoskeleton—The hybrid assistive limb: A study of safety and feasibility. J. Neuroeng. Rehabil. 2014, 11, 92. [Google Scholar] [CrossRef] [Green Version]
- Fisahn, C.; Aach, M.; Jansen, O.; Moisi, M.; Mayadev, A.; Pagarigan, K.T.; Dettori, J.R.; Schildhauer, T.A. The effectiveness and safety of exoskeletons as assistive and rehabilitation devices in the treatment of neurologic gait disorders in patients with spinal cord injury: A systematic review. Glob. Spine J. 2016, 6, 822–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, A.D.; Escalon, M.X.; Bryce, T.N.; Weinrauch, W.; Suarez, S.J.; Kozlowski, A.J. Safety and feasibility of exoskeleton-assisted walking during acute/sub-acute SCI in an inpatient rehabilitation facility: A single-group preliminary study. J. Spinal Cord. Med. 2020, 43, 657–666. [Google Scholar] [CrossRef]
- Hogue, R.E.; McCandless, S. Genu recurvatum: Auditory biofeedback treatment for adult patients with stroke or head injuries. Arch. Phys. Med. Rehabil. 1983, 3, 368–370. [Google Scholar]
- Basaglia, N.; Mazzini, N.; Boldrini, P.; Bacciglieri, P.; Contenti, E.; Ferraresi, G. Biofeedback treatment of genu-recurvatum using an electrogoniometric device with an acoustic signal. One-year follow-up. Scand. J. Rehabil. Med. 1989, 21, 125–130. [Google Scholar] [PubMed]
- Kim, H.; Park, G.; Shin, J.H.; You, J.H. Neuroplastic effects of end-effector robotic gait training for hemiparetic stroke: A randomised controlled trial. Sci. Rep. 2020, 10, 12461. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Kadone, H.; Watanabe, H.; Marushima, A.; Hada, Y.; Yamazaki, M.; Sankai, Y.; Matsumura, A.; Suzuki, K. Differences in muscle synergy symmetry between subacute post-stroke patients with bioelectrically-controlled exoskeleton gait training and conventional gait training. Front. Bioeng. Biotechnol. 2020, 8, 770. [Google Scholar] [CrossRef] [PubMed]
- Li, D.X.; Zha, F.B.; Long, J.J.; Liu, F.; Cao, J.; Wang, Y.L. Effect of robot assisted gait training on motor and walking function in patients with subacute stroke: A random controlled study. J. Stroke Cerebrovasc. Dis. 2021, 30, 105807. [Google Scholar] [CrossRef]
- Li, Y.; Fan, T.; Qi, Q.; Wang, J.; Qiu, H.; Zhang, L.; Wu, X.; Ye, J.; Chen, G.; Long, J.; et al. Efficacy of a novel exoskeletal robot for locomotor rehabilitation in stroke patients: A multi-center, non-inferiority, randomized controlled trial. Front. Aging Neurosci. 2021, 13, 706569. [Google Scholar] [CrossRef]
- Maggio, M.G.; Naro, A.; Manuli, A.; Maresca, G.; Balletta, T.; Latella, D.; Luca, R.D.; Calabro, R.S. Effects of robotic neurorehabilitation on body representation in individuals with stroke: A preliminary study focusing on an EEG-based approach. Brain Topogr. 2021, 34, 348–362. [Google Scholar] [CrossRef]
- Oh, W.; Park, C.; Oh, S.; You, S.J.H. Stage 2: Who are the best candidates for robotic gait training rehabilitation in hemiparetic stroke? J. Clin. Med. 2021, 10, 5715. [Google Scholar] [CrossRef]
- Mustafaoglu, R.; Erhan, B.; Yeldan, I.; Gunduz, B.; Tarakci, E. Does robot-assisted gait training improve mobility, activities of daily living and quality of life in stroke? A single-blinded, randomized controlled trial. Acta Neurol. Belg. 2020, 120, 335–344. [Google Scholar] [CrossRef]
- O’Dwyer, N.J.; Ada, L.; Neilson, P.D. Spasticity and muscle contracture following stroke. Brain 1996, 119, 1737–1749. [Google Scholar] [CrossRef]
- Dietz, V.; Sinkjaer, T. Spastic movement disorder: Impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007, 6, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, D.J. Demystifying spasticity: Reply to Dietz. J. Neurophysiol. 2008, 99, 1041–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, M.Y.; Ashe, M.C.; Eng, J.J. Compromised bone strength index in the hemiparetic distal tibia epiphysis among chronic stroke patients: The association with cardiovascular function, muscle atrophy, mobility, and spasticity. Osteoporos. Int. 2010, 21, 997–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, J.; Burnfield, J.M. GAIT ANALYSYS: Normal and Pathological Function, 2nd ed.; Slack Incorporated: West Deptford, NJ, USA, 2010. [Google Scholar]
- Druzbicki, M.; Guzik, A.; Przysada, G.; Kwolek, A.; Brzozowska-Magon, A.; Sobolewski, M. Changes in gait symmetry after training on a treadmill with biofeedback in chronic stroke patients: A 6-month follow-up from a randomized controlled trial. J. Pharmacol. Exp. Ther. 2016, 22, 4859–4868. [Google Scholar] [CrossRef] [PubMed]
| ID | Diagnosis | Age (Years) | Sex | Affected Side | TFO (Month) | FMA-LE | Modified Ashworth Scale | Experiment | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Quad | Hamst | TS | ||||||||
| A | Cerebral hemorrhage | 50 | Female | Right | 40 | 19 | 0 | 0 | 1+ | 1 |
| B | Cerebral infarction | 21 | Male | Right | 47 | 25 | 0 | 0 | 2 | 1 |
| C | Cerebral hemorrhage | 18 | Female | Left | 12 | 23 | 1 | 1 | 1+ | 1 |
| D | Cerebral infarction | 64 | Male | Left | 24 | 20 | 1+ | 0 | 2 | 1 |
| E | Cerebral hemorrhage | 53 | Female | Left | 30 | 29 | 0 | 1+ | 1 | 1 |
| F | Cerebral hemorrhage | 30 | Female | Right | 19 | 20 | 0 | 0 | 2 | 1 |
| G | Cerebral infarction | 68 | Male | Right | 15 | 19 | 0 | 0 | 1 | 1 |
| H | Cerebral hemorrhage | 64 | Male | Right | 88 | 18 | 1+ | 0 | 3 | 1 |
| I | Cerebral infarction | 47 | Female | Left | 44 | 20 | 2 | 1 | 1+ | 2 |
| Spec | Value |
|---|---|
| Weight | |
| -Total | 5.0 kg |
| -Nested chamber pneumatic artificial muscles (NcPAMs) unit | 2.1 kg |
| -Exoskeleton and modular exoskeletal joint | 2.9 kg |
| Encoder | Optical 3 phase 16,000 counts per revolution |
| Max pressure | 0.8 MPa |
| Max peak torque | 48.0 Nm |
| NcPAM diameter | 20 mm each |
| Clinical Assessments | Pre | Post | |
|---|---|---|---|
| Fugl-Meyer Assessment of lower extremity | 20 | 25 | |
| (Full score 34 points) | |||
| (E-I/E-IIa/E-IIb/E-III/E-IV/E-V/F) | (2/4/7/2/1/0/4) | (4/5/7/2/2/0/5) | |
| Modified Ashworth scale | Quadriceps muscle | 2 | 0 |
| Hamstrings muscle | 1 | 0 | |
| Triceps surae muscle | 1+ | 1 | |
| 10-m gait speed test | Normal | ||
| Time (s) | 14.7 | 14.5 | |
| Steps (steps) | 21 | 21 | |
| Cadence (steps/min) | 85.7 | 86.9 | |
| Fast | |||
| Time (s) | 12.9 | 12.7 | |
| Steps (steps) | 21 | 20 | |
| Cadence (steps/min) | 97.7 | 94.5 | |
| 6-min walk test | Barefoot, no cane (m) | 266 | 277 |
| With ankle-foot orthosis, T-cane (m) | 345 | 343 | |
| Gait Assessment and Intervention Tool | 36 | 32 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, Y.; Okada, K.; Noda, T.; Teramae, T.; Nakamura, T.; Haruyama, K.; Okuyama, K.; Tsujimoto, K.; Mizuno, K.; Morimoto, J.; et al. Robotized Knee-Ankle-Foot Orthosis-Assisted Gait Training on Genu Recurvatum during Gait in Patients with Chronic Stroke: A Feasibility Study and Case Report. J. Clin. Med. 2023, 12, 415. https://doi.org/10.3390/jcm12020415
Takahashi Y, Okada K, Noda T, Teramae T, Nakamura T, Haruyama K, Okuyama K, Tsujimoto K, Mizuno K, Morimoto J, et al. Robotized Knee-Ankle-Foot Orthosis-Assisted Gait Training on Genu Recurvatum during Gait in Patients with Chronic Stroke: A Feasibility Study and Case Report. Journal of Clinical Medicine. 2023; 12(2):415. https://doi.org/10.3390/jcm12020415
Chicago/Turabian StyleTakahashi, Yoko, Kohsuke Okada, Tomoyuki Noda, Tatsuya Teramae, Takuya Nakamura, Koshiro Haruyama, Kohei Okuyama, Kengo Tsujimoto, Katsuhiro Mizuno, Jun Morimoto, and et al. 2023. "Robotized Knee-Ankle-Foot Orthosis-Assisted Gait Training on Genu Recurvatum during Gait in Patients with Chronic Stroke: A Feasibility Study and Case Report" Journal of Clinical Medicine 12, no. 2: 415. https://doi.org/10.3390/jcm12020415





