Ventilator Acquired Pneumonia in COVID-19 ICU Patients: A Retrospective Cohort Study during Pandemia in France
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Study Design
2.2. Diagnosis and Definitions
2.3. Collected Data
2.4. Microbiological Investigations
2.5. Statistical Analysis
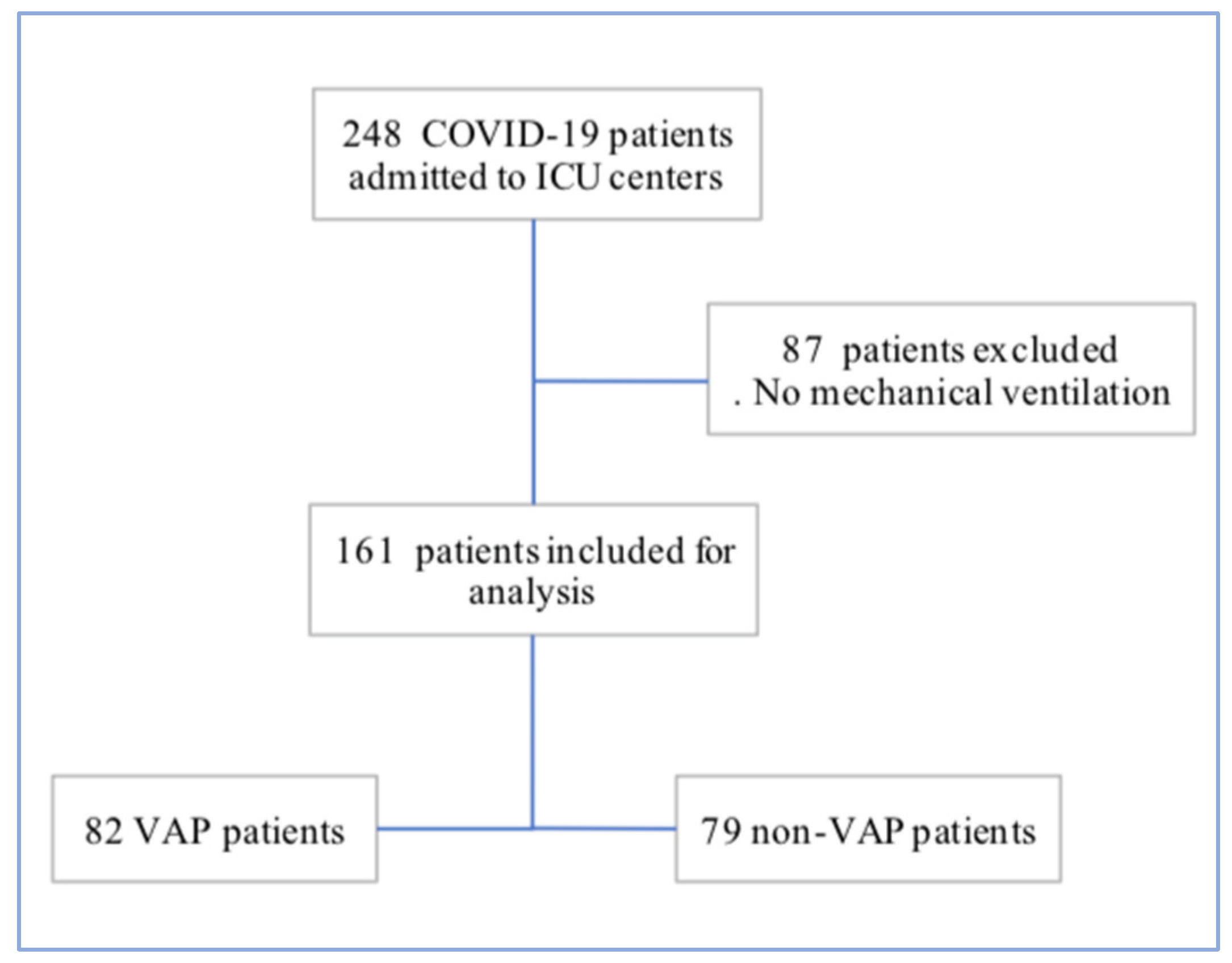
3. Results
3.1. Patient Characteristics
3.2. Infectious Disease Management
3.3. Patient Management, Complications and Outcome during ICU Stay
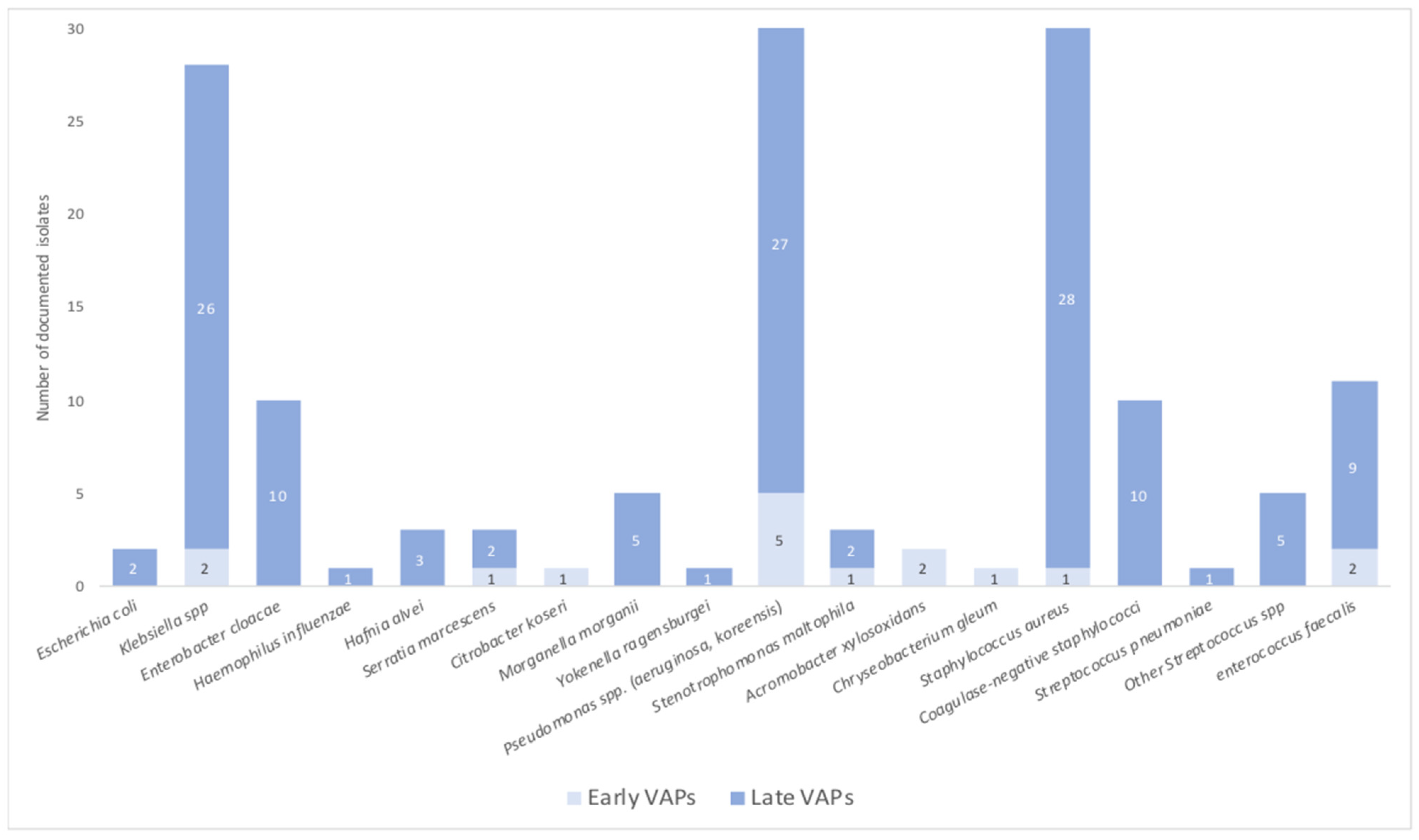
3.4. VAP Characteristics and Bacterial Documentation
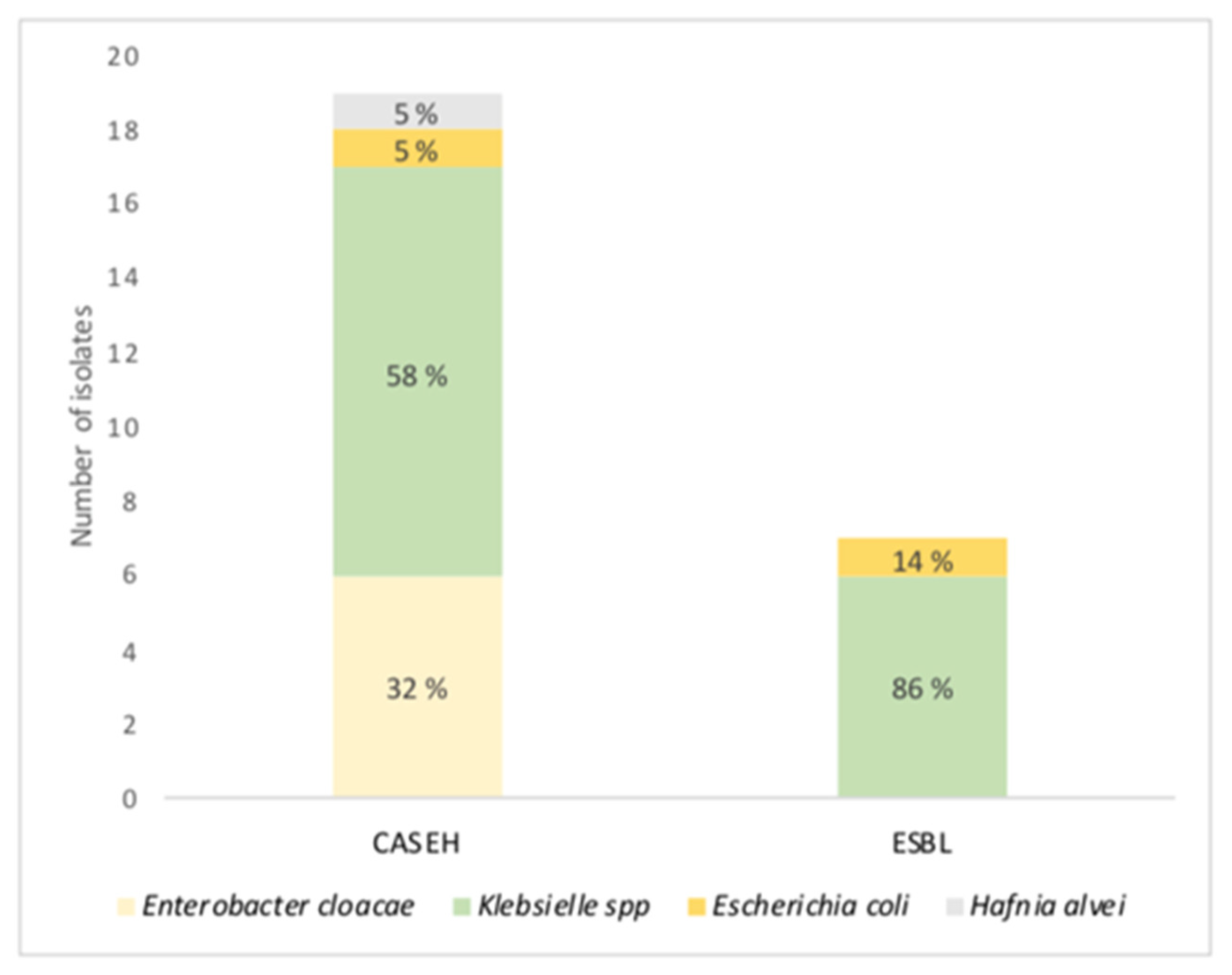
3.5. Antibiotic Resistance Mechanisms
3.6. Multivariate Analysis
4. Discussion
4.1. Ventilation-Acquired Pneumonia Characteristics
4.2. Ventilation-Acquired Pneumonia Risk Factors
4.3. Incidence of Ventilation-Acquired Pneumonia
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19 Map. Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 30 July 2022).
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. SARS-CoV-2 Variants of Concern as of 28 July 2022. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 30 July 2022).
- Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 30 July 2022).
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Lf, A.-M.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 1. [Google Scholar] [CrossRef]
- Le Terrier, C.; Suh, N.; Wozniak, H.; Boroli, F.; Giudicelli-Bailly, A.; Sangla, F.; Legouis, D.; Bendjelid, K.; Quintard, H.; Pugin, J. Delayed intubation is associated with mortality in patients with severe COVID-19: A single-centre observational study in Switzerland. Anaesth. Crit. Care Pain Med. 2022, 41. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581, Erratum in JAMA 2021, 325, 2120. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Rodriguez, A.H.; Torres, A. New guidelines for hospital-acquired pneumonia/ventilator-associated pneumonia: USA vs. Europe. Curr. Opin. Crit. Care 2018, 24, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W. the Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.; Klarin, B.; Koeman, M.; A Krueger, W.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Gamberini, L.; Tonetti, T.; Spadaro, S.; Zani, G.; Mazzoli, C.A.; Capozzi, C.; Giampalma, E.; Reggiani, M.L.B.; Bertellini, E.; Castelli, A.; et al. Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: Multicenter observational study in fifteen Italian ICUs. J. Intensive Care 2020, 8, 80, Erratum in J. Intensive Care 2020, 8, 96. [Google Scholar] [CrossRef]
- Schenck, E.J.; Hoffman, K.; Goyal, P.; Choi, J.; Torres, L.; Rajwani, K.; Tam, C.W.; Ivascu, N.; Martinez, F.J.; Berlin, D.A. Respiratory Mechanics and Gas Exchange in COVID-19–associated Respiratory Failure. Ann. Am. Thorac. Soc. 2020, 17, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care 2021, 25, 25, Erratum in Crit. Care 2021, 25, 130. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Moreno, G.; Rodríguez, A.; Reyes, L.F.; Gomez, J.; Sole-Violan, J.; Díaz, E.; Bodí, M.; Trefler, S.; Guardiola, J.; on behalf of the GETGAG Study Group; et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: A propensity score matching study. Care Med. 2018, 44, 1470–1482. [Google Scholar] [CrossRef]
- Plachouras, D.; Lepape, A.; Suetens, C. ECDC definitions and methods for the surveillance of healthcare-associated infections in intensive care units. Intensive Care Med. 2018, 44, 2216–2218. [Google Scholar] [CrossRef]
- de Jager, C.P.; van Wijk, P.T.; Mathoera, R.B.; de Jongh-Leuvenink, J.; van der Poll, T.; Wever, P.C. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit. Care 2010, 14, R192. [Google Scholar] [CrossRef]
- Predictive Value of Systemic Immune-Inflammation index and Neutrophil-to-Lymphocyte Ratio in Patients with Severe COVID-19. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221111391. [CrossRef]
- van Arkel, A.L.E.; Rijpstra, T.A.; Belderbos, H.N.A.; van Wijngaarden, P.; Verweij, P.E.; Bentvelsen, R.G. COVID-19-associated Pulmonary Aspergillosis. Am. J. Respir. Crit. Care Med. 2020, 202, 132–135. [Google Scholar] [CrossRef]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.C.; Zhang, H.X.; Zhang, Z.; Rinkiko, S.; Cui, Y.M.; Zhu, Y.Z. The Two-Way Switch Role of ACE2 in the Treatment of Novel Coronavirus Pneumonia and Underlying Comorbidities. Molecules 2021, 26, 142. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; Pineles, L.; Belton, B.; Johnson, J.K.; Shardell, M.; Loeb, M.; Newhouse, R.; Dembry, L.; Braun, B.; Perencevich, E.; et al. Universal Glove and Gown Use and Acquisition of Antibiotic-Resistant Bacteria in the ICU. JAMA 2013, 310, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, C.; Zhang, S.; Zhong, Y. Risk Factors of Ventilator-Associated Pneumonia in Critically III Patients. Front. Pharmacol. 2019, 10, 482. [Google Scholar] [CrossRef]
- Forel, J.-M.; Voillet, F.; Pulina, D.; Gacouin, A.; Perrin, G.; Barrau, K.; Jaber, S.; Arnal, J.-M.; Fathallah, M.; Auquier, P.; et al. Ventilator-associated pneumonia and ICU mortality in severe ARDS patients ventilated according to a lung-protective strategy. Crit. Care 2012, 16, R65. [Google Scholar] [CrossRef] [PubMed]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Roca, O.; Caralt, B.; Messika, J.; Samper, M.; Sztrymf, B.; Hernández, G.; García-De-Acilu, M.; Frat, J.-P.; Masclans, J.R.; Ricard, J.-D. An Index Combining Respiratory Rate and Oxygenation to Predict Outcome of Nasal High-Flow Therapy. Am. J. Respir. Crit. Care Med. 2019, 199, 1368–1376. [Google Scholar] [CrossRef]
- Reyes, L.F.; Rodriguez, A.; Bastidas, A.; Parra-Tanoux, D.; Fuentes, Y.V.; García-Gallo, E.; Moreno, G.; Ospina-Tascon, G.; Hernandez, G.; Silva, E.; et al. Dexamethasone as risk-factor for ICU-acquired respiratory tract infections in severe COVID-19. J. Crit. Care 2022, 69. [Google Scholar] [CrossRef] [PubMed]
- Gragueb-Chatti, I.; Lopez, A.; Hamidi, D.; Guervilly, C.; Loundou, A.; Daviet, F.; Cassir, N.; Papazian, L.; Forel, J.-M.; Leone, M.; et al. Impact of dexamethasone on the incidence of ventilator-associated pneumonia and blood stream infections in COVID-19 patients requiring invasive mechanical ventilation: A multicenter retrospective study. Ann. Intensive Care 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Consoli, G.M.L.; Cafiso, V.; Stefani, S.; Geraci, C. Synthesis of a calix[4]arene derivative exposing multiple units of fucose and preliminary investigation as a potential broad-spectrum antibiofilm agent. Carbohydr. Res. 2019, 476, 60–64. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A.H. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Leone, M.; Bouadma, L.; Bouhemad, B.; Brissaud, O.; Dauger, S.; Gibot, S.; Hraiech, S.; Jung, B.; Kipnis, E.; Launey, Y.; et al. Pneumonies associées aux soins de réanimation. Anesthésie Réanimation 2018, 4, 421–441. [Google Scholar] [CrossRef]
- Jimeno, S.; Ventura, P.S.; Castellano, J.M.; García-Adasme, S.I.; Miranda, M.; Touza, P.; Lllana, I.; López-Escobar, A. Prognostic implications of neutrophil-lymphocyte ratio in COVID-19. Eur. J. Clin. Investig. 2020, 51. [Google Scholar] [CrossRef]
- Pickens, C.O.; Gao, C.A.; Cuttica, M.J.; Smith, S.B.; Pesce, L.L.; Grant, R.A.; Kang, M.; Morales-Nebreda, L.; Bavishi, A.A.; Arnold, J.M.; et al. Bacterial Superinfection Pneumonia in Patients Mechanically Ventilated for COVID-19 Pneumonia. Am. J. Respir. Crit. Care Med. 2021, 204, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Razazi, K.; Arrestier, R.; Haudebourg, A.F.; Benelli, B.; Carteaux, G.; Decousser, J.-W.; Fourati, S.; Woerther, P.L.; Schlemmer, F.; Charles-Nelson, A.; et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit. Care 2020, 24, 699, Erratum in Crit. Care 2021, 25, 118. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef] [PubMed]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Geronimi, C.B.; et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef]

| Variable | All Patients | VAP Patients | Non VAP Patients | p-Value | OR (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| N = 161 | N = 82 | N = 79 | ||||||
| (51%) | (49%) | |||||||
| Age—YEAR, median | 63 | (57–72) | 65 | (58–73) | 62 | (54–69) | 0.02 | 1.03 (1.00–1.06) |
| Sex—Male | 124 | (77) | 68 | (82.9) | 56 | (70.9) | 0.069 | |
| BMI kg/m2, median | 28 | (25–32) | 27.7 | (25.3–32.1) | 28 | (25.8–33.8) | 0.51 | 0.98 (0.93–1.03) |
| 18–24.9 | 31 | (19) | 16 | (23.2) | 15 | (21.4) | 0.8 | |
| 25–29.9 | 52 | (32) | 27 | (39.1) | 25 | (35.7) | 0.67 | |
| 30–40 | 49 | (30) | 23 | (33.3) | 26 | (37.1) | 0.63 | |
| >40 | 7 | (4) | 3 | (3.9) | 4 | (5.3) | 0.71 | |
| Comorbidities | 147 | (91.3) | 75 | (91.5) | 72 | (91.1) | ||
| Hypertension | 88 | (54) | 45 | (54.9) | 43 | (54.4) | 0.95 | 1.01 (0.54–1.89) |
| Diabetes | 58 | (36) | 25 | (30.5) | 33 | (41.8) | 0.13 | 0.61 (0.32–1.17) |
| Cardiovascular disease | 31 | (19) | 19 | (23.2) | 12 | (15.2) | 0.2 | 1.68 (0.75–3.74) |
| Coronary disease | 20 | (12.4) | 11 | (13.4) | 9 | (11.4) | 0.69 | |
| Chronic heart failure | 3 | (19) | 1 | (1.2) | 2 | (2.5) | 0.61 | |
| Occlusive arteriopathy | 4 | (2.5) | 3 | (3.7) | 1 | (1.3) | 0.62 | |
| Metabolic syndrome | 36 | (22.4) | 16 | (19.5) | 20 | (25.3) | 0.37 | |
| Current smoking | 6 | (3.7) | 5 | (6.1) | 1 | (1.3) | 0.14 | 5.06 (0.57–44.3) |
| Chronic lung disease | 39 | (24) | 20 | (24.4) | 19 | (24.1) | 0.96 | 1.01 (0.49–2.09) |
| Obstructive sleep apnea | 21 | (13) | 11 | (13.4) | 10 | (12.7) | 0.88 | |
| Chronic kidney disease | 12 | (7.5) | 4 | (4.9) | 8 | (10.1) | 0.21 | 0.45 (0.13–1.57) |
| Immunocompromised * | 20 | (12.4) | 11 | (13.4) | 9 | (11.4) | 0.69 | 1.2 (0.47–3.08) |
| Chronic treatment | 103 | (64) | 52 | (63.4) | 51 | (64.6) | 0.88 | 0.95 (0.5–1.81) |
| Status at ICU admission | ||||||||
| Delay between hospitalisation and ICU, d | 0 | (1–3) | 0 | (0–1.25) | 1 | (0–4) | 0.12 | |
| IGS 2 | 35 | (28–43) | 34 | (30–43) | 35 | (27–43) | 0.86 | |
| CHARLSON | 3 | (1–4) | 3 | (2–4) | 2 | (1–4) | 0.1 | |
| SOFA | 4 | (3–7) | 4 | (3–7) | 4 | (2–7) | 0.43 | |
| ROX | 4.6 | (3–9.3) | 3.7 | (3–7) | 5.8 | (3–10.1) | 0.012 | |
| PaO2/FiO2 ratio (mmHg) | 131 | (96–180) | 132.5 | (91.5–179) | 130 | (100–181.5) | 0.42 | 0.99 (0.99–1) |
| Maximum Temperature (°C) | 38.3 | (37.5–39) | 38.3 | (37.6–38.7) | 38.4 | (37.3–39) | 0.7 | 0.93 (0.67–1.31) |
| Neutrophil to lymphocyte ratio | 7.3 | (5.1–13) | 8.3 | (5.5–15.6) | 6 | (4.6–9.2) | 0.016 | |
| Systemic immune-inflammation index (SII) | 1610 | (971–3270) | 2113 | (1116–3900) | 1281 | (834–2054) | 0.016 | 1.11 (1.01–1.2) |
| Ferritin (µg/L) | 1427 | (1013–2345) | 1241 | (796–1611) | 2273 | (976–4141) | 0.19 | |
| Fibrinogen (g/L) | 6.6 | (5.9–7.7) | 6.7 | (5.9–8) | 6.6 | (5.7–7.3) | 0.59 | |
| D-dimer (µg/L) | 1.5 | (1–4) | 1.5 | (0.8–4) | 1.6 | (1.1–2.9) | 0.9 | |
| Procalcitonin (ng/mL) | 0.3 | (0.19–0.58) | 0.3 | (0.2–0.5) | 0.3 | (0.2–1.3) | 0.49 | |
| C-reactive protein (mg/dL) | 170 | (91.6–227) | 185.9 | (120–251) | 98.7 | (83.6–184) | 0.03 | 0.89 (0.64–1.23) |
| Infection within 48 h of ICU admission | 26 | (16.1) | 13 | (15.9) | 13 | (16.5) | 0.91 | |
| CT scan lung lesion (%) | 31.2 | 37.3 | 27.8 | 0.038 | ||||
| Variable | All Patients | VAP Patients | Non VAP Patients | p-Value | OR (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| N = 161 | N = 82 | N = 79 | ||||||
| (51%) | (49%) | |||||||
| Early ICU management | ||||||||
| Antibiotic treatment within 48 h of ICU admission | 137 | (85.6) | 68 | (82.9) | 69 | (88.5) | 0.32 | |
| Corticosteroids | ||||||||
| Dexamethasone 6 mg | 51 | (31.7) | 31 | (37.8) | 20 | (25.3) | 0.09 | 1.8 (0.9–3.5) |
| Median duration of dexamethasone 6 mg | 10 | (7–10) | 10 | (7–10) | 10 | (6–10) | 0.22 | |
| Immunomodulators | 44 | (27.3) | 27 | (32.9) | 17 | (21.5) | 0.1 | |
| Anakinra (anti IL-1) | 19 | (11.8) | 10 | (12.2) | 9 | (11.4) | 0.87 | |
| Tocilizumab (anti IL-6) | 4 | (2.5) | 1 | (1.2) | 3 | (3.8) | 0.31 | |
| Ruxolitinib (Jakavi) | 19 | (11.8) | 12 | (14.6) | 7 | (8.9) | 0.26 | |
| Lopinavir/Ritonavir | 18 | (11.2) | 13 | (15.9) | 5 | (6.3) | 0.063 | |
| Hydroxychloroquine | 107 | (66.5) | 50 | (61) | 57 | (72) | 0.13 | |
| Pre ICU management | ||||||||
| Antibiotic treatment | 90 | (56.3) | 39 | (47.6) | 51 | (65.4) | 0.024 | 0.48 (0.25–0.91) |
| 3rd generation cephalosporin | 60 | (37.2) | 25 | (30) | 35 | (44) | 0.069 | |
| Azithromycin | 60 | (37.2) | 24 | (29) | 36 | (45.5) | 0.035 | |
| Penicillin A +/− Clavulanic acid | 20 | (12.4) | 9 | (10.9) | 11 | (13.9) | 0.57 | |
| Piperacillin—Tazobactam | 11 | (6.8) | 6 | (7.3) | 5 | (6.3) | 0.8 | |
| Duration of pre ICU antibiotic treatment, d (IQR) | 4 | (2–5) | 3 | (2–5) | 4 | (2–5.5) | 0.16 | |
| Hydroxychloroquine | 52 | (32) | 23 | (28) | 29 | (36.7) | 0.24 | |
| Dexamethasone 6 mg | 8 | (5) | 3 | (3) | 5 | (6.3) | 0.48 | |
| Variable | All Patients | VAP Patients | Non VAP Patients | p-Value | OR (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| N = 161 | N = 82 | N = 79 | ||||||
| (51%) | (49%) | |||||||
| Respiratory management | ||||||||
| Treated with High-flow nasal cannula (HFNC) | 105 | (65.2) | 52 | (63.4) | 53 | (67.1) | 0.62 | 0.85 (0.44–1.6) |
| Treated with Non-invasive ventilation (NIV) | 43 | (26.7) | 23 | (28) | 20 | (25.3) | 0.69 | 1.15 (0.57–2.3) |
| Neuromuscular blockers (NMB) | 152 | (94.4) | 79 | (96.3) | 73 | (92.4) | 0.32 | |
| Prone position | 132 | (82) | 72 | (87.8) | 60 | (75.9) | 0.05 | |
| Nitric oxyde (NO) | 52 | (32) | 35 | (42.6) | 17 | (21.5) | 0.004 | |
| Extracorporeal membrane oxygenation (ECMO) | 24 | (14.9) | 13 | (15.9) | 11 | (13.9) | 0.73 | |
| Solumedrol 2 mg/kg | 21 | (13) | 17 | (20.7) | 4 | (5.1) | 0.006 | 4.9 (1.5–15.3) |
| ICU complications and outcome | ||||||||
| Norephinephrin > 1 mg/h | 82 | (50.9) | 52 | (63.4) | 30 | (37.9) | 0.001 | |
| Septic shock | 50 | (31) | 30 | (36.6) | 20 | (25.3) | 0.12 | |
| Blood stream infections | 40 | (25.5) | 32 | (80) | 8 | (20) | < 0.001 | |
| Acute renal failure | 76 | (47.5) | 43 | (52.4) | 33 | (42.3) | 0.2 | |
| Renal replacement therapy | 24 | (14.9) | 13 | (15.9) | 11 | (13.9) | 0.73 | |
| Pneumothorax | 11 | (6.9) | 10 | (12.2) | 1 | (1.3) | 0.006 | |
| Rythm disorders | 39 | (24.2) | 30 | (36.6) | 9 | (11.4) | < 0.001 | |
| Conduction disorders | 14 | (8.7) | 10 | (12.2) | 4 | (5.1) | 0.108 | |
| HSV-1 reactivation | 19 | (11.8) | 13 | (15.9) | 6 | (7.6) | 0.104 | |
| CMV reactivation | 18 | (11.2) | 13 | (15.9) | 5 | (6.3) | 0.055 | |
| SARS-CoV 2 viral loads (Ct/mL) | 28.9 | (24.9–31.7) | 29.7 | (24.8–32) | 28 | (24.9–31) | 0.38 | 1.03 (0.96–1.10) |
| Duration of PCR positivity, d | 12 | (6.5–17) | 13 | (8–17) | 11 | (5.7–17) | 0.2 | 1.02 (0.98–1.07) |
| Delay between ICU and IMV | 0 | (0–1.5) | 0 | (0–2) | 1 | (0–1) | 0.2 | |
| IMV duration, d | 18 | (8.2–33) | 31.5 | (15–47.2) | 10 | (5–18.2) | < 0.001 | |
| Length of stay—ICU, d | 24.5 | (15–43) | 39.5 | (21–57) | 17 | (8–25) | < 0.001 | |
| Length of stay—Hospital, d | 35 | (21–50) | 43 | (30–62.5) | 24 | (14–37.5) | < 0.001 | |
| Death during ICU stay | 41 | (25.5) | 21 | (25.6) | 20 | (25.3) | 0.96 | |
| Variable | All VAPs | Early VAPs | Late VAPs | p-Value | |||
|---|---|---|---|---|---|---|---|
| N = 82 | N = 10 | N = 72 | |||||
| Average delay of VAPs, d | 9 | (6–14) | 3 | (3–3) | 10 | (7–17) | < 0.001 |
| Polymicrobial VAPs (> 2 isolates) | 15 | (18) | 2 | (20) | 13 | (18) | 1 |
| Multi-drug resistant (MDR) VAPs | 26 | (31.7) | 2 | (20) | 24 | (33) | 0.49 |
| VAP associated blood stream infections (BSI) | 20 | (24) | - | 20 | (27.7) | ||
| VAP associated MDR BSIs | 8 | (33) | 8 | (33) | |||
| Documented microbiological isolates | N = 148 | N = 16 | N = 132 | ||||
| Gram negative bacteria (%) | (62) | (81) | (59) | ||||
| Fermenters | |||||||
| Escherichia coli | 2 | 2 | (2.7) | 1 | |||
| Klebsiella spp. | 28 | (18.7) | 2 | (12) | 26 | (19) | 0.87 |
| Enterobacter cloacae | 10 | (6.7) | 10 | (7) | 0.45 | ||
| Haemophilus influenzae | 1 | 1 | (0.7) | 1 | |||
| Hafnia alvei | 3 | 3 | (2.2) | 1 | |||
| Serratia marcescens | 3 | 1 | (6) | 2 | (2.7) | 0.8 | |
| Citrobacter koseri | 1 | 1 | (6) | 0.24 | |||
| Morganella morganii | 5 | 5 | (3.7) | 0.87 | |||
| Yokenella ragensburgei | 1 | 1 | (0.7) | 1 | |||
| Non fermenters | |||||||
| Pseudomonas spp. (aeruginosa, koreensis) | 32 | (21.4) | 5 | (31) | 27 | (20) | 0.67 |
| Stenotrophomonas maltophila | 3 | 1 | (10) | 2 | (2.7) | 0.8 | |
| Acromobacter xylosoxidans | 2 | 2 | (12) | 0.005 | |||
| Chryseobacterium gleum | 1 | 1 | (6) | 0.24 | |||
| Gram positive bacteria (%) | (38) | (19) | (41) | ||||
| Staphylococcus aureus | 29 | (20) | 1 | (6) | 29 | (22) | 0.08 |
| Methicillin-sensitive Staphylococcus aureus | 24 | 1 | 23 | 0.68 | |||
| Methicillin-resistant Staphylococcus aureus | 5 | 5 | 0.76 | ||||
| Coagulase-negative staphylococci | 10 | (6.7) | 10 | (7) | 0.45 | ||
| Streptococcus pneumoniae | 1 | 1 | (0.7) | 1 | |||
| Other Streptococcus spp. | 5 | 5 | (3.7) | 1 | |||
| Enterococcus faecalis | 11 | (7.4) | 2 | (12) | 9 | (6.7) | 0.87 |
| Variable | VAP Patients | Non VAP Patients | OR (95% CI) | Multivariate | ||
|---|---|---|---|---|---|---|
| N = 82 | N = 79 | Adjusted | ||||
| (51%) | (49%) | p-Value | ||||
| Age—YEAR, median | 65 | (58–73) | 62 | (54–69) | 1.08 (1.01–1.15) | 0.014 |
| Sex—Male | 34 | (30–43) | 35 | (27–43) | 3.99 (1.26–12.6) | 0.018 |
| IGS 2 | 34 | (30–43) | 35 | (27–43) | 0.94 (0.89–0.99) | 0.037 |
| CHARLSON | 3 | (2–4) | 2 | (1–4) | 1.19 (0.79–1.78) | 0.39 |
| ROX | 3.7 | (3–7) | 5.8 | (3–10.1) | 0.85 (0.75–0.97) | 0.015 |
| Dexamethasone 6 mg | 31 | (37.8) | 20 | (25.3) | 3.2 (1.01–10) | 0.046 |
| Immunomodulatory therapy | 27 | (32.9) | 17 | (21.5) | 2.73 (0.84–8.8) | 0.092 |
| Antibiotic course prior to ICU | 39 | (47.6) | 51 | (65.4) | 0.23 (0.07–0.7) | 0.009 |
| Neutrophil Lymphocyte ratio | 8.3 | (5.5–15.6) | 6 | (4.6–9.2) | 0.99 (0.95–1.03) | 0.82 |
| Duration of PCR positivity, d | 13 | (8–17) | 11 | (5.7–17) | 1.02 (0.96–1.08) | 0.41 |
| Delay between hospitalization and ICU, d | 0 | (0–1.25) | 1 | (0–4) | 0.96 (0.79–1.16) | 0.7 |
| Delay between ICU and IMV, d | 0 | (0–2) | 1 | (0–1) | 1.18 (0.92–1.51) | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, J.; Carvelli, J.; Lesaux, A.; Boucekine, M.; Tonon, D.; Bichon, A.; Gainnier, M.; Bourenne, J. Ventilator Acquired Pneumonia in COVID-19 ICU Patients: A Retrospective Cohort Study during Pandemia in France. J. Clin. Med. 2023, 12, 421. https://doi.org/10.3390/jcm12020421
Moreno J, Carvelli J, Lesaux A, Boucekine M, Tonon D, Bichon A, Gainnier M, Bourenne J. Ventilator Acquired Pneumonia in COVID-19 ICU Patients: A Retrospective Cohort Study during Pandemia in France. Journal of Clinical Medicine. 2023; 12(2):421. https://doi.org/10.3390/jcm12020421
Chicago/Turabian StyleMoreno, Jacques, Julien Carvelli, Audrey Lesaux, Mohamed Boucekine, David Tonon, Amandine Bichon, Marc Gainnier, and Jeremy Bourenne. 2023. "Ventilator Acquired Pneumonia in COVID-19 ICU Patients: A Retrospective Cohort Study during Pandemia in France" Journal of Clinical Medicine 12, no. 2: 421. https://doi.org/10.3390/jcm12020421
APA StyleMoreno, J., Carvelli, J., Lesaux, A., Boucekine, M., Tonon, D., Bichon, A., Gainnier, M., & Bourenne, J. (2023). Ventilator Acquired Pneumonia in COVID-19 ICU Patients: A Retrospective Cohort Study during Pandemia in France. Journal of Clinical Medicine, 12(2), 421. https://doi.org/10.3390/jcm12020421






