A Novel Predictive Model of Pathological Lymph Node Metastasis Constructed with Preoperative Independent Predictors in Patients with Renal Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
3. Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU Guidelines on Renal Cell Carcinoma: 2014 Update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Jeldres, C.; Patard, J.-J.; Perrotte, P.; Zini, L.; de La Taille, A.; Ficarra, V.; Cindolo, L.; Bensalah, K.; Artibani, W.; et al. Stage-specific effect of nodal metastases on survival in patients with non-metastatic renal cell carcinoma. Br. J. Urol. 2009, 103, 33–37. [Google Scholar] [CrossRef]
- Capitanio, U.; Becker, F.; Blute, M.L.; Mulders, P.; Patard, J.-J.; Russo, P.; Studer, U.E.; Van Poppel, H. Lymph Node Dissection in Renal Cell Carcinoma. Eur. Urol. 2011, 60, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Rosiello, G.; Palumbo, C.; Knipper, S.; Pecoraro, A.; Luzzago, S.; Tian, Z.; Larcher, A.; Capitanio, U.; Montorsi, F.; Shariat, S.F.; et al. Histotype predicts the rate of lymph node invasion at nephrectomy in patients with nonmetastatic renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 537–544. [Google Scholar] [CrossRef]
- Capitanio, U.; Leibovich, B.C. The rationale and the role of lymph node dissection in renal cell carcinoma. World J. Urol. 2016, 35, 497–506. [Google Scholar] [CrossRef]
- Kang, H.W.; The KORCC (KOrean Renal Cell Carcinoma) Group; Kim, S.M.; Kim, W.T.; Yun, S.J.; Lee, S.-C.; Kim, W.-J.; Hwang, E.C.; Kang, S.H.; Hong, S.-H.; et al. The age-adjusted Charlson comorbidity index as a predictor of overall survival of surgically treated non-metastatic clear cell renal cell carcinoma. J. Cancer Res. Clin. Oncol. 2019, 146, 187–196. [Google Scholar] [CrossRef]
- Kidd, A.C.; McGettrick, M.; Tsim, S.; Halligan, D.; Bylesjo, M.; Blyth, K.G. Survival prediction in mesothelioma using a scalable Lasso regression model: Instructions for use and initial performance using clinical predictors. BMJ Open Respir. Res. 2018, 5, e000240. [Google Scholar] [CrossRef]
- Lei, Z.; Li, J.; Wu, D.; Xia, Y.; Wang, Q.; Si, A.; Wang, K.; Wan, X.; Lau, W.Y.; Wu, M.; et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus–Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016, 151, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Steyerberg, E.W.; Vergouwe, Y. Towards better clinical prediction models: Seven steps for development and an ABCD for validation. Eur. Hear. J. 2014, 35, 1925–1931. [Google Scholar] [CrossRef]
- Muttin, F.; Pecoraro, A.; Larcher, A.; Dell’Oglio, P.; Nini, A.; Cianflone, F.; Trevisani, F.; Dehò, F.; Briganti, A.; Salonia, A.; et al. Predictive and prognostic effect of inflammatory lymphadenopathies in renal cell carcinoma. World J. Urol. 2018, 37, 701–708. [Google Scholar] [CrossRef]
- Kuusk, T.; Klatte, T.; Zondervan, P.; Lagerveld, B.; Graafland, N.; Hendricksen, K.; Capitanio, U.; Minervini, A.; Stewart, G.D.; Ljungberg, B.; et al. Outcome after resection of occult and non-occult lymph node metastases at the time of nephrectomy. World J. Urol. 2021, 39, 3377–3383. [Google Scholar] [CrossRef]
- Tadayoni, A.; Paschall, A.K.; Malayeri, A.A. Assessing lymph node status in patients with kidney cancer. Transl. Androl. Urol. 2018, 7, 766–773. [Google Scholar] [CrossRef]
- Capitanio, U.; Abdollah, F.; Matloob, R.; Suardi, N.; Castiglione, F.; Di Trapani, E.; Capogrosso, P.; Gallina, A.; Dell’Oglio, P.; Briganti, A.; et al. When to perform lymph node dissection in patients with renal cell carcinoma: A novel approach to the preoperative assessment of risk of lymph node invasion at surgery and of lymph node progression during follow-up. BJU Int. 2013, 112, E59–E66. [Google Scholar] [CrossRef] [Green Version]
- Terrone, C.; Guercio, S.; De Luca, S.; Poggio, M.; Castelli, E.; Scoffone, C.; Tarabuzzi, R.; Scarpa, R.; Fontana, D.; Rossetti, S.R. The number of lymph nodes examined and staging accuracy in renal cell carcinoma. Br. J. Urol. 2003, 91, 37–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blute, M.L.; Leibovich, B.C.; Cheville, J.C.; Lohse, C.M.; Zincke, H. A protocol for performing extended lymph node dissection using primary tumor pathological features for patients treated with radical nephrectomy for clear cell renal cell carcinoma. J. Urol. 2004, 172, 465–469. [Google Scholar] [CrossRef]
- Gershman, B.; Takahashi, N.; Moreira, D.; Thompson, R.H.; Boorjian, S.A.; Lohse, C.M.; Costello, B.A.; Cheville, J.C.; Leibovich, B.C. Radiographic size of retroperitoneal lymph nodes predicts pathological nodal involvement for patients with renal cell carcinoma: Development of a risk prediction model. BJU Int. 2016, 118, 742–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Peng, C.; Xie, Y.; Wang, L.; Gu, L.; Wu, S.; Shen, D.; Xuan, Y.; Ma, X.; Zhang, X. A Novel Preoperative Nomogram for Predicting Lymph Node Invasion in Renal Cell Carcinoma Patients Without Metastasis. Cancer Manag. Res. 2019, 11, 9961–9967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliò, A.; Segala, D.; Munari, E.; Brunelli, M.; Martignoni, G. MiT Family Translocation Renal Cell Carcinoma: From the Early Descriptions to the Current Knowledge. Cancers 2019, 11, 1110. [Google Scholar] [CrossRef] [Green Version]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bensalah, K.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur. Urol. 2019, 75, 799–810. [Google Scholar] [CrossRef]
- Babaian, K.N.; Kim, D.Y.; Kenney, P.A.; Wood, C.G.; Wong, J.; Sanchez, C.; Fang, J.E.; Gerber, J.A.; Didic, A.; Wahab, A.; et al. Preoperative Predictors of Pathological Lymph Node Metastasis in Patients with Renal Cell Carcinoma Undergoing Retroperitoneal Lymph Node Dissection. J. Urol. 2014, 193, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Kuusk, T.; Neves, J.B.; Tran, M.; Bex, A. Radiomics to better characterize small renal masses. World J. Urol. 2021, 39, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
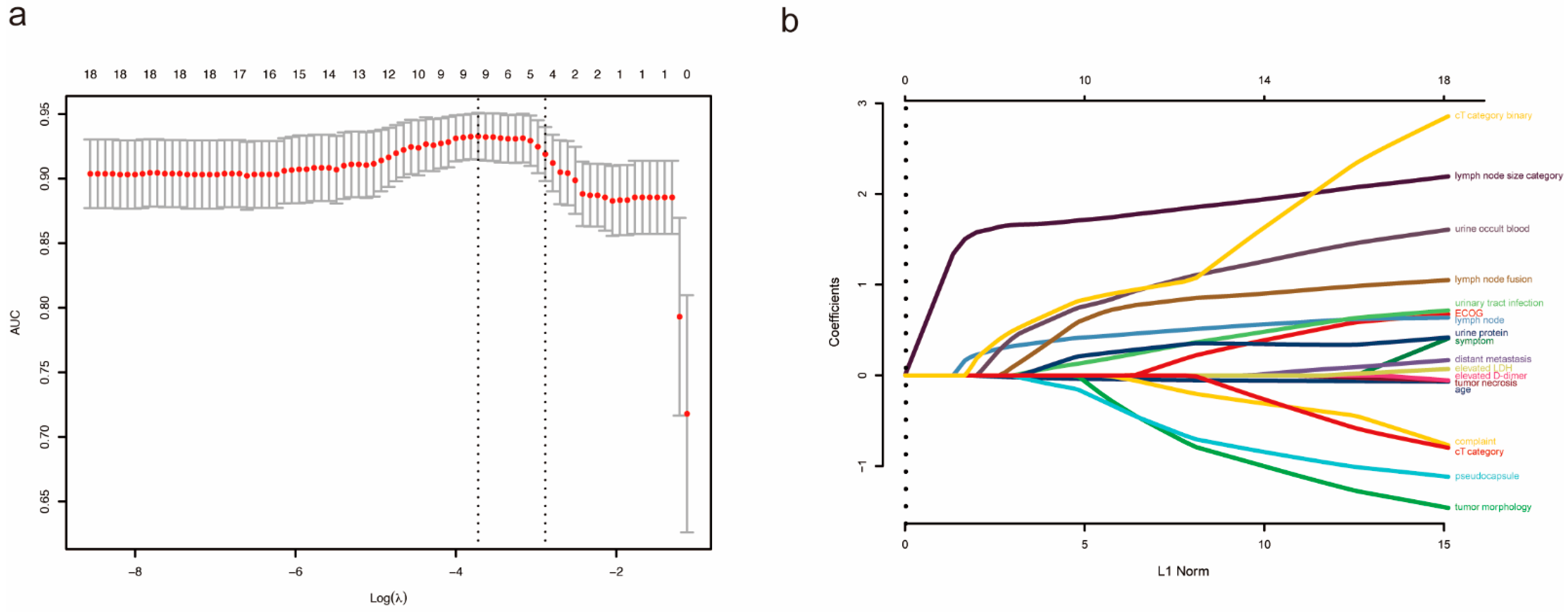
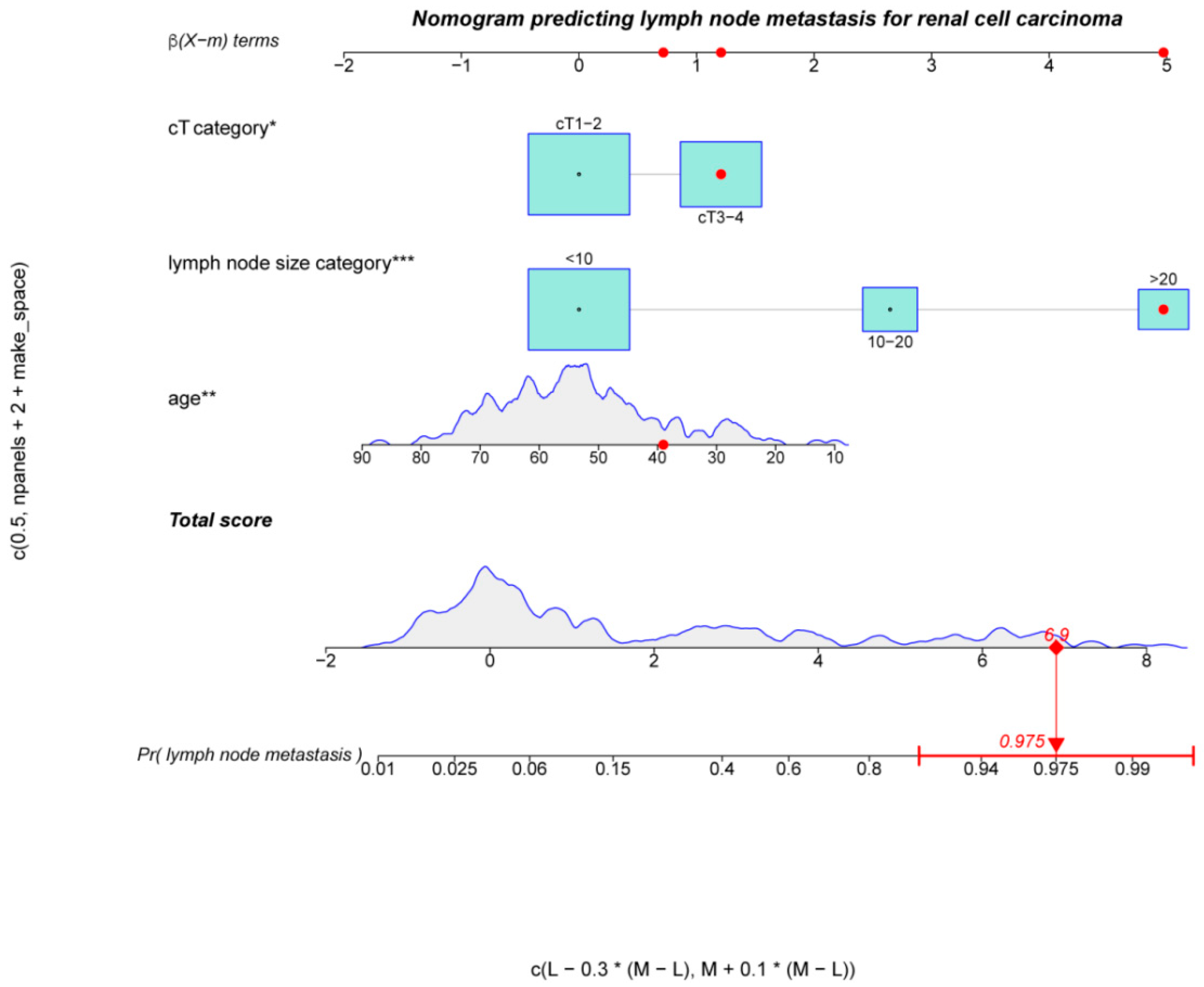

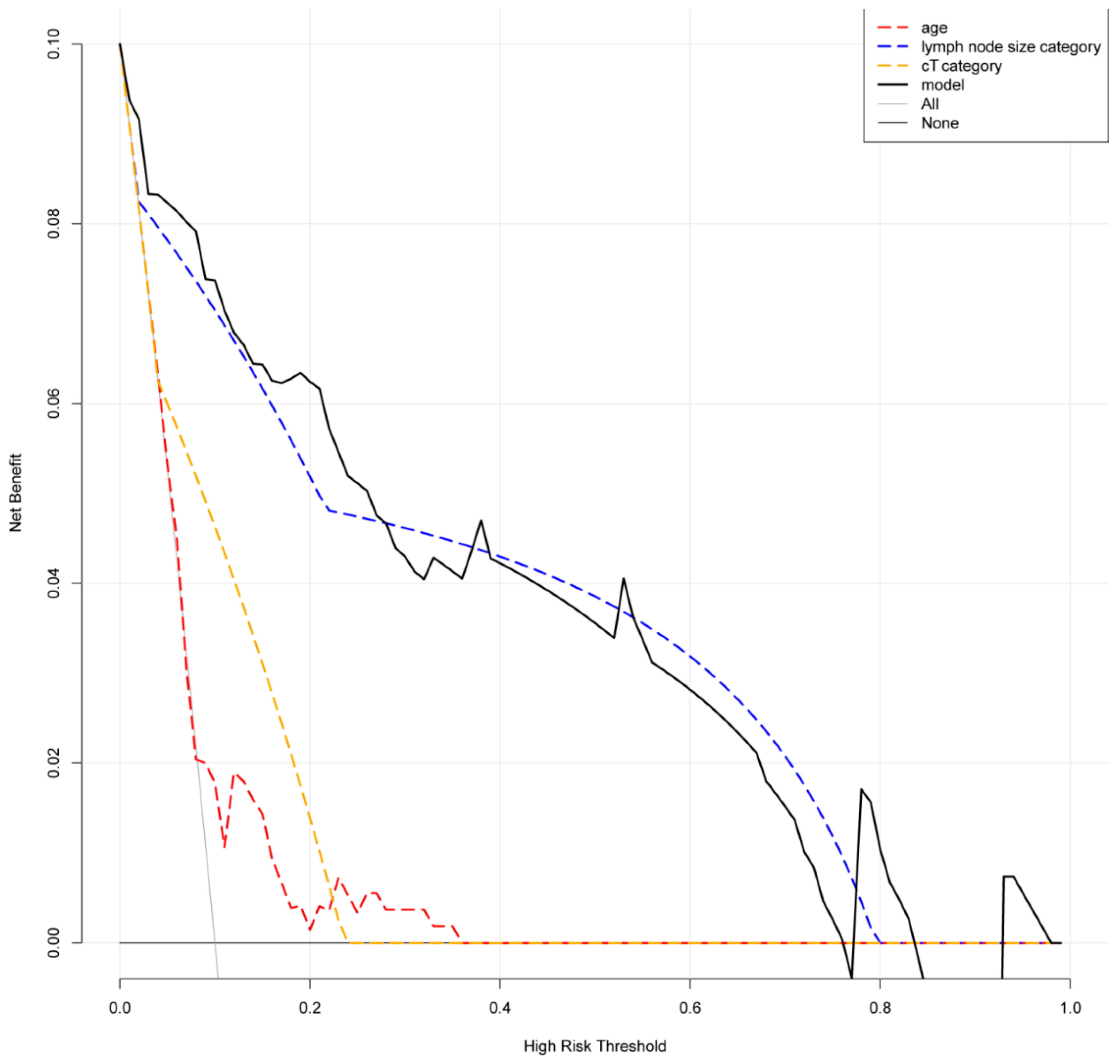
| All Patients (n = 189) | pN0 (n = 135) | pN1 (n = 54) | p Value | |
|---|---|---|---|---|
| Gender, n (%) | 0.172 | |||
| Male | 121 (64.0) | 91 (67.4) | 30 (55.6) | |
| Female | 68 (36.0) | 44 (32.6) | 24 (44.4) | |
| Age, median [IQR] (years) | 54.00 [46.00, 63.00] | 55.00 [48.00, 64.50] | 51.00 [37.50, 61.00] | 0.007 |
| ECOG PS, n (%) | 0.038 b | |||
| 0 | 131 (69.3) | 100 (74.1) | 31 (57.4) | |
| 1 | 58 (30.7) | 35 (25.9) | 23 (42.6) | |
| Complaint, n (%) | 0.006 | |||
| Medical examination | 92 (48.7) | 75 (55.6) | 17 (31.5) | |
| Low back pain | 37 (19.6) | 22 (16.3) | 15 (27.8) | |
| Hematuria | 36 (19.0) | 26 (19.3) | 10 (18.5) | |
| Others | 24 (12.7) | 12 (8.9) | 12 (22.2) | |
| Symptom, n (%) | 0.002 | |||
| None | 95 (50.3) | 78 (57.8) | 17 (31.5) | |
| Local symptoms | 70 (37.0) | 45 (33.3) | 25 (46.3) | |
| Systemic symptoms | 24 (12.7) | 12 (8.9) | 12 (22.2) | |
| History of tumor, n (%) | 0.355 a | |||
| No | 183 (96.8) | 132 (97.8) | 51 (94.4) | |
| Yes | 6 (3.2) | 3 (2.2) | 3 (5.6) | |
| History of smoking and drinking, n (%) | 0.625 | |||
| No | 137 (72.5) | 96 (71.1) | 41 (75.9) | |
| Yes | 52 (27.5) | 39 (28.9) | 13 (24.1) | |
| History of abdominal surgery, n (%) | 1 | |||
| No | 162 (85.7) | 116 (85.9) | 46 (85.2) | |
| Yes | 27 (14.3) | 19 (14.1) | 8 (14.8) | |
| Hypertension, n (%) | 0.635 | |||
| No | 148 (78.3) | 104 (77.0) | 44 (81.5) | |
| Yes | 41 (21.7) | 31 (23.0) | 10 (18.5) | |
| Diabetes, n (%) | 1 | |||
| No | 165 (87.3) | 118 (87.4) | 47 (87.0) | |
| Yes | 24 (12.7) | 17 (12.6) | 7 (13.0) | |
| Coronary heart disease, n (%) | 1 a | |||
| No | 184 (97.4) | 131 (97.0) | 53 (98.1) | |
| Yes | 5 (2.6) | 4 (3.0) | 1 (1.9) | |
| Respiratory diseases, n (%) | 0.41 a | |||
| No | 182 (96.3) | 131 (97.0) | 51 (94.4) | |
| Yes | 7 (3.7) | 4 (3.0) | 3 (5.6) | |
| Infectious disease, n (%) | 1 a | |||
| No | 184 (97.4) | 131 (97.0) | 53 (98.1) | |
| Yes | 5 (2.6) | 4 (3.0) | 1 (1.9) | |
| Urolithiasis, n (%) | 0.45 a | |||
| No | 180 (95.2) | 127 (94.1) | 53 (98.1) | |
| Yes | 9 (4.8) | 8 (5.9) | 1 (1.9) | |
| aCCI, n (%) | 0.285 b | |||
| 0–1 | 102 (54.0) | 68 (50.4) | 34 (63.0) | |
| 2–3 | 67 (35.4) | 52 (38.5) | 15 (27.8) | |
| ≥4 | 20 (10.6) | 15 (11.1) | 5 (9.3) | |
| Tumor side, n (%) | 0.121 | |||
| Left | 104 (55.0) | 69 (51.1) | 35 (64.8) | |
| Right | 85 (45.0) | 66 (48.9) | 19 (35.2) | |
| Tumor size, median [IQR] (mm) | 70.00 [50.00, 98.00] | 69.00 [50.00, 92.00] | 70.00 [46.00, 102.50] | 0.739 |
| Exophytic or endophytic property, n (%) | 0.123 a | |||
| 50% or more exophytic | 36 (19.0) | 26 (19.3) | 10 (18.5) | |
| Less than 50% exophytic | 140 (74.1) | 103 (76.3) | 37 (68.5) | |
| Entirely endophytic | 13 (6.9) | 6 (4.4) | 7 (13.0) | |
| Renal collecting system under pressure, n (%) | 0.13 | |||
| No | 23 (12.2) | 20 (14.8) | 3 (5.6) | |
| Yes | 166 (87.8) | 115 (85.2) | 51 (94.4) | |
| Renal hilum invasion, n (%) | 0.103 | |||
| No | 64 (33.9) | 51 (37.8) | 13 (24.1) | |
| Yes | 125 (66.1) | 84 (62.2) | 41 (75.9) | |
| Tumor morphology, n (%) | <0.001 | |||
| Round | 74 (39.2) | 65 (48.1) | 9 (16.7) | |
| Irregular | 115 (60.8) | 70 (51.9) | 45 (83.3) | |
| Pseudocapsule, n (%) | <0.001 | |||
| No | 87 (46.0) | 45 (33.3) | 42 (77.8) | |
| Yes | 102 (54.0) | 90 (66.7) | 12 (22.2) | |
| Tumor necrosis, n (%) | 0.062 | |||
| No | 35 (18.5) | 30 (22.2) | 5 (9.3) | |
| Yes | 154 (81.5) | 105 (77.8) | 49 (90.7) | |
| Venous tumor thrombus, n (%) | 0.464 a | |||
| No | 162 (85.7) | 118 (87.4) | 44 (81.5) | |
| Renal vein | 26 (13.8) | 16 (11.9) | 10 (18.5) | |
| Vena cava | 1 (0.5) | 1 (0.7) | 0 (0.0) | |
| Lymph node status by pre-operative imaging, n (%) | <0.001 | |||
| Negative | 92 (48.7) | 85 (63.0) | 7 (13.0) | |
| Enlarged and increased lymph nodes | 56 (29.6) | 44 (32.6) | 12 (22.2) | |
| Metastasis | 41 (21.7) | 6 (4.4) | 35 (64.8) | |
| Lymph node size by pre-operative imaging, median [IQR] (mm) | 5.00 [0.00, 15.00] | 0.00 [0.00, 8.00] | 21.00 [17.00, 30.00] | <0.001 |
| Lymph node size category, n (%) | <0.001 b | |||
| ≤10 mm | 123 (65.1) | 115 (85.2) | 8 (14.8) | |
| 10–20 mm | 36 (19.0) | 18 (13.3) | 18 (33.3) | |
| >20 mm | 30 (15.9) | 2 (1.5) | 28 (51.9) | |
| Lymph node fusion, n (%) | <0.001 | |||
| No | 170 (89.9) | 134 (99.3) | 36 (66.7) | |
| Yes | 19 (10.1) | 1 (0.7) | 18 (33.3) | |
| Clinical M stage, n (%) | 0.005 b | |||
| cM0 | 163 (86.2) | 123 (91.1) | 40 (74.1) | |
| cM1 | 26 (13.8) | 12 (8.9) | 14 (25.9) | |
| Neutrophil, median [IQR] (109 cells/L) | 3.97 [3.10, 5.11] | 3.87 [2.90, 5.08] | 4.33 [3.44, 5.12] | 0.128 |
| Neutrophil, n (%) | 1 | |||
| Normal | 166 (87.8) | 119 (88.1) | 47 (87.0) | |
| Abnormal | 23 (12.2) | 16 (11.9) | 7 (13.0) | |
| Lymphocyte, mean (SD) (109 cells/L) | 1.60 (0.50) | 1.62 (0.52) | 1.56 (0.45) | 0.523 |
| Lymphocyte, n (%) | 0.975 | |||
| Normal | 159 (84.1) | 113 (83.7) | 46 (85.2) | |
| Abnormal | 30 (15.9) | 22 (16.3) | 8 (14.8) | |
| NLR, median [IQR] | 2.57 [1.82, 3.68] | 2.51 [1.73, 3.50] | 2.73 [2.04, 4.00] | 0.181 |
| Hemoglobin, median [IQR] (g/L) | 126.00 [104.00, 139.00] | 127.00 [108.00, 140.50] | 120.00 [102.00, 136.00] | 0.206 |
| Anemia, n (%) | 0.447 | |||
| No | 101 (53.4) | 75 (55.6) | 26 (48.1) | |
| Yes | 88 (46.6) | 60 (44.4) | 28 (51.9) | |
| Platelet, median [IQR] (109 cells/L) | 258.00 [190.00, 315.00] | 248.00 [186.00, 310.50] | 272.00 [195.00, 330.50] | 0.136 |
| Platelet, n (%) | 0.747 | |||
| Normal | 153 (81.0) | 108 (80.0) | 45 (83.3) | |
| Abnormal | 36 (19.0) | 27 (20.0) | 9 (16.7) | |
| Fibrinogen, median [IQR] (g/L) | 4.31 [3.22, 6.13] | 4.08 [3.09, 6.06] | 4.96 [3.60, 6.22] | 0.139 |
| Fibrinogen, n (%) | 0.156 | |||
| Normal | 80 (42.3) | 62 (45.9) | 18 (33.3) | |
| Abnormal | 109 (57.7) | 73 (54.1) | 36 (66.7) | |
| D-dimer, median [IQR] (mg/L) | 0.55 [0.30, 0.97] | 0.46 [0.30, 0.92] | 0.66 [0.38, 1.09] | 0.073 |
| D-dimer, n (%) | 0.011 | |||
| Normal | 89 (47.1) | 72 (53.3) | 17 (31.5) | |
| High | 100 (52.9) | 63 (46.7) | 37 (68.5) | |
| Clotting time, n (%) | 0.568 | |||
| Normal | 130 (68.8) | 95 (70.4) | 35 (64.8) | |
| Prolonged | 59 (31.2) | 40 (29.6) | 19 (35.2) | |
| Albumin, median [IQR] (g/L) | 39.00 [35.10, 41.40] | 39.40 [35.80, 41.80] | 38.35 [34.73, 40.30] | 0.096 |
| Albumin, n (%) | 0.641 | |||
| Normal | 146 (77.2) | 106 (78.5) | 40 (74.1) | |
| Low | 43 (22.8) | 29 (21.5) | 14 (25.9) | |
| Globulin, median [IQR] (g/L) | 31.80 [27.60, 37.00] | 30.90 [27.25, 36.00] | 33.15 [28.75, 39.65] | 0.173 |
| Globulin, n (%) | 0.132 | |||
| Normal | 129 (68.3) | 97 (71.9) | 32 (59.3) | |
| High | 60 (31.7) | 38 (28.1) | 22 (40.7) | |
| AGR, mean (SD) | 1.23 (0.36) | 1.26 (0.36) | 1.17 (0.36) | 0.155 |
| AGR < 1.5, n (%) | 0.321 | |||
| No | 46 (24.3) | 36 (26.7) | 10 (18.5) | |
| Yes | 143 (75.7) | 99 (73.3) | 44 (81.5) | |
| ALP, median [IQR] (U/L) | 72.00 [61.00, 94.00] | 71.00 [61.00, 91.00] | 77.50 [63.00, 98.00] | 0.49 |
| ALP, n (%) | 0.239 a | |||
| Normal | 174 (92.1) | 122 (90.4) | 52 (96.3) | |
| High | 15 (7.9) | 13 (9.6) | 2 (3.7) | |
| LDH, median [IQR] (U/L) | 171.00 [143.00, 200.00] | 167.00 [138.00, 189.50] | 178.00 [156.75, 253.75] | 0.006 |
| LDH, n (%) | 0.004 | |||
| Normal | 155 (82.0) | 118 (87.4) | 37 (68.5) | |
| High | 34 (18.0) | 17 (12.6) | 17 (31.5) | |
| Calcium, median [IQR] (mmol/L) | 2.38 [2.30, 2.49] | 2.37 [2.29, 2.48] | 2.43 [2.36, 2.52] | 0.021 |
| Calcium, n (%) | 0.427 | |||
| Normal | 165 (87.3) | 120 (88.9) | 45 (83.3) | |
| High | 24 (12.7) | 15 (11.1) | 9 (16.7) | |
| Creatinine, median [IQR] (μmol/L) | 76.00 [66.00, 92.00] | 76.00 [66.00, 93.00] | 79.00 [65.00, 90.75] | 0.962 |
| Creatine, n (%) | 1 | |||
| Normal | 159 (84.1) | 114 (84.4) | 45 (83.3) | |
| High | 30 (15.9) | 21 (15.6) | 9 (16.7) | |
| eGFR, median [IQR] | 91.70 [73.80, 102.90] | 92.60 [73.80, 100.65] | 88.75 [74.02, 104.80] | 0.753 |
| Urine RBC, median [IQR] (cells/hpf) | 10.20 [3.00, 49.90] | 8.10 [3.00, 30.00] | 31.50 [2.88, 416.50] | 0.012 |
| Urine occult blood, n (%) | 0.001 | |||
| No | 117 (61.9) | 94 (69.6) | 23 (42.6) | |
| Yes | 72 (38.1) | 41 (30.4) | 31 (57.4) | |
| Urine WBC, median [IQR] (cells/hpf) | 6.30 [1.70, 35.50] | 6.00 [1.70, 19.85] | 11.50 [1.35, 97.30] | 0.075 |
| Urinary tract infection, n (%) | 0.015 | |||
| No | 128 (67.7) | 99 (73.3) | 29 (53.7) | |
| Yes | 61 (32.3) | 36 (26.7) | 25 (46.3) | |
| Urine protein, n (%) | 0.001 | |||
| No | 145 (76.7) | 113 (83.7) | 32 (59.3) | |
| Yes | 44 (23.3) | 22 (16.3) | 22 (40.7) | |
| Surgery type and technique, n (%) | <0.001 a | |||
| Open radical nephrectomy | 67 (35.4) | 37 (27.4) | 30 (55.6) | |
| Open partial nephrectomy | 2 (1.1) | 2 (1.5) | 0 (0.0) | |
| Laparoscopic radical nephrectomy | 88 (46.6) | 75 (55.6) | 13 (24.1) | |
| Laparoscopic partial nephrectomy | 10 (5.3) | 10 (7.4) | 0 (0.0) | |
| Robot-assisted radical nephrectomy | 17 (9.0) | 7 (5.2) | 10 (18.5) | |
| Robot-assisted partial nephrectomy | 5 (2.6) | 4 (3.0) | 1 (1.9) | |
| Resected lymph nodes, median [IQR] | 2.00 [1.00, 6.00] | 2.00 [1.00, 6.00] | 3.00 [1.00, 8.75] | 0.162 |
| Positive lymph nodes, median [IQR] | 0.00 [0.00, 1.00] | 0.00 [0.00, 0.00] | 2.00 [1.00, 3.00] | <0.001 |
| Clinical T stage, n (%) | <0.001 b | |||
| cT1 | 75 (39.7) | 64 (47.4) | 11 (20.4) | |
| cT2 | 40 (21.2) | 36 (26.7) | 4 (7.4) | |
| cT3 | 56 (29.6) | 28 (20.7) | 28 (51.9) | |
| cT4 | 18 (9.5) | 7 (5.2) | 11 (20.4) | |
| Histology type, n (%) | <0.001 a | |||
| Clear cell carcinoma | 126 (66.7) | 112 (83.0) | 14 (25.9) | |
| Medullary carcinoma | 2 (1.1) | 1 (0.7) | 1 (1.9) | |
| Papillary | 25 (13.2) | 6 (4.4) | 19 (35.2) | |
| MiT | 9 (4.8) | 1 (0.7) | 8 (14.8) | |
| Collecting duct carcinoma | 3 (1.6) | 0 (0.0) | 3 (5.6) | |
| Unclassified | 15 (7.9) | 6 (4.4) | 9 (16.7) | |
| Chromophobe | 6 (3.2) | 6 (4.4) | 0 (0.0) | |
| Others | 3 (1.6) | 3 (2.2) | 0 (0.0) |
| Variables | B | SE | OR | 95% CI | p Value |
|---|---|---|---|---|---|
| Age | −0.0574 | 0.0211 | 0.3769 | [0.1864–0.7622] | 0.0066 |
| Urine protein | 0.6814 | 0.6861 | 1.9766 | [0.5151–7.5841] | 0.3207 |
| Lymph node status in pre-operative imaging | |||||
| Negative | - | - | - | - | Ref. |
| Enlarged and increased | −1.5404 | 1.1512 | 0.2143 | [0.0224–2.0460] | 0.1809 |
| Metastasis | 0.4349 | 1.3311 | 1.5449 | [0.1137–20.9840] | 0.7438 |
| Lymph node size category | |||||
| ≤10 | - | - | - | - | Ref. |
| 10–20 | 2.7083 | 1.1528 | 15.0040 | [1.5666–143.7000] | 0.0188 |
| >20 | 4.4013 | 1.4858 | 4.4013 | [1.4892–7.3134] | 0.0031 |
| Pseudocapsule (Yes vs. No) | −0.4616 | 0.5898 | 1.5866 | [0.4994–5.0410] | 0.4338 |
| Urine occult blood (Positive vs. Negative) | 1.3974 | 0.7238 | 4.0445 | [0.9790–16.7090] | 0.0535 |
| Urinary tract infection (Yes vs. No) | 0.2208 | 0.6332 | 1.2471 | [0.3605–4.3141] | 0.7273 |
| Lymph node fusion (Yes vs. No) | 0.7686 | 1.3527 | 2.1566 | [0.1522–30.5660] | 0.5699 |
| Clinical T stage (cT1–2 vs. cT3–4) | 1.1519 | 0.5708 | 3.1641 | [1.0336–9.6860] | 0.0436 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.-X.; Liu, C.-Q.; Zhang, Z.-B.; Xia, Q.-D.; Xu, J.-Z.; An, Y.; Xu, M.-Y.; Zhong, X.-Y.; Zeng, N.; Ma, S.-Y.; et al. A Novel Predictive Model of Pathological Lymph Node Metastasis Constructed with Preoperative Independent Predictors in Patients with Renal Cell Carcinoma. J. Clin. Med. 2023, 12, 441. https://doi.org/10.3390/jcm12020441
Sun J-X, Liu C-Q, Zhang Z-B, Xia Q-D, Xu J-Z, An Y, Xu M-Y, Zhong X-Y, Zeng N, Ma S-Y, et al. A Novel Predictive Model of Pathological Lymph Node Metastasis Constructed with Preoperative Independent Predictors in Patients with Renal Cell Carcinoma. Journal of Clinical Medicine. 2023; 12(2):441. https://doi.org/10.3390/jcm12020441
Chicago/Turabian StyleSun, Jian-Xuan, Chen-Qian Liu, Zong-Biao Zhang, Qi-Dong Xia, Jin-Zhou Xu, Ye An, Meng-Yao Xu, Xing-Yu Zhong, Na Zeng, Si-Yang Ma, and et al. 2023. "A Novel Predictive Model of Pathological Lymph Node Metastasis Constructed with Preoperative Independent Predictors in Patients with Renal Cell Carcinoma" Journal of Clinical Medicine 12, no. 2: 441. https://doi.org/10.3390/jcm12020441






