Superior Mesenteric Artery Injury during Robotic Radical Nephrectomy: Scenarios and Management Strategies
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Case #1: Timely Recognition of SMA Misidentification
3.2. Case #2: SMA Clipping
3.3. Case #3: SMA Clipping and Complete Transection
4. Systematic Workup Algorithm Management of SMA Injury
4.1. SMA Clipping
4.2. SMA Transection
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, S.; Uzzo, R.G.; Allaf, M.E.; Bass, E.B.; Cadeddu, J.A.; Chang, A.; Clark, P.E.; Davis, B.J.; Derweesh, I.H.; Giambarresi, L. Renal mass and localized renal cancer: AUA guideline. J. Urol. 2017, 198, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Gill, K.; Gill, I.S. Robotic versus open urological oncological surgery: Study protocol of a systematic review and meta-analysis. BMJ Open 2020, 10, e036609. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, U.; Crocerossa, F.; Campi, R.; Veccia, A.; Cacciamani, G.E.; Amparore, D.; Checcucci, E.; Loizzo, D.; Pecoraro, A.; Marchioni, M. Retroperitoneal Robot-assisted Partial Nephrectomy: A Systematic Review and Pooled Analysis of Comparative Outcomes. Eur. Urol. Open Sci. 2022, 40, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.; Challacombe, B.; Khan, M.; Dasgupta, P. Robotic technology in urology. Postgrad. Med. J. 2006, 82, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Brassetti, A.; Cacciamani, G.E.; Mari, A.; Garisto, J.D.; Bertolo, R.; Sundaram, C.P.; Derweesh, I.; Bindayi, A.; Dasgupta, P.; Porter, J.; et al. On-Clamp vs. Off-Clamp Robot-Assisted Partial Nephrectomy for cT2 Renal Tumors: Retrospective Propensity-Score-Matched Multicenter Outcome Analysis. Cancers 2022, 14, 4431. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Sebben, M.; Tafuri, A.; Nassiri, N.; Cocci, A.; Russo, G.I.; Hung, A.; de Castro Abreu, A.L.; Gill, I.S.; Artibani, W. Consulting ‘Dr. Google’ for minimally invasive urological oncological surgeries: A contemporary web-based trend analysis. Int. J. Med. Robot 2021, 17, e2250. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Medina, L.G.; Gill, T.S.; Mendelsohn, A.; Husain, F.; Bhardwaj, L.; Artibani, W.; Sotelo, R.; Gill, I.S. Impact of renal hilar control on outcomes of robotic partial nephrectomy: Systematic review and cumulative meta-analysis. Eur. Urol. Focus 2019, 5, 619–635. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Shakir, A.; Tafuri, A.; Gill, K.; Han, J.; Ahmadi, N.; Hueber, P.A.; Gallucci, M.; Simone, G.; Campi, R.; et al. Best practices in near-infrared fluorescence imaging with indocyanine green (NIRF/ICG)-guided robotic urologic surgery: A systematic review-based expert consensus. World J. Urol. 2020, 38, 883–896. [Google Scholar] [CrossRef]
- Puliatti, S.; Eissa, A.; Checcucci, E.; Piazza, P.; Amato, M.; Ferretti, S.; Scarcella, S.; Rivas, J.G.; Taratkin, M.; Marenco, J.; et al. New imaging technologies for robotic kidney cancer surgery. Asian J. Urol. 2022, 9, 253–262. [Google Scholar] [CrossRef]
- Mayor, N.; Sapre, N.; Sandford, B.; Challacombe, B. Superior Mesenteric Artery Injury During Robot-assisted Laparoscopic Nephrectomy: A Robotic Nightmare. Eur. Urol. Open Sci. 2022, 38, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Veccia, A.; Dell’oglio, P.; Antonelli, A.; Minervini, A.; Simone, G.; Challacombe, B.; Perdonà, S.; Porter, J.; Zhang, C.; Capitanio, U.; et al. Robotic partial nephrectomy versus radical nephrectomy in elderly patients with large renal masses. Minerva Urol. Nefrol. 2020, 72, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.L.; Medina, L.G.; Chopra, S.; Gill, K.; Cacciamani, G.E.; Azhar, R.A.; Ashrafi, A.; Winter, M.; Fay, C.; Weaver, F.; et al. Robotic Renal Artery Aneurysm Repair. Eur. Urol. 2020, 78, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Gill, T.; Medina, L.; Ashrafi, A.; Winter, M.; Sotelo, R.; Artibani, W.; Gill, I.S. Impact of host factors on robotic partial nephrectomy outcomes: Comprehensive systematic review and meta-analysis. J. Urol. 2018, 200, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Medina, L.G.; Gill, T.; Abreu, A.; Sotelo, R.; Artibani, W.; Gill, I.S. Impact of surgical factors on robotic partial nephrectomy outcomes: Comprehensive systematic review and meta-analysis. J. Urol. 2018, 200, 258–274. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Chang, P.; Yang, J.; Zheng, D.; Zhang, D.; Wen, S.; Jing, S. A Novel Approach for Repairing Superior Mesenteric Artery Injury During Left Nephrectomy—6-year Follow-up. Urology 2020, 144, 241–244. [Google Scholar] [CrossRef]
- Blunt Jr, L.W.; Matsumura, J.; Carter, M.F.; Gonzalez, C.M.; Smith, N.D. Repair of superior mesenteric artery ligation during left nephrectomy with a native renal vein patch. Urology 2004, 64, 377–378. [Google Scholar] [CrossRef]
- Paul, J.S.; Webb, T.P.; Aprahamian, C.; Weigelt, J.A. Intraabdominal vascular injury: Are we getting any better? J. Trauma Acute Care Surg. 2010, 69, 1393–1397. [Google Scholar] [CrossRef]
- Ramani, A.P.; Ryndin, I.; Veetil, R.T.; Han, H.; Hendlin, K.; Monga, M. Novel technique for removal of misdirected laparoscopic weck clips. Urology 2007, 70, 168–169. [Google Scholar] [CrossRef]
- Aichroth, J.; Fox, T. Retroaortic Left Renal Vein. J. Diagn. Med. Sonogr. 2013, 29, 11–14. [Google Scholar] [CrossRef]
- Kumar, S.; Navariya, S.C.; Bhirud, D.P.; Ranjan, S.K.; Mittal, A.; Mammen, K.J. Revascularisation of iatrogenic superior mesenteric artery injury by end to end anastomosis during robot assisted nephrectomy. Int. J. Surg. Case Rep. 2019, 63, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Bourland, W.A.; Kispert, J.F.; Hyde, G.L.; Kazmers, A. Trauma to the proximal superior mesenteric artery: A case report and review of the literature. J. Vasc. Surg. 1992, 15, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.; Pereira, J.; Eufrásio, P.; Constantino, J.; Rebelo, P. Splenomesenteric bypass as revascularisation technique after iatrogenic injury of the superior mesenteric artery during radical nephrectomy: A case report. Int. J. Surg. Case Rep. 2019, 60, 34–37. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, A.K.; Acharya, N.; Thingnam, S.K.; Bhalla, V.; Singh, S.K. Superior mesenteric artery injury during en bloc excision of a massive left adrenal tumor. Urol. Int. 2007, 78, 182–184. [Google Scholar] [CrossRef]
- Shaikh, H.; Wehrle, C.J.; Khorasani-Zadeh, A. Anatomy, Abdomen and Pelvis, Superior Mesenteric Artery; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Mann, M.R.; Kawzowicz, M.; Komosa, A.J.; Sherer, Y.M.; Łazarz, D.P.; Loukas, M.; Tubbs, R.S.; Pasternak, A. The marginal artery of Drummond revisited: A systematic review. Transl. Res. Anat. 2021, 24, 100118. [Google Scholar] [CrossRef]
- Hostiuc, S.; Rusu, M.C.; Negoi, I.; Dorobanțu, B.; Grigoriu, M. Anatomical variants of renal veins: A meta-analysis of prevalence. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nevoux, P.; Zini, L.; Villers, A.; Boleslawski, E.; Nunes, B.; Zerbib, P. Celiac axis and superior mesenteric artery: Danger zone for left nephrectomy. J. Endourol. 2008, 22, 2571–2574. [Google Scholar] [CrossRef]
- Asensio, J.A.; Britt, L.; Borzotta, A.; Peitzman, A.; Miller, F.B.; Mackersie, R.C.; Pasquale, M.D.; Pachter, H.L.; Hoyt, D.B.; Rodriguez, J.L. Multiinstitutional experience with the management of superior mesenteric artery injuries. J. Am. Coll. Surg. 2001, 193, 354–365. [Google Scholar] [CrossRef]
- Fullen, W.D.; Hunt, J.; Altemeier, W.A. The clinical spectrum of penetrating injury to the superior mesenteric arterial circulation. J. Trauma Acute Care Surg. 1972, 12, 656–664. [Google Scholar] [CrossRef]
- Melmer, P.D.; Clatterbuck, B.; Parker, V.; Castater, C.A.; Klingensmith, N.J.; Ramos, C.R.; Busby, S.; Hurst, S.D.; Koganti, D.; Williams, K.N. Superior Mesenteric Artery and Vein Injuries: Operative Strategies and Outcomes. Vasc. Endovasc. Surg. 2022, 56, 40–48. [Google Scholar] [CrossRef]
- Sayegh, A.S.; Eppler, M.; Ballon, J.; Hemal, S.; Goldenberg, M.; Sotelo, R.; Cacciamani, G.E. Strategies for Improving the Standardization of Perioperative Adverse Events in Surgery and Anesthesiology:“The Long Road from Assessment to Collection, Grading and Reporting”. J. Clin. Med. 2022, 11, 5115. [Google Scholar] [CrossRef] [PubMed]
- Eppler, M.; Sayegh, A.S.; Goldenberg, M.; Sholklapper, T.; Hemal, S.; Cacciamani, G.E. If You Know Them, You Avoid Them: The Imperative Need to Improve the Narrative Regarding Perioperative Adverse Events. J. Clin. Med. 2022, 11, 4978. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Sholklapper, T.; Dell-Kuster, S.; Biyani, S.C.; Francis, N.; Kaafarani, H.M.; Desai, M.; Gill, I.; Collaboration, I.G.S. Standardizing The Intraoperative Adverse Events Assessment to Create a Positive Culture of Reporting Errors in Surgery and Anesthesiology. Ann. Surg. 2022, 276, e75–e76. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.; Sholklapper, T.; Dell-Kuster, S.; Biyani, C.; Francis, N.; Kaafarani, H.; Desai, M.; Sotelo, R.; Gill, I. Assessing, grading, and reporting intraoperative adverse events during and after surgery. Br. J. Surg. 2021, 109, 301–302. [Google Scholar]
- Cacciamani, G.E. Intraoperative adverse events grading tools and their role in honest and accurate reporting of surgical outcomes. Surgery 2022, 172, 1035–1036. [Google Scholar] [CrossRef] [PubMed]
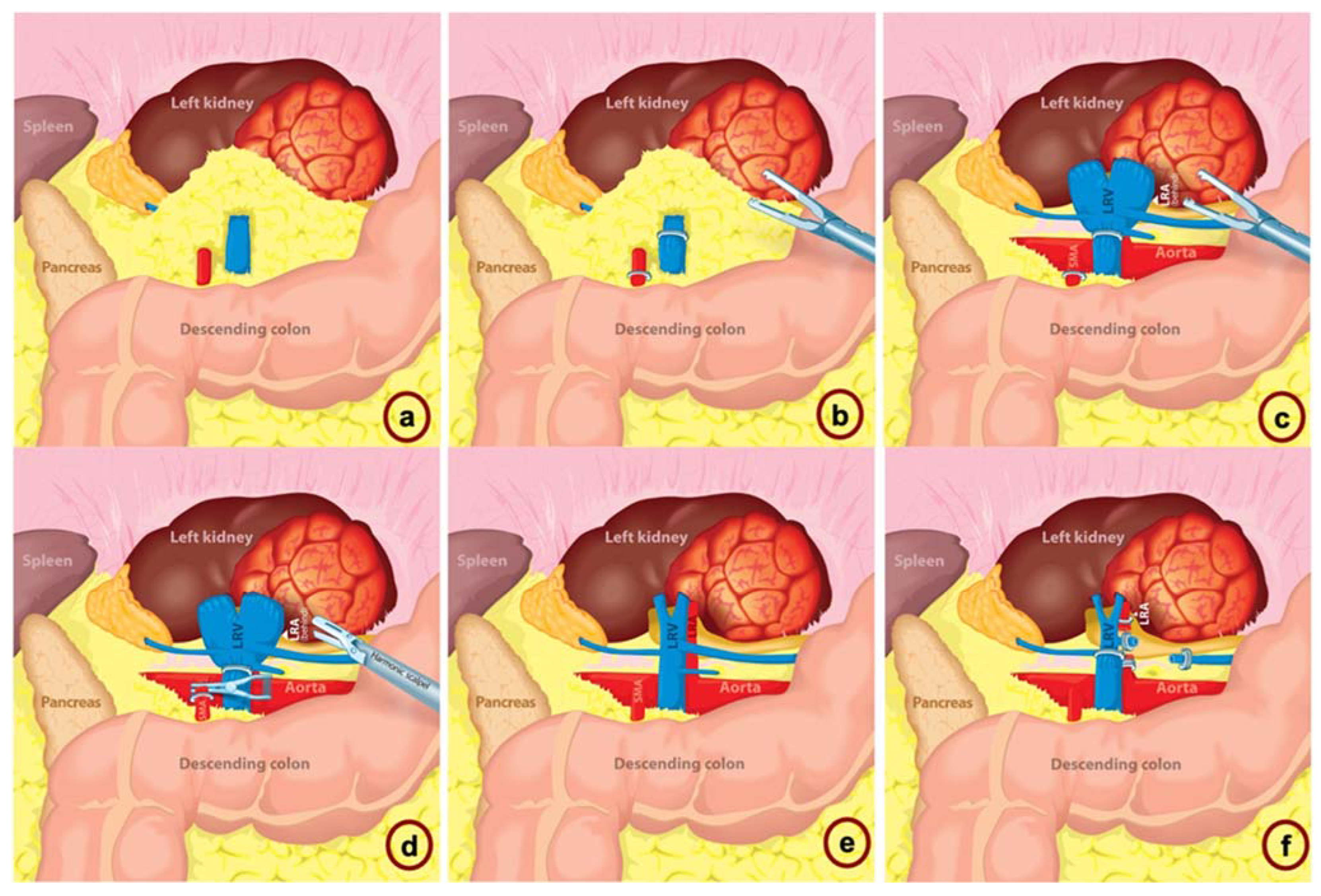



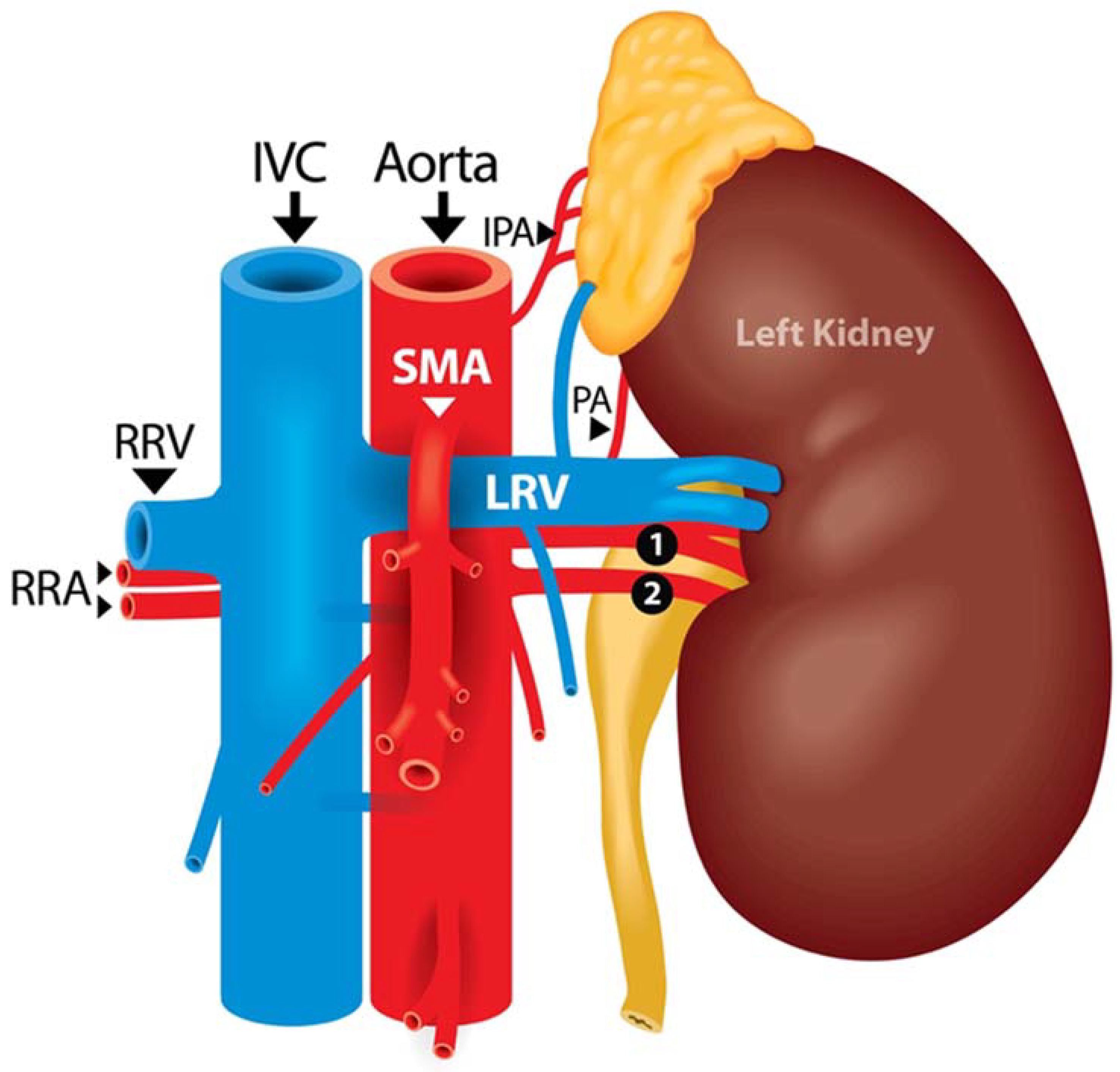

| When to Suspect SMA? |
|---|
| An artery is identified anterior to the renal vein |
| An artery has an atypical orientation (i.e., transverse) |
| An artery is medial to the abdominal aorta |
| More than one large artery encountered, not previously seen on CT |
| The abdominal aorta has not been fully identified |
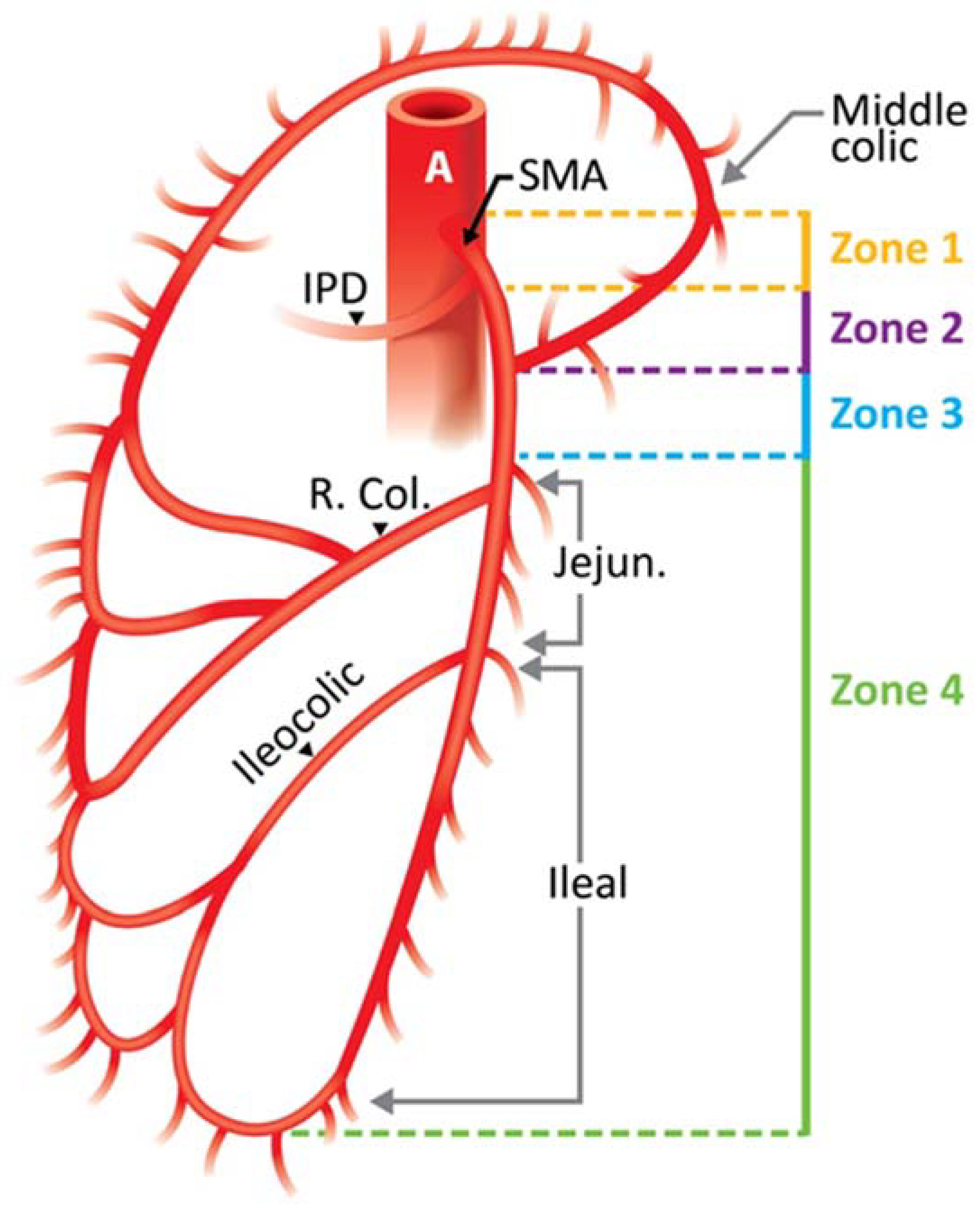
| Zone | Segment of the SMA Involved | Grade | Ischemic Category | Bowel Segment Affected |
|---|---|---|---|---|
| I | Trunk proximal to first major branch | I | Maximal | Jejunum, ileum, right colon |
| II | Trunk between pancreaticoduodenal and middle colic | II | Moderate | Major segment, small bowel and/or right colon |
| III | Trunk distal to middle colic | III | Minimal | Minor segment or segments, small bowel, or right colon |
| IV | Segmental branches, jejunal, ileal, or colic | IV | None | No ischemic bowel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayegh, A.S.; Medina, L.G.; La Riva, A.; Perez, L.C.; Poncel, J.; Forsyth, E.; Cacciamani, G.E.; Challacombe, B.; Stifelman, M.; Gill, I.; et al. Superior Mesenteric Artery Injury during Robotic Radical Nephrectomy: Scenarios and Management Strategies. J. Clin. Med. 2023, 12, 427. https://doi.org/10.3390/jcm12020427
Sayegh AS, Medina LG, La Riva A, Perez LC, Poncel J, Forsyth E, Cacciamani GE, Challacombe B, Stifelman M, Gill I, et al. Superior Mesenteric Artery Injury during Robotic Radical Nephrectomy: Scenarios and Management Strategies. Journal of Clinical Medicine. 2023; 12(2):427. https://doi.org/10.3390/jcm12020427
Chicago/Turabian StyleSayegh, Aref S., Luis G. Medina, Anibal La Riva, Laura C. Perez, Jaime Poncel, Edward Forsyth, Giovanni E. Cacciamani, Ben Challacombe, Michael Stifelman, Inderbir Gill, and et al. 2023. "Superior Mesenteric Artery Injury during Robotic Radical Nephrectomy: Scenarios and Management Strategies" Journal of Clinical Medicine 12, no. 2: 427. https://doi.org/10.3390/jcm12020427
APA StyleSayegh, A. S., Medina, L. G., La Riva, A., Perez, L. C., Poncel, J., Forsyth, E., Cacciamani, G. E., Challacombe, B., Stifelman, M., Gill, I., & Sotelo, R. (2023). Superior Mesenteric Artery Injury during Robotic Radical Nephrectomy: Scenarios and Management Strategies. Journal of Clinical Medicine, 12(2), 427. https://doi.org/10.3390/jcm12020427








