Development and Internal Validation of a Prediction Model for Surgical Success of Maxillomandibular Advancement for the Treatment of Moderate to Severe Obstructive Sleep Apnea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants Enrolment
2.2. Treatment Protocol
2.3. Predictors
2.3.1. Patient-Related Variables
2.3.2. Respiratory Parameters
2.3.3. Cephalometric Variables
2.3.4. Drug-induced Sleep Endoscopy
2.4. Outcomes
2.5. Statistical Analysis
2.5.1. Missing Data
2.5.2. Development of the Model
Screening of Potential Predictors and Modelling
Shrinkage Factor
Performance of the Prediction Model
Clinical (Added) Values
Score Chart and Line Chart
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Potential Predictors | Number (%) or Mean (±SD) | Surgical Success | Missing Values (n) | ||
|---|---|---|---|---|---|
| Yes (±SD/%) (n = 67) | No (±SD/%) (n = 33) | ||||
| Age (n = 100) | 50.5 (9.9) | 49.3 (9.8) | 53.1 (9.7) | 0 | |
| Gender (n = 100) | Male | 82 (82.0) | 54 (80.6) | 28 (84.8) | 0 |
| Female | 18 (18.0) | 13 (19.4) | 5 (15.2) | ||
| BMI (n = 100) * | 29.7 (27.4–32.2) | 29.7 (27.4–32.4) | 29.8 (28.2–32.00) | 0 | |
| ASA classification score (n = 100) | I | 23 (23.0) | 17 (25.4) | 6 (18.2) | 0 |
| II | 56 (56.0) | 38 (56.7) | 18 (54.5) | ||
| III | 21 (21.0) | 12 (17.9) | 9 (27.3) | ||
| Previous upper airway surgery (n = 100) | Yes | 42 (42.0) | 27 (40.3) | 15 (45.5) | 0 |
| No | 58 (58.0) | 40 (59.7) | 18 (54.5) | ||
| Dentulous (full + partially) (n = 98) | Yes | 81 (82.7) | 54 (83.1) | 27 (81.8) | 2 |
| No | 17 (17.3) | 11 (16.9) | 6 (18.2) | ||
| Polysomnographic variables | |||||
| AHI pre-operative (n = 100) | 52.9 (21.4) | 54.2 (20.9) | 50.3 (22.6) | 0 | |
| Positional dependent OSA (n = 80) | Yes | 34 (42.5) | 22 (43.1) | 12 (41.4) | 20 |
| No | 46 (57.5) | 29 (56.9) | 17 (58.6) | ||
| CAI ≥ 5 events/hour (n = 84) | Yes | 13 (15.5) | 5 (9.1) | 8 (27.6) | 16 |
| No | 71 (84.5) | 50 (90.9) | 21 (72.4) | ||
| CAI (n = 84) | 2.2 (3.5) | 1.4 (2.4) | 3.5 (4.8) | 16 | |
| Cephalometric variables | |||||
| Anterior total face height (n = 82) | 123.5 (8.4) | 122.6 (7.6) | 125.5 (9.6) | 18 | |
| Anterior lower face height (n = 82) | 72.8 (7.4) | 71.9 (7.1) | 74.8 (8.0) | 18 | |
| SPAS (n = 95) | 8.3 (2.9) | 7.7 (2.7) | 9.7 (3.1) | 5 | |
| Presence of maxillomandibular deficiency (n = 82) | Yes | 66 (80.5) | 21 (65.6) | 45 (90) | 18 |
| No | 16 (19.5) | 11 (34.4) | 5 (10) | ||
| DISE variables | |||||
| Concentric collapse velum (n = 64) | Yes | 18 (28.1) | 10 (23.8) | 8 (33.3) | 36 |
| No | 46 (71.9) | 32 (76.2) | 14 (66.7) | ||
| Complete anteroposterior epiglottis collapse (n = 64) | Yes | 12 (18.8) | 8 (19.0) | 4 (18.2) | 36 |
| No | 52 (81.3) | 34 (81.0) | 18 (81.8) | ||
References
- Malhotra, A.; White, D.P. Obstructive sleep apnoea. Lancet 2002, 360, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Finn, L.; Peppard, P.E.; Szklo-Coxe, M.; Austin, D.; Nieto, F.J.; Stubbs, R.; Hla, K.M. Sleep disordered breathing and mortality: Eighteen-year follow-up of the wisconsin sleep cohort. Sleep 2008, 31, 291–292. [Google Scholar]
- Marshall, N.S.; Wong, K.K.H.; Liu, P.Y.; Cullen, S.R.J.; Knuiman, M.W.; Grunstein, R.R. Sleep apnea as an independent risk factor for all-cause mortality: The Busselton Health Study. Sleep 2008, 31, 1079–1085. [Google Scholar] [PubMed] [Green Version]
- Shamsuzzaman, A.S.M.; Gersh, B.J.; Somers, V.K. Obstructive Sleep Apnea: Implications for Cardiac and Vascular Disease. J. Am. Med. Assoc. 2003, 290, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Young, T.; Matthews, C.G.; Weber, S.M.; Woodard, A.R.; Palta, M. Sleep-disordered breathing and neuropsychological deficits: A population-based study. Am. J. Respir. Crit. Care Med. 1997, 156, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.M.; Griffith, K.A.; Nieto, F.J.; O’Connor, G.T.; Walsleben, J.A.; Redline, S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the sleep heart health study. Sleep 2001, 24, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Epstein, L.J.; Kristo, D.; Strollo, P.J.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar]
- Ravesloot, M.J.L.; de Vries, N. Reliable Calculation of the Efficacy of Non-Surgical and Surgical Treatment of Obstructive Sleep Apnea Revisited. Sleep 2011, 34, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Randerath, W.; de Lange, J.; Hedner, J.; Ho, J.P.T.F.; Marklund, M.; Schiza, S.; Steier, J.; Verbraecken, J. Current and novel treatment options for obstructive sleep apnoea. ERJ Open Res. 2022, 8, 00126–02022. [Google Scholar] [CrossRef]
- Zhou, N.; Ho, J.P.T.F.; Huang, Z.; Spijker, R.; de Vries, N.; Aarab, G.; Lobbezoo, F.; Ravesloot, M.J.L.; de Lange, J. Maxillomandibular advancement versus multilevel surgery for treatment of obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 57, 101471. [Google Scholar] [CrossRef] [PubMed]
- Makovey, I.; Shelgikar, A.V.; Stanley, J.J.; Robinson, A.; Aronovich, S. Maxillomandibular Advancement Surgery for Patients Who Are Refractory to Continuous Positive Airway Pressure: Are There Predictors of Success? J. Oral Maxillofac. Surg. 2017, 75, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, J.; Diminutto, M.; Comiti, S.; Pépin, J.L.; Deschaux, C.; Raphaël, B.; Bettega, G. Lateral cephalometric radiography of the upper airways for evaluation of surgical treatment of obstructive sleep apnea syndrome. Rev. Stomatol. Chir. Maxillofac. 2007, 108, 13–20. [Google Scholar] [CrossRef]
- Sher, A.E.; Schechtman, K.B.; Piccirillo, J.F. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996, 19, 156–177. [Google Scholar] [CrossRef] [Green Version]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): The TRIPOD Statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Trauner, R.; Obwegeser, H. The surgical correction of mandibular prognathism and retrognathia with consideration of genioplasty. I. Surgical procedures to correct mandibular prognathism and reshaping of the chin. Oral Surg. Oral Med. Oral Pathol. 1957, 10, 677–689. [Google Scholar] [CrossRef]
- Böckmann, R.; Meyns, J.; Dik, E.; Kessler, P. The Modifications of the Sagittal Ramus Split Osteotomy: A Literature Review. Plast. Reconstr. Surg. Glob. Open 2014, 2, e271. [Google Scholar] [CrossRef]
- Liu, S.Y.C.; Awad, M.; Riley, R.W. Maxillomandibular Advancement: Contemporary Approach at Stanford. Atlas Oral Maxillofac. Surg. Clin. North Am. 2019, 27, 29–36. [Google Scholar] [CrossRef]
- Kuik, K.; Ho, J.P.T.F.; de Ruiter, M.H.T.; Klop, C.; Kleverlaan, C.J.; de Lange, J.; Hoekema, A. Stability of fixation methods in large mandibular advancements after sagittal split ramus osteotomy: An in vitro biomechanical study. Br. J. Oral Maxillofac. Surg. 2021, 59, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Van Ewijk, L.J.; van Riet, T.C.T.; van der Tol, I.G.H.; Ho, J.P.T.F.; Becking, A.G. Power chains as an alternative to steel-wire ligatures in temporary maxillomandibular fixation: A pilot study. Int. J. Oral Maxillofac. Surg. 2022, 51, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Holty, J.-E.C.; Guilleminault, C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2010, 14, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Ho, J.P.T.F.; de Vries, N.; Bosschieter, P.F.N.; Ravesloot, M.J.L.; de Lange, J. Evaluation of drug-induced sleep endoscopy as a tool for selecting patients with obstructive sleep apnea for maxillomandibular advancement. J. Clin. Sleep Med. 2022, 18, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Kloesel, B.; Todd, M.M.; Cole, D.J.; Prielipp, R.C. The Evolution, Current Value, and Future of the American Society of Anesthesiologists Physical Status Classification System. Anesthesiology 2021, 135, 904–919. [Google Scholar] [CrossRef] [PubMed]
- Iber, C.; Chesson, A.Q.S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- American Academy of Sleep Medicine International Classification of Sleep Disorders—Third Edition (ICSD-3); American Academy of Sleep Medicine: Chicago, IL, USA, 2015.
- Oksenberg, A.; Silverberg, D.S.; Arons, E.; Radwan, H. Positional vs Nonpositional Obstructive Sleep Apnea Patients: Anthropomorphic, Nocturnal Polysomnographic and Multiple Sleep Latency Test Data. Chest 1997, 112, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Livas, C.; Delli, K.; Spijkervet, F.K.L.; Vissink, A.; Dijkstra, P.U. Concurrent validity and reliability of cephalometric analysis using smartphone apps and computer software. Angle Orthod. 2019, 89, 889. [Google Scholar] [CrossRef] [Green Version]
- Dantas, J.F.C.; de Carvalho, S.H.G.; Oliveira, L.S.D.A.F.; Barbosa, D.B.M.; de Souza, R.F.; Sarmento, V.A. Accuracy of Two Cephalometric Analyses in the Treatment of Patients with Skeletal Class III Malocclusion. Braz. Dent. J. 2015, 26, 186–192. [Google Scholar] [CrossRef]
- Hair, J.F.J.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate Data Analysis, 3rd ed.; Macmillan: New York, NY, USA, 1995. [Google Scholar]
- Pavlou, M.; Ambler, G.; Seaman, S.R.; Guttmann, O.; Elliott, P.; King, M.; Omar, R.Z. How to develop a more accurate risk prediction model when there are few events. BMJ 2015, 351, h3868. [Google Scholar] [CrossRef] [Green Version]
- Steyerberg, E.W.; Harrell, F.E.; Borsboom, G.J.J.M.; Eijkemans, M.J.C.; Vergouwe, Y.; Habbema, J.D.F. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol. 2001, 54, 774–781. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siregar, S.; Groenwold, R.H.H.; de Heer, F.; Bots, M.L.; van der Graaf, Y.; van Herwerden, L.A. Performance of the original EuroSCORE. Eur. J. Cardiothorac. Surg. 2012, 41, 746–754. [Google Scholar] [CrossRef]
- Debray, T.P.A.; Damen, J.A.A.G.; Snell, K.I.E.; Ensor, J.; Hooft, L.; Reitsma, J.B.; Riley, R.D.; Moons, K.G.M. A guide to systematic review and meta-analysis of prediction model performance. BMJ 2017, 356, 6460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Assessing the Fit of the Model. In Applied Logistic Regression, 2nd ed.; Wiley: Hoboken, NJ, USA, 2000; pp. 143–202. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/0471722146.ch5 (accessed on 3 October 2022).
- Ho, J.P.T.F.; Zhou, N.; Verbraecken, J.; de Vries, N.; de Lange, J. Central and mixed sleep apnea related to patients treated with maxillomandibular advancement for obstructive sleep apnea: A retrospective cohort study. J. Cranio Maxillofac. Surg. 2022, 50, 537–542. [Google Scholar] [CrossRef]
- Xie, A.; Bedekar, A.; Skatrud, J.B.; Teodorescu, M.; Gong, Y.; Dempsey, J.A. The heterogeneity of obstructive sleep apnea (predominant obstructive vs pure obstructive apnea). Sleep 2011, 34, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, B.C.; Kharbanda, O.P.; Sardana, H.K.; Balachandran, R.; Sardana, V.; Kapoor, P.; Gupta, A.; Vasamsetti, S. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med. Rev. 2017, 31, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, R.R.; Masterson, D.; Trindade Mattos, C.; de Vasconcellos Vilella, O. Facial growth direction after surgical intervention to relieve mouth breathing: A systematic review and meta-analysis. J. Orofac. Orthop. 2018, 79, 412–426. [Google Scholar] [CrossRef]
- Van der Heijden, G.J.M.G.; Donders, A.R.T.; Stijnen, T.; Moons, K.G.M. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: A clinical example. J. Clin. Epidemiol. 2006, 59, 1102–1109. [Google Scholar] [CrossRef]
- Ogundimu, E.O.; Altman, D.G.; Collins, G.S. Adequate sample size for developing prediction models is not simply related to events per variable. J. Clin. Epidemiol. 2016, 76, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstem, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.B.; Walters, A.S.; Waite, P.; Harding, S.M.; Song, Y. Long-Term Effectiveness and Safety of Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 699. [Google Scholar] [CrossRef] [PubMed]
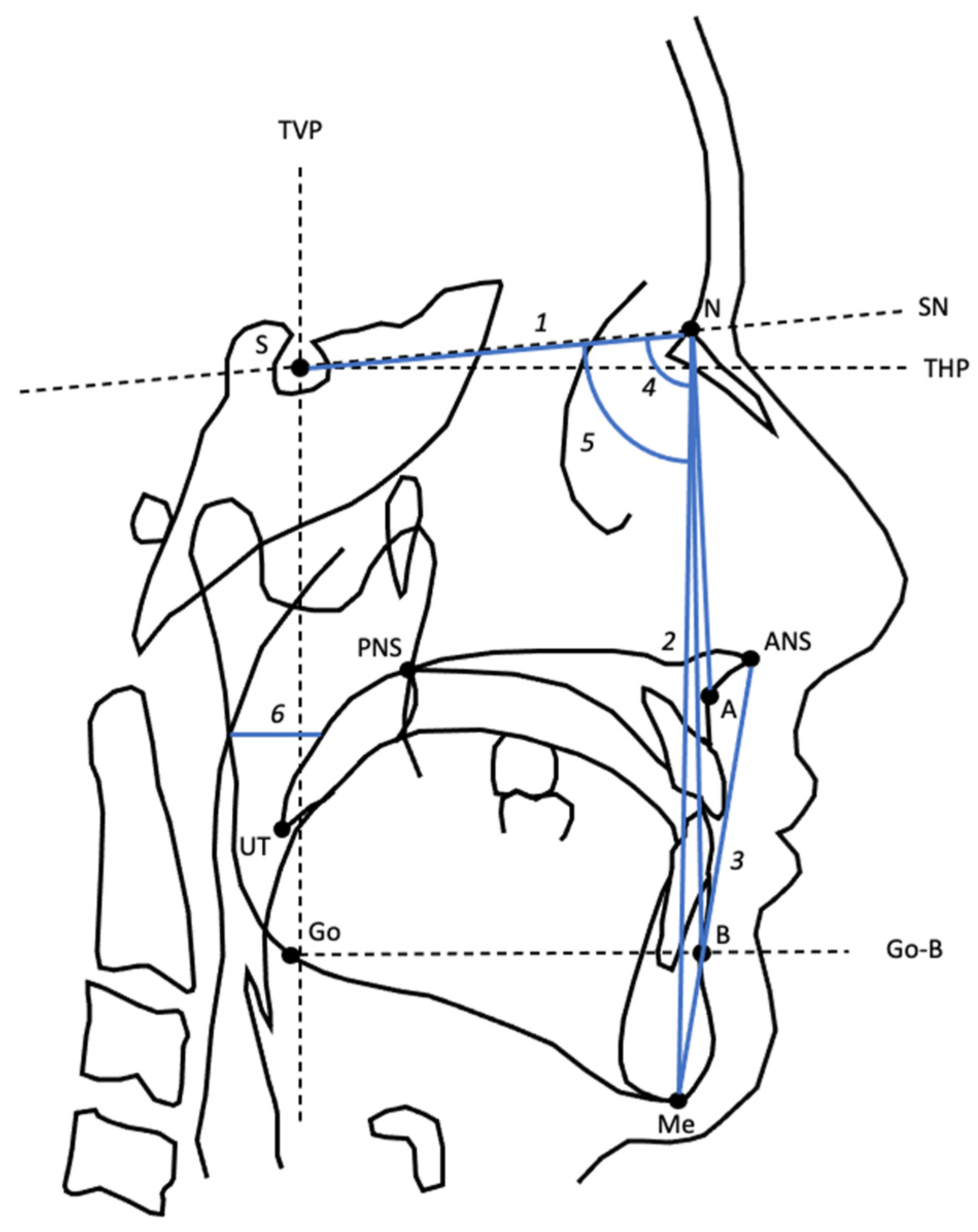
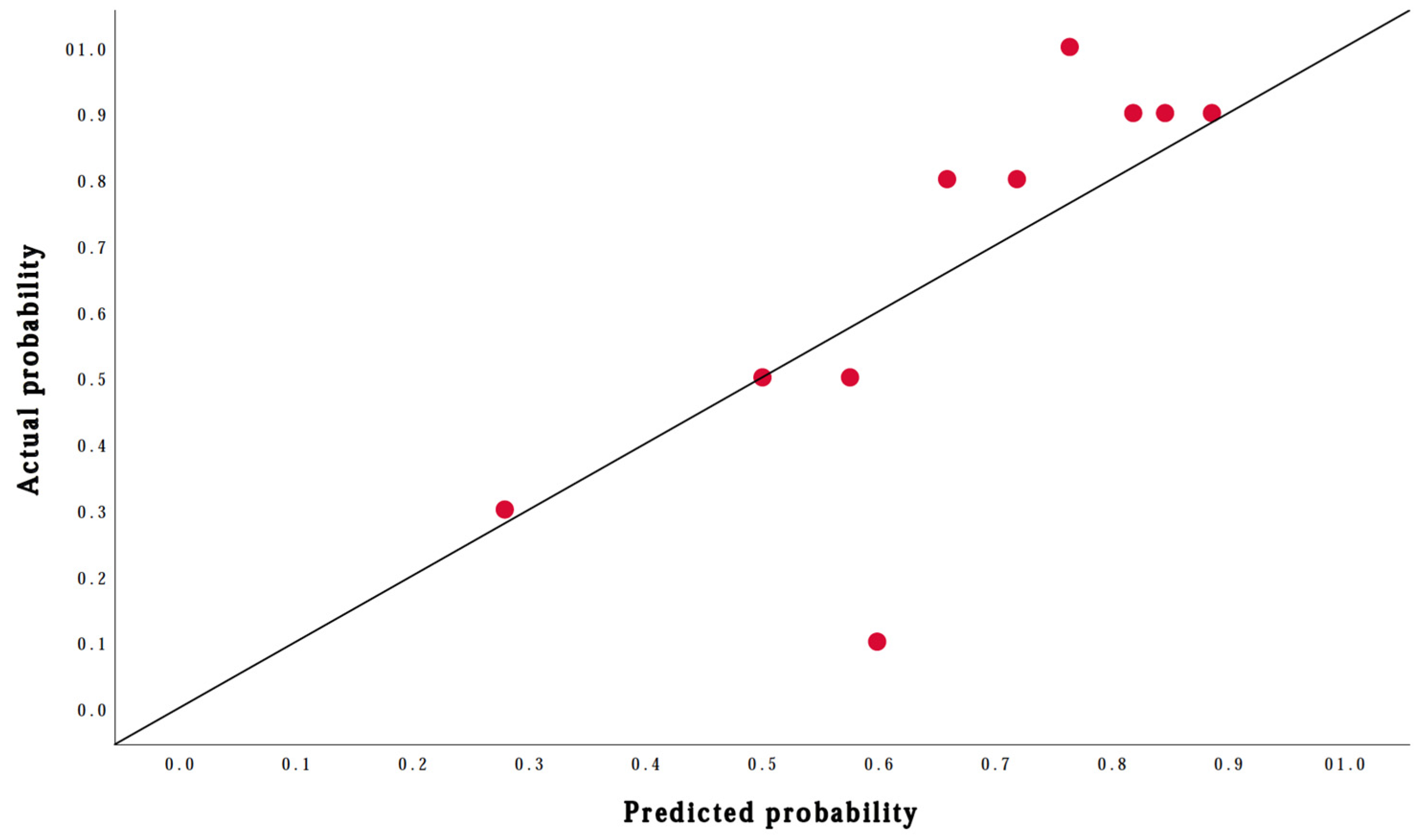
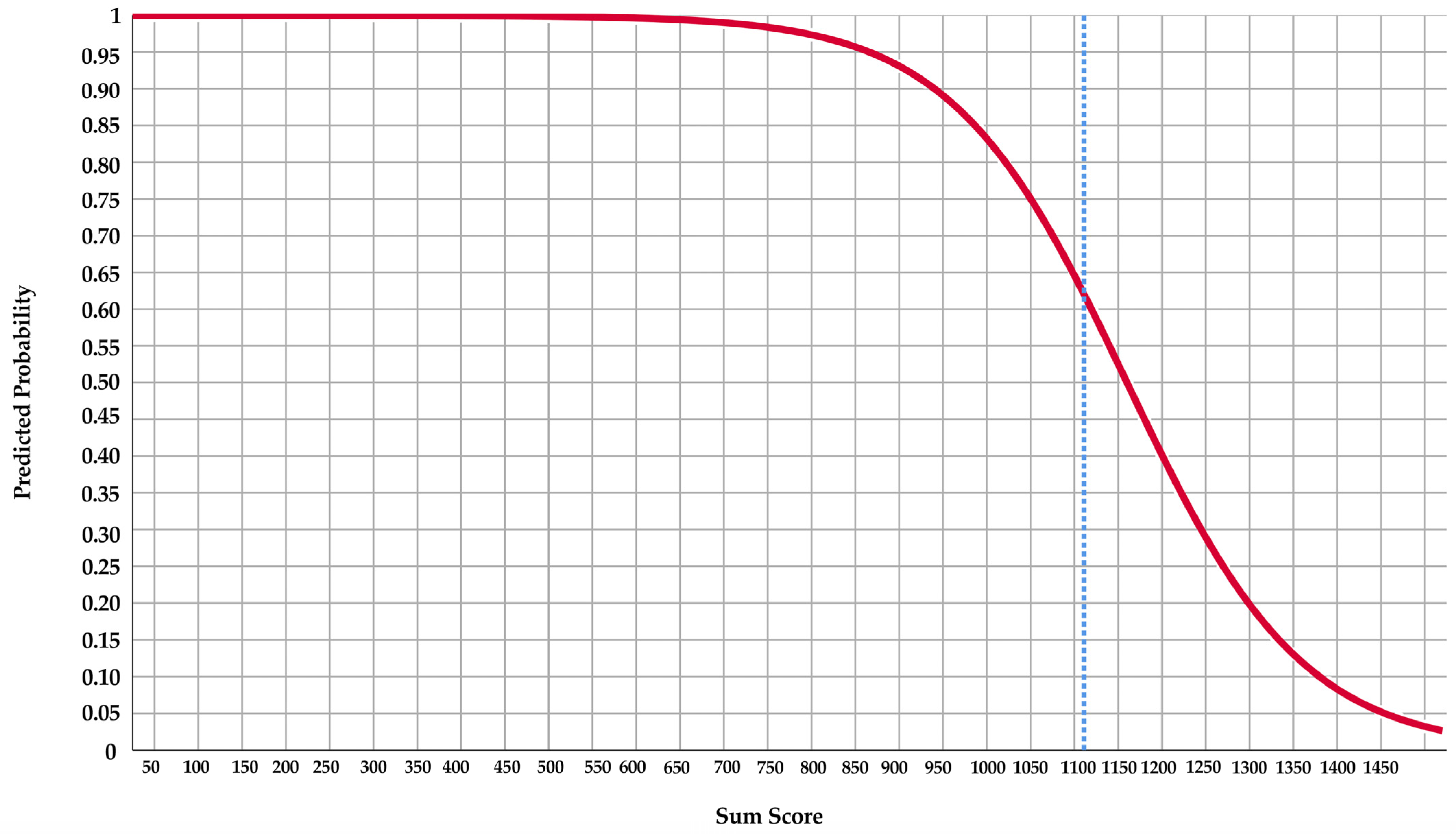
| Variable | Definition |
|---|---|
| S-N | Distance between S and N |
| ATFH | Distance between N and Me |
| ALFH | Distance between ANS and Me |
| SNA | Angle from S to N to A |
| SNB | Angle from S to N to B |
| SPAS | Width of the posterior airway at the level of the midpoint of UT and PNS, parallel to line Go-B. |
| Potential Predictors (n = 100) | Number (%) or Mean (±SD) | Surgical Success | ||
|---|---|---|---|---|
| Yes (±SD/%) (n = 67) | No (±SD/%) (n = 33) | |||
| Age | 50.5 (9.9) | 49.3 (9.8) | 53.1 (9.7) | |
| Gender | Male | 82 (82.0) | 54 (80.6) | 28 (84.8) |
| Female | 18 (18.0) | 13 (19.4) | 5 (15.2) | |
| BMI * | 29.7 (27.4–32.2) | 29.7 (27.4–32.4) | 29.8 (28.2–32.0) | |
| ASA classification score | I | 23 (23.0) | 17 (25.4) | 6 (18.2) |
| II | 56 (56.0) | 38 (56.7) | 18 (54.5) | |
| III | 21 (21.0) | 12 (17.9) | 9 (27.3) | |
| Previous upper airway surgery | Yes | 42 (42.0) | 27 (40.3) | 15 (45.5) |
| No | 58 (58.0) | 40 (59.7) | 18 (54.5) | |
| Dentulous (full + partially) ** | Yes | 82.6 (82.6) | 55.6 (83.0) | 27 (81.8) |
| No | 17.4 (17.4) | 11.4 (17) | 6 (18.2) | |
| Polysomnographic variables | ||||
| AHI pre-operative | 52.9 (21.4) | 54.2 (20.9) | 50.3 (22.6) | |
| Positional dependent OSA ** | Yes | 43.9 (43.9) | 29.5 (44) | 14.5 (43.9) |
| No | 56.1 (56.1) | 37.5 (66) | 18.5 (56.1) | |
| CAI ≥ 5 events/hour ** | Yes | 16 (16) | 7 (10.4) | 9 (27.3) |
| No | 84 (84) | 60 (89.6) | 24 (72.7) | |
| Cephalometric variables | ||||
| Anterior total face height ** | 123.9 (8.3) | 122.8 (7.7) | 126.1 (9.2) | |
| Anterior lower face height ** | 73.0 (7.4) | 72.0 (7.2) | 75.0 (7.7) | |
| SPAS ** | 8.3 (2.9) | 7.7 (2.7) | 9.6 (3.3) | |
| Presence of maxillomandibular deficiency ** | Yes | 75.4 (75.4) | 50.5 (75.4) | 24.9 (75.5) |
| No | 24.6 (24.6) | 16.5 (24.6) | 8.1 (24.5) | |
| DISE variables | ||||
| Concentric collapse velum ** | Yes | 30.5 (30.5) | 17.7 (26.4) | 12.9 (39.1) |
| No | 69.5 (69.5) | 49.3 (73.6) | 20.1 (60.9) | |
| Complete anteroposterior epiglottis collapse ** | Yes | 24.2 (24.2) | 15.9 (23.7) | 8.3 (25.2) |
| No | 75.8 (75.8) | 51.1 (76.3) | 24.7 (74.8) | |
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | Coding | B (SE) | OR (95%CI) | p-Value | B (SE) | Shrunken B | OR (95%CI) | p-Value |
| Intercept | 14.258 (5.082) | 11.6005 | <0.01 | |||||
| Age | −0.041 (0.023) | 0.959 (0.917–1.003) | 0.070 | −0.041 (0.027) | −0.033 | 0.96 (0.91–1.01) | 0.13 | |
| Gender | Female Male | Ref. −0.299 (0.575) | 0.742 (0.240–2.291) | 0.604 | ||||
| BMI | −0.004 (0.051) | 0.996 (0.901–1.101) | 0.941 | |||||
| ASA classification score | I II III | Ref. −0.294 (0.554) −0.754 (0.648) | 0.745 (0.251–2.209) 0.471 (0.132–1.676) | 0.596 0.245 | ||||
| Previous upper airway surgery | No Yes | Ref. −0.211 (0.429) | 0.810 (0.349–1.879) | 0.623 | ||||
| Dentulous (full + partially) | No Yes | Ref. 0.082 (0.560) | 1.085 (0.362–3.252) | 0.884 | ||||
| AHI pre-operative | 0.009 (0.010) | 1.009 (0.989–1.029) | 0.389 | |||||
| Positional dependent OSA | No Yes | Ref. 0.002 (0.451) | 1.002 (0.414–2.428) | 0.996 | ||||
| CAI ≥ 5 events/hour | No Yes | Ref. −1.185 (0.636) | 0.306 (0.088–1.065) | 0.063 | Ref. −1.830 (0.865) | −1.473 | 0.16 (0.03–0.91) | 0.04 |
| Anterior total face height | −0.048 (0.028) | 0.953 (0.901–1.008) | 0.091 | |||||
| Anterior lower face height | −0.056 (0.032) | 0.945 (0.888–1.006) | 0.075 | −0.071 (0.036) | −0.057 | 0.93 (0.87–1.00) | 0.05 | |
| SPAS | −0.235 (0.083) | 0.791 (0.672–0.931) | 0.005 | −0.280 (0.099) | −0.225 | 0.76 (0.62–0.92) | 0.01 | |
| Presence of maxillomandibular deficiency | No Yes | Ref. −0.016 (0.558) | 0.984 (0.329–2.945) | 0.978 | ||||
| Concentric collapse velum | No Yes | Ref. −0.587 (0.535) | 0.556 (0.194–1.591) | 0.273 | ||||
| Complete anteroposterior epiglottis collapse | No Yes | Ref. −0.050 (0.612) | 0.951 (0.285–3.169) | 0.935 | ||||
| Outcome | Prevalence (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Added Value for Ruling in the Outcome (95% CI) | Added Value for Ruling Out the Outcome (95% CI) |
|---|---|---|---|---|---|---|---|
| Surgical success | 0.67 (0.57–0.76) | 0.79 (0.68–0.88) | 0.79 (0.62–0.90) | 0.88 (0.78–0.95) | 0.65 (0.49–0.79) | 0.21 (0.09–0.34) | 0.32 (0.15–0.49) |
| Predictors | Score | |
|---|---|---|
| Anterior lower face height | 6 | |
| SPAS | 23 | |
| Age | 3 | |
| CAI ≥ 5 events/hour | No | 0 |
| Yes | 147 | |
| Sum score |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visscher, W.P.; Ho, J.-P.T.F.; Zhou, N.; Ravesloot, M.J.L.; Schulten, E.A.J.M.; Lange, J.d.; Su, N. Development and Internal Validation of a Prediction Model for Surgical Success of Maxillomandibular Advancement for the Treatment of Moderate to Severe Obstructive Sleep Apnea. J. Clin. Med. 2023, 12, 503. https://doi.org/10.3390/jcm12020503
Visscher WP, Ho J-PTF, Zhou N, Ravesloot MJL, Schulten EAJM, Lange Jd, Su N. Development and Internal Validation of a Prediction Model for Surgical Success of Maxillomandibular Advancement for the Treatment of Moderate to Severe Obstructive Sleep Apnea. Journal of Clinical Medicine. 2023; 12(2):503. https://doi.org/10.3390/jcm12020503
Chicago/Turabian StyleVisscher, Wouter P., Jean-Pierre T. F. Ho, Ning Zhou, Madeline J. L. Ravesloot, Engelbert A. J. M. Schulten, Jan de Lange, and Naichuan Su. 2023. "Development and Internal Validation of a Prediction Model for Surgical Success of Maxillomandibular Advancement for the Treatment of Moderate to Severe Obstructive Sleep Apnea" Journal of Clinical Medicine 12, no. 2: 503. https://doi.org/10.3390/jcm12020503






