Delayed Leucoencephalopathy as a Complication after Endovascular Therapy of Intracranial Aneurysms—A Case Series
Abstract
1. Introduction
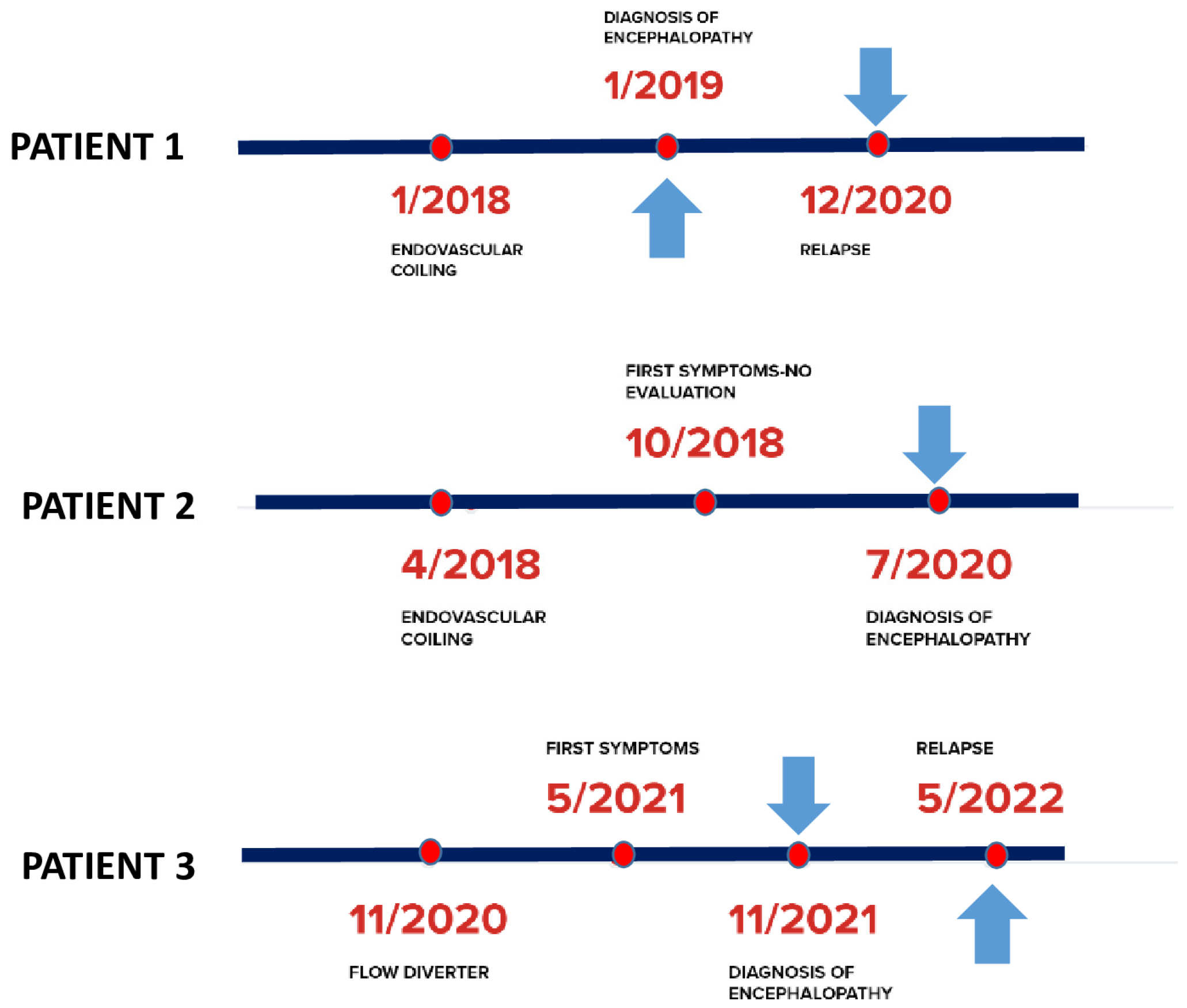
2. Case Descriptions
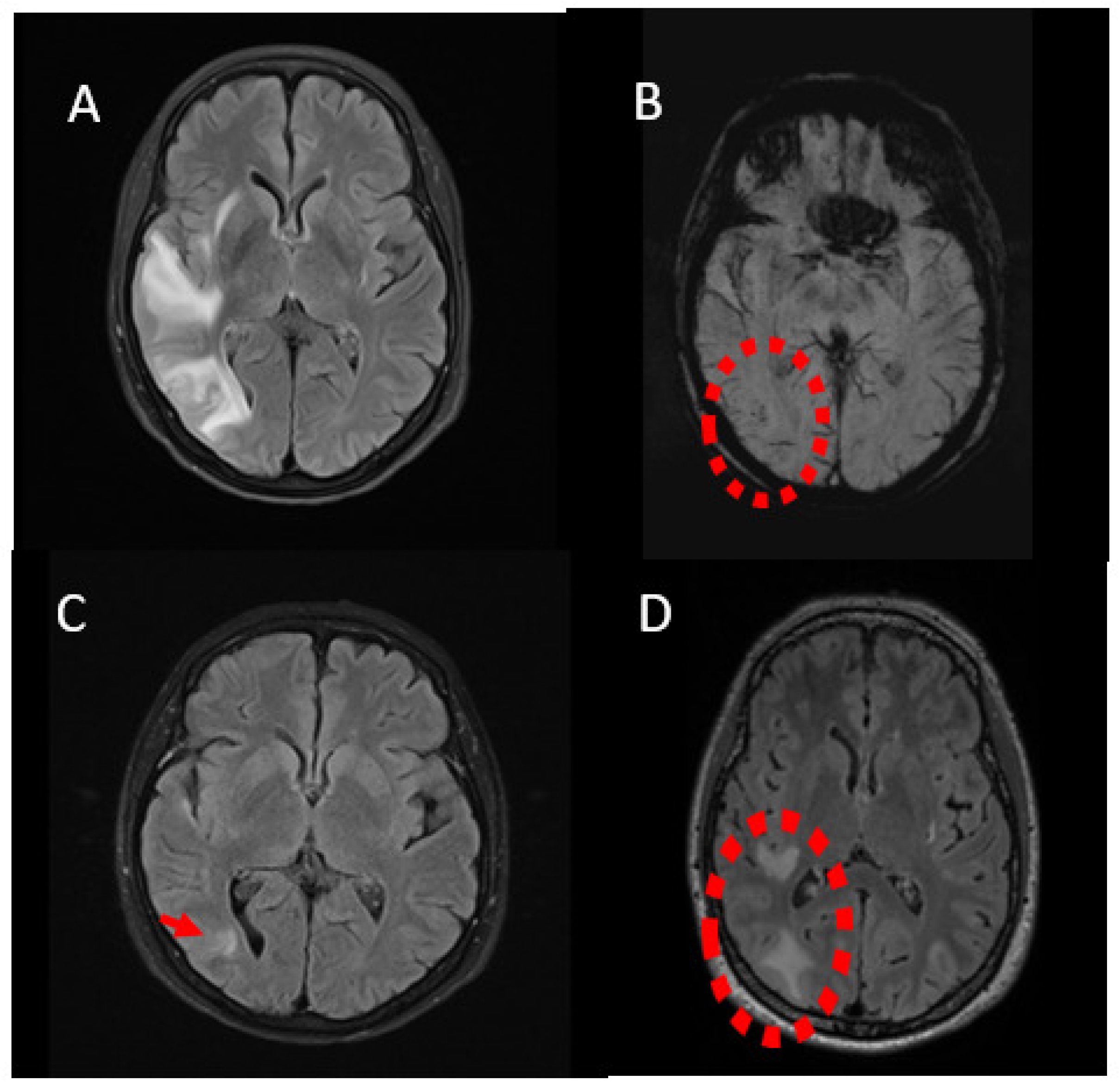
2.1. Case 1
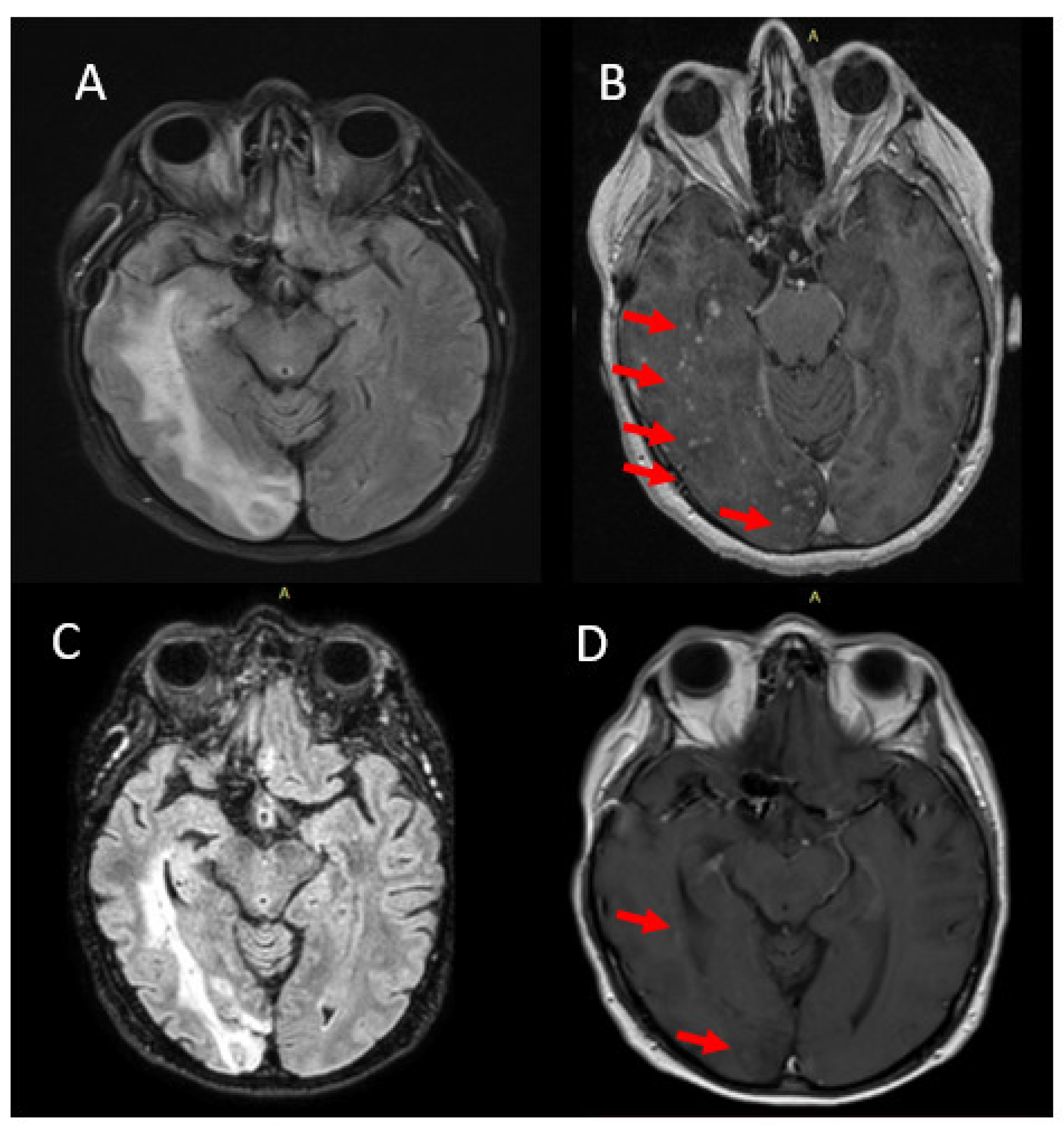
2.2. Case 2
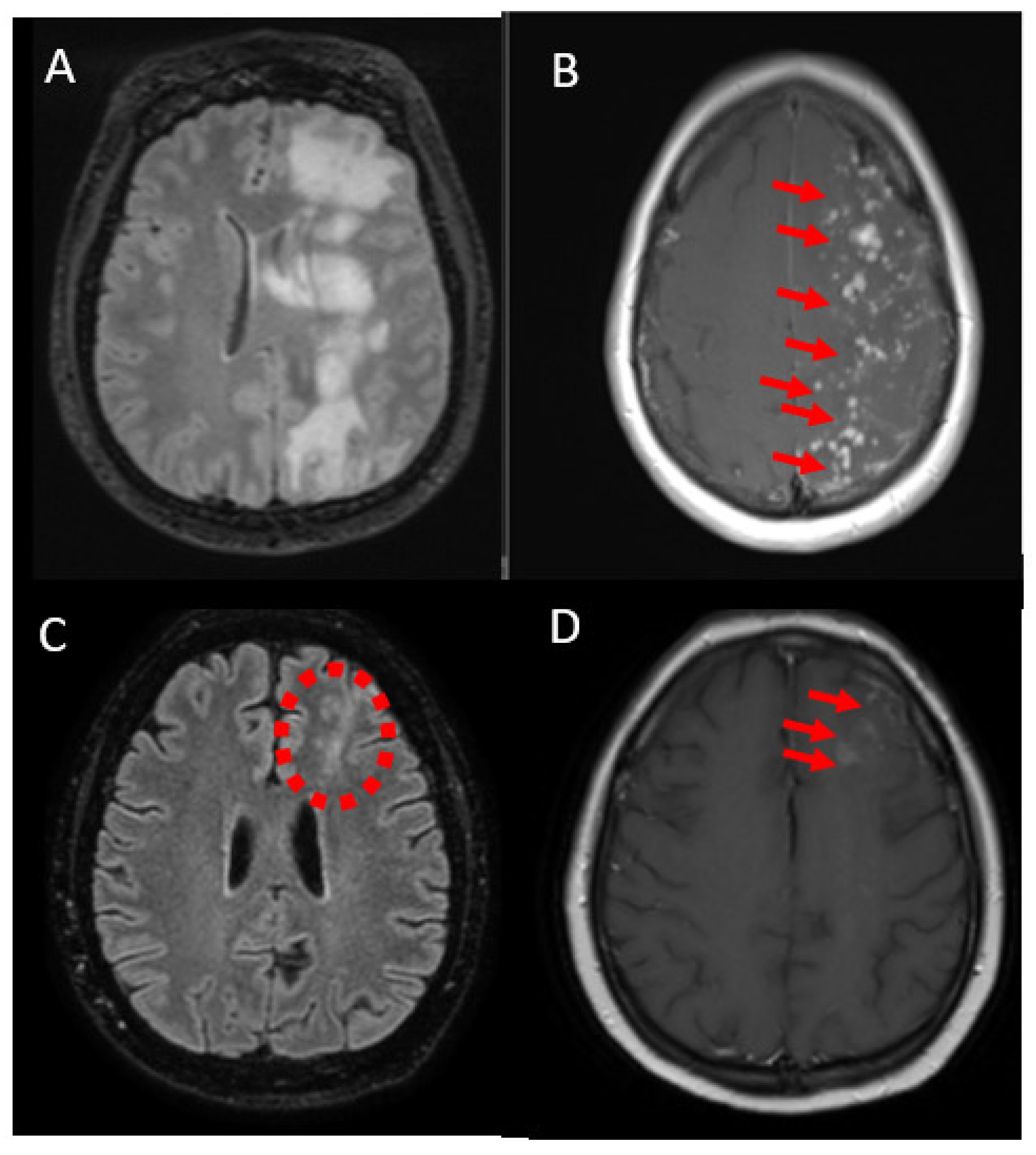
2.3. Case 3
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, A.S.; Campos, J.K.; Colby, G.P.; Coon, A.L.; Lin, L.M. Cerebral aneurysm treatment trends in National Inpatient Sample 2007–2016: Endovascular therapies favored over surgery. J. Neurointerv. Surg. 2020, 12, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Pierot, L.; Wakhloo, A.K. Endovascular treatment of intracranial aneurysms: Current status. Stroke 2013, 44, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, A.; Ishibashi, T.; Otani, K.; Yuki, I.; Kodama, T.; Kan, I.; Kato, N.; Murayama, Y. Delayed Leukoencephalopathy: A Rare Complication after Coiling of Cerebral Aneurysms. AJNR Am. J. Neuroradiol. 2020, 41, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Bakola, E.; Katsanos, A.H.; Palaiodimou, L.; Theodorou, A.; Stefanou, M.I.; Chondrogianni, M.; Andreadou, E.; Papadopoulou, M.; Konstantakos, V.; Voumvourakis, K.; et al. Delayed recurrent enhancing white matter lesions complicating coiling of intracranial aneurysm. Eur. J. Neurol. 2021, 28, 2388–2391. [Google Scholar] [CrossRef] [PubMed]
- Shotar, E.; Law-Ye, B.; Baronnet-Chauvet, F.; Zeidan, S.; Psimaras, D.; Bielle, F.; Pecquet, C.; Navarro, S.; Rosso, C.; Cohen, F.; et al. Non-ischemic cerebral enhancing lesions secondary to endovascular aneurysm therapy: Nickel allergy or foreign body reaction? Case series and review of the literature. Neuroradiology 2016, 58, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Park, H.S.; Kotsugi, M.; Morisaki, Y.; Wada, T.; Aketa, S.; Takayama, K.; Fujimoto, K.; Deguchi, J.; Kichikawa, K.; et al. Delayed Intracranial Parenchymal Changes after Aneurysmal Coil Embolization Procedures for Unruptured Intracranial Aneurysms. Oper. Neurosurg. 2020, 19, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Bayas, A.; Christ, M.; Berlis, A.; Naumann, M.; Ertl, M.; Joachimsk, F.; Müller, M.; Welzel, J.; Ann Gerdes, L.; Seelos, K.; et al. Incidence, clinical spectrum, and immunotherapy of non-ischemic cerebral enhancing lesions after endovascular therapy. Ther. Adv. Neurol Disord. 2022, 15, 17562864211072372. [Google Scholar] [CrossRef] [PubMed]
- Shotar, E.; Labeyrie, M.A.; Biondi, A.; Velascoet, S.; Saliou, G.; Boulouis, G.; Daumas-Duport, B.; Bourcier, R.; Janot, K.; Herbreteau, D.; et al. Non-ischemic cerebral enhancing lesions after intracranial aneurysm endovascular repair: A retrospective French national registry. J. Neurointerv. Surg. 2022, 14, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.; Ollenschleger, M.D.; Baccin, C.; Becske, T.; Spiegel, G.R.; Wang, Y.; Song, X.; Raz, E.; Zumofen, D.; Potts, M.B.; et al. Foreign Body Emboli following Cerebrovascular Interventions: Clinical, Radiographic, and Histopathologic Features. AJNR Am. J. Neuroradiol. 2015, 36, 2121–2126. [Google Scholar] [CrossRef]
- Grewal, S.S.; López Del Valle, E.M.; Gupta, V.; Ramon, N.; Freeman, W.D.; Tawk, R.G. Neurological Changes with Abnormal Brain Reactivity Following Coiling of Cerebral Aneurysm. Possible Reactivity to Endovascular Devices and Material? J. Vasc. Interv. Neurol. 2015, 8, 28–36. [Google Scholar]
- Fealey, M.E.; Edwards, W.D.; Giannini, C.; Piepgras, D.G.; Cloft, H.; Rihal, C.S. Complications of endovascular polymers associated with vascular introducer sheaths and metallic coils in 3 patients, with literature review. Am. J. Surg. Pathol. 2008, 32, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Skolarus, L.E.; Gemmete, J.J.; Braley, T.; Morgenstern, L.B.; Pandey, A. Abnormal white matter changes after cerebral aneurysm treatment with polyglycolic-polylactic acid coils. World Neurosurg. 2010, 74, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Ulus, S.; Yakupoğlu, A.; Kararslan, E.; Işlak, C.; Siva, A.; Koçer, N. Reversible intracranial parenchymal changes in MRI after MCA aneurysm treatment with stent-assisted coiling technique; possible nickel allergy. Neuroradiology 2012, 54, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.P.; Marotta, T.; O’Kelly, C.; Holtmannspötter, M.; Saliou, G.; Willinsky, R.; Krings, T.; Agid, R. Enhancing brain lesions after endovascular treatment of aneurysms. AJNR Am. J. Neuroradiol. 2014, 35, 1954–1958. [Google Scholar] [CrossRef] [PubMed]
- Lobotesis, K.; Mahady, K.; Ganesalingam, J.; Amlani, S.; Carlton-Jones, L.; Davies, N.W.; Bentley, P. Coiling-associated delayed cerebral hypersensitivity: Is nickel the link? Neurology 2015, 84, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Nakagawa, I.; Yokoyama, S.; Wajima, D.; Wada, T.; Motoyama, Y.; Kichikawa, K.; Nakase, H. Nickel-associated delayed multiple white matter lesions after stent-assisted coil embolization of intracranial unruptured aneurysm. J. Neurointerv. Surg. 2018, 10, e1. [Google Scholar] [CrossRef] [PubMed]
- Ridwan, S.; Kandyba, J.A.; Schug, A.; Malsagov, E.; Karageorgos, N.; Hans, F.J. Delayed Leukoencephalopathy and Foreign Body Reaction after Endovascular Treatment in Patients with Intracranial Aneurysms and Aneurysmal Subarachnoid Hemorrhage—A Systematic Review of the Literature. Front. Surg. 2021, 8, 732603. [Google Scholar] [CrossRef] [PubMed]
| Scheme | EVT Procedure/ Parent Vessel | Interval between Procedure and Leucoencephalopathy | Symptoms | MRI Findings | Biopsy | Treatment/ Response | Follow Up Duration |
|---|---|---|---|---|---|---|---|
| Case 1 * F/64 | Endovascular coiling/ right PCom | 12 months | headache, auditory hallucinations, paresthesias and weakness of the left upper limb | Enhancing lesions, vasogenic edema, low SWI signals | No | Steroids **/Initial improvement, recurrence after tapering | 3.5 years |
| Case 2 F/62 | Endovascular coiling/ right PCom | 6 months | left homonymous hemianopsia, left-sided hemiparesis, auditory hallucinations and seizures | Enhancing lesions, vasogenic edema, low SWI signals | No | Steroids **/improvement | 3 years |
| Case 3 F/49 | Flow diverter stent/ supraclinoid segment of left ICA | 6 months | gait disorders, right-sided hemiparesis, partial seizures of the right upper limb, mild cognitive impairment | Enhancing lesions, vasogenic edema, low SWI signals | Yes-chronic necrotizing granulomatous inflammation | Steroids **/improvement, recurrence after tapering | 1 year |
| Authors, Year | Type of Study | Patients (Sex/Age) |
|---|---|---|
| Ikemura et al., 2020 [3] | Cohort study | 16 out of 1722 procedures F 71.3%, mean age 59 |
| Shotar et al., 2016 [5] | Case series | 2 out of 374 patients, M/45, F/54 |
| Nakagawa et al., 2020 [6] | Cohort study | 7 patients out of 305 F 100%-mean age 59 |
| Bayas et al., 2022 [7] | Cohort study | 5 out of 746 patients, F 100%, mean age 51 |
| Shotar et al., 2022 [8] | Cohort study | 31 out of 58815 procedures 84% F, mean age 45 |
| Shapiro et al., 2015 [9] | Case series | 5 patients |
| Grewal et al., 2015 [10] | Case report | F/65 |
| Fealy et al., 2008 [11] | Case report | F/58 |
| Skolarus et al., 2010 [12] | Case report | F/46, F/56 |
| Ulus et al., 2012 [13] | Case report | F/41 |
| Cruz et al., 2014 [14] | Case series | 7 patients, F 86%, mean age 54 |
| Lobotesis et al., 2015 [15] | Case report | F/60 |
| Park et al., 2018 [16] | Case report | F/64, F/52 |
| Bakola et al., 2021 [4] | Case report | F/64 |
| Ridwan et al., 2021 [17] | Systematic review and case report | F/53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakola, E.; Papagiannopoulou, G.; Palaiodimou, L.; Lagios, K.; Archontakis, E.; Theodorou, A.; Katsanos, A.H.; Triantafyllou, S.; Zouvelou, V.; Lachanis, S.; et al. Delayed Leucoencephalopathy as a Complication after Endovascular Therapy of Intracranial Aneurysms—A Case Series. J. Clin. Med. 2023, 12, 496. https://doi.org/10.3390/jcm12020496
Bakola E, Papagiannopoulou G, Palaiodimou L, Lagios K, Archontakis E, Theodorou A, Katsanos AH, Triantafyllou S, Zouvelou V, Lachanis S, et al. Delayed Leucoencephalopathy as a Complication after Endovascular Therapy of Intracranial Aneurysms—A Case Series. Journal of Clinical Medicine. 2023; 12(2):496. https://doi.org/10.3390/jcm12020496
Chicago/Turabian StyleBakola, Eleni, Georgia Papagiannopoulou, Lina Palaiodimou, Konstantinos Lagios, Eftychios Archontakis, Aikaterini Theodorou, Aristeidis H. Katsanos, Sokratis Triantafyllou, Vasiliki Zouvelou, Stefanos Lachanis, and et al. 2023. "Delayed Leucoencephalopathy as a Complication after Endovascular Therapy of Intracranial Aneurysms—A Case Series" Journal of Clinical Medicine 12, no. 2: 496. https://doi.org/10.3390/jcm12020496
APA StyleBakola, E., Papagiannopoulou, G., Palaiodimou, L., Lagios, K., Archontakis, E., Theodorou, A., Katsanos, A. H., Triantafyllou, S., Zouvelou, V., Lachanis, S., Tzanetakos, D., Tzartos, J. S., Giannopoulos, S., & Tsivgoulis, G. (2023). Delayed Leucoencephalopathy as a Complication after Endovascular Therapy of Intracranial Aneurysms—A Case Series. Journal of Clinical Medicine, 12(2), 496. https://doi.org/10.3390/jcm12020496









