Handheld Ultrasound or Conventional Ultrasound Devices in Patients Undergoing HCT: A Validation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. HHUS Device
2.3. Conventional Device
2.4. Operators
2.5. Time Points of Validation
2.6. Parameters Considered in the Ultrasound Examination
2.7. Patients
2.8. Statistical Analysis
3. Results
3.1. Clinical and Laboratory Features
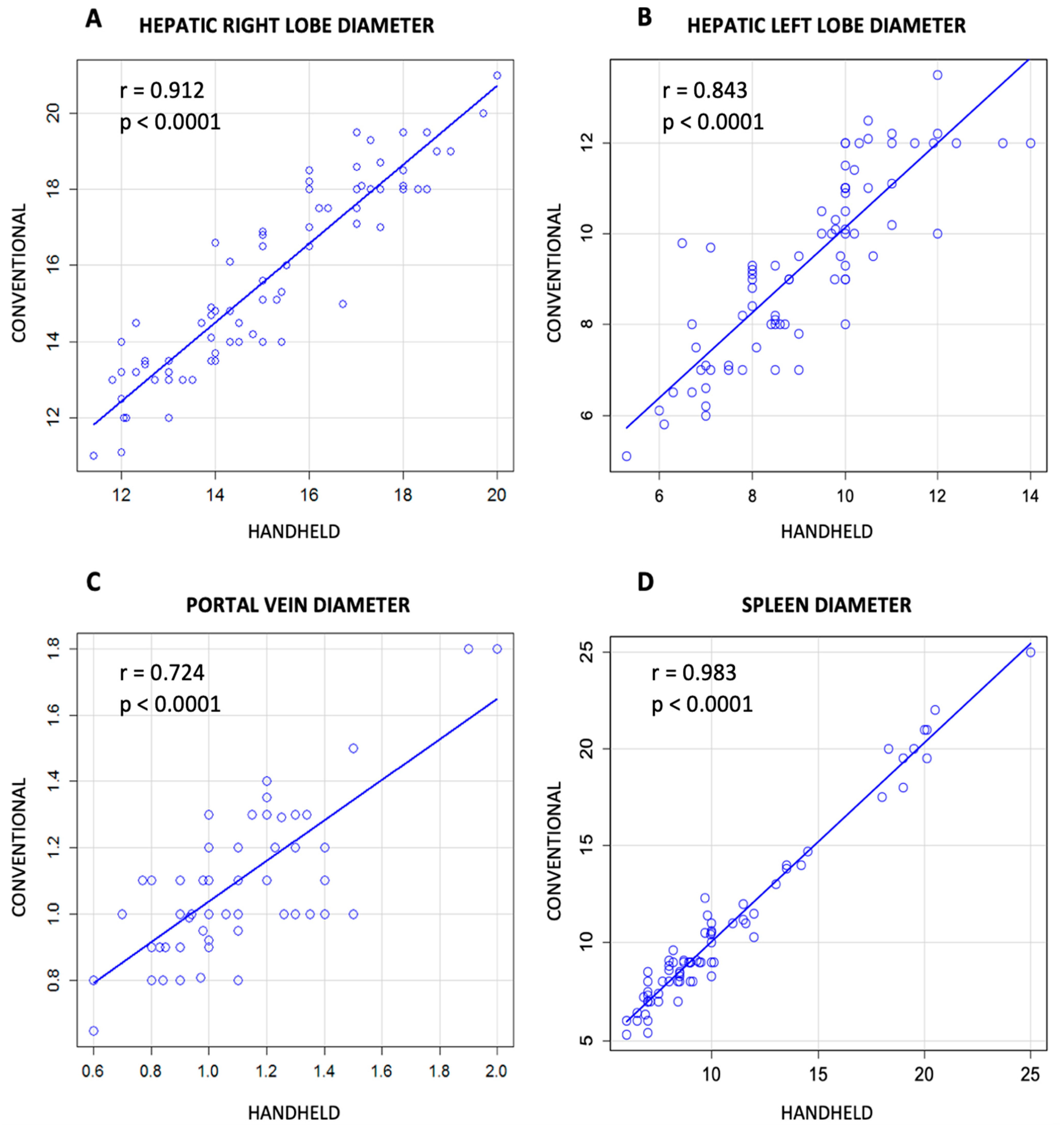
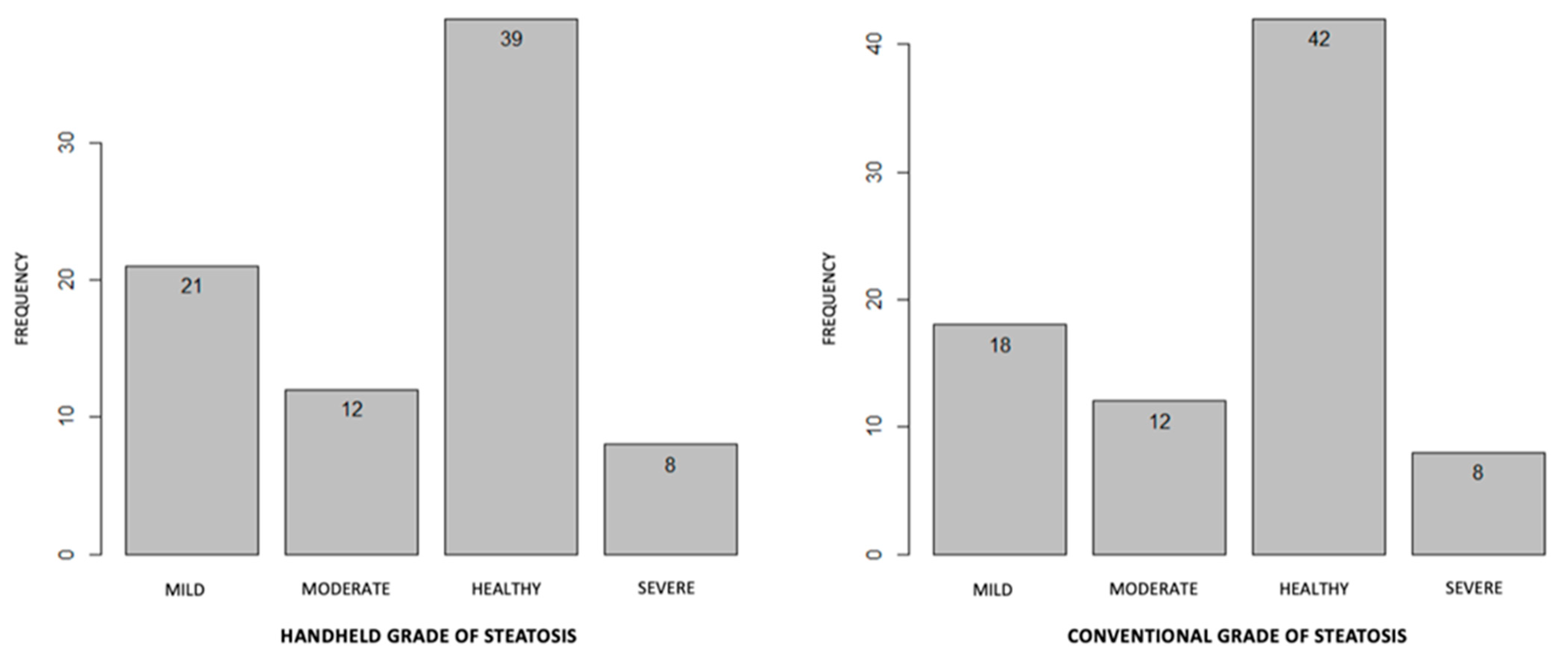
3.2. Comparison between Conventional and Handheld Ultrasound Measurement
3.3. HHUS in the Diagnosis of Other Radiologic Abnormalities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shulman, H.M.; McDonald, G.B.; Matthews, D.; Doney, K.C.; Kopecky, K.J.; Gauvreau, J.M.; Thomas, E.D. An analysis of hepatic veno-occlusive disease and centrilobular hepatic degeneration following bone marrow transplantation. Gastroenterology 1980, 79, 1178–1191. [Google Scholar] [CrossRef]
- McDonald, G.B. Hepatobiliary Complications of Hematopoietic Cell Transplantation, 40 Years On. Hepatology 2010, 51, 1450–1460. [Google Scholar] [CrossRef] [Green Version]
- Carreras, E.; Bertz, H.; Arcese, W.; Vernant, J.-P.; Tomás, J.-F.; Hagglund, H.; Bandini, G.; Esperou, H.; Russell, J.; de la Rubia, J.; et al. Incidence and Outcome of Hepatic Veno-Occlusive Disease After Blood or Marrow Transplantation: A Prospective Cohort Study of the European Group for Blood and Marrow Transplantation. Blood 1998, 92, 3599–3604. [Google Scholar] [CrossRef]
- Jones, R.J.; Lee, K.S.K.; Beschorner, W.E.; Vogel, V.G.; Grochow, L.B.; Braine, H.G.; Vogelsang, G.B.; Sensenbrenner, L.L.; Santos, G.W.; Saral, R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987, 44, 778–783. [Google Scholar] [CrossRef]
- Kernan, N.A.; Grupp, S.; Smith, A.R.; Arai, S.; Triplett, B.; Antin, J.H.; Lehmann, L.; Shore, T.; Ho, V.T.; Bunin, N.; et al. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br. J. Haematol. 2018, 181, 816–827. [Google Scholar] [CrossRef]
- Corbacioglu, S.; Greil, J.; Peters, C.; Wulffraat, N.M.; Laws, H.J.; Dilloo, D.; Strahm, B.; Gross-Wieltsch, U.; Sykora, K.W.; Ridolfi-Lüthy, A.; et al. Defibrotide in the treatment of children with veno-occlusive disease (VOD): A retrospective multicentre study demonstrates therapeutic efficacy upon early intervention. Bone Marrow Transplant. 2003, 33, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, G.B.; Hinds, M.S.; Fisher, L.D.; Schoch, H.G.; Wolford, J.L.; Banaji, M.; Hardin, B.J.; Shulman, H.M.; Clift, R.A. Veno-occlusive Disease of the Liver and Multiorgan Failure after Bone Marrow Transplantation: A Cohort Study of 355 Patients. Ann. Intern. Med. 1993, 118, 255–267. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.B.; Sharma, P.; Matthews, D.E.; Shulman, H.M.; Thomas, E.D. Veno-occlusive disease of the liver after bone marrow transplantation: Diagnosis, incidence, and predisposing factors. Hepatology 1984, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Malard, F.; Abecassis, M.; Aerts, E.; Alaskar, A.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016, 51, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Carreras, E.; Grañena, A.; Navasa, M.; Bruguera, M.; Marco, V.; Sierra, J.; Tassies, M.D.; García-Pagán, J.C.; Martí, J.M.; Bosch, J.; et al. On the reliability of clinical criteria for the diagnosis of hepatic veno-occlusive disease. Ann. Hematol. 1993, 66, 77–80. [Google Scholar] [CrossRef]
- Lassau, N.; Leclère, J.; Auperin, A.; Bourhis, J.H.; Hartmann, O.; Valteau-Couanet, D.; Benhamou, E.; Bosq, J.; Ibrahim, A.; Girinski, T.; et al. Hepatic veno-occlusive disease after myeloablative treatment and bone marrow transplantation: Value of gray-scale and Doppler US in 100 patients. Radiology 1997, 204, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Dignan, F.L.; Wynn, R.; Hadzic, N.; Karani, J.; Quaglia, A.; Pagliuca, A.; Veys, P.; Potter, M.N. Haemato-Oncology Task The Haemato-oncology Task Force of the British Committee for Standards in Haematology and the British Society for Blood and Marrow Transplantation BCSH/BSBMT guideline: Diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br. J. Haematol. 2013, 163, 444–457. [Google Scholar] [PubMed]
- Nishida, M.; Kahata, K.; Hayase, E.; Shigematsu, A.; Sato, M.; Kudo, Y.; Omotehara, S.; Iwai, T.; Sugita, J.; Shibuya, H.; et al. Novel Ultrasonographic Scoring System of Sinusoidal Obstruction Syndrome after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 1896–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mjolstad, O.C.; Dalen, H.; Graven, T.; Kleinau, J.O.; Salvesen, O.; Haugen, B.O. Routinely adding ultrasound examinations by pocket-sized ultrasound devices improves inpatient diagnostics in a medical department. Eur. J. Intern. Med. 2012, 23, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Andersen, G.N.; Graven, T.; Skjetne, K.; Mjølstad, O.C.; Kleinau, J.O.; Olsen, Ø.; Haugen, B.O.; Dalen, H. Diagnostic Influence of Routine Point-of-Care Pocket-size Ultrasound Examinations Performed by Medical Residents. J. Ultrasound Med. 2015, 34, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Stock, K.F.; Klein, B.; Steubl, D.; Lersch, C.; Heemann, U.; Wagenpfeil, S.; Eyer, F.; Clevert, D.A. Comparison of a pocket-size ultrasound device with a premium ultrasound machine: Diagnostic value and time required in bedside ultrasound examination. Abdom. Imaging 2015, 40, 2861–2866. [Google Scholar] [CrossRef] [PubMed]
- Rykkje, A.; Carlsen, J.F.; Nielsen, M.B. Hand-Held Ultrasound Devices Compared with High-End Ultrasound Systems: A Systematic Review. Diagnostics 2019, 9, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashiguchi, M.; Okamura, T.; Yoshimoto, K.; Ono, N.; Imamura, R.; Yakushiji, K.; Ogata, H.; Seki, R.; Otsubo, K.; Oku, E.; et al. Demonstration of reversed flow in segmental branches of the portal vein with hand-held color Doppler ultrasonography after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005, 36, 1071–1075. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, K.; Yakushiji, K.; Ijuin, H.; Ono, N.; Hashiguchi, M.; Imamura, R.; Ogata, H.; Okamura, T.; Sata, M.; Hashimoto, H. Colour Doppler ultrasonography of a segmental branch of the portal vein is useful for early diagnosis and monitoring of the therapeutic course of veno-occlusive disease after allogenic haematopoietic stem cell transplantation. Br. J. Haematol. 2001, 115, 945–948. [Google Scholar] [CrossRef]
- Chan, S.S.; Colecchia, A.; Duarte, R.F.; Bonifazi, F.; Ravaioli, F.; Bourhis, J.H. Imaging in Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol. Blood Marrow Transplant. 2020, 26, 1770–1779. [Google Scholar] [CrossRef]
- Ferraioli, G.; Monteiro, L.B.S. Ultrasound-based techniques for the diagnosis of liver steatosis. World J. Gastroenterol. 2019, 25, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Corbacioglu, S.; Kernan, N.A.; Pagliuca, A.; Ryan, R.J.; Tappe, W.; Richardson, P.G. Incidence of Anicteric Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome and Outcomes with Defibrotide following Hematopoietic Cell Transplantation in Adult and Pediatric Patients. Biol. Blood Marrow Transplant. 2020, 26, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- Carreras, E.; Díaz-Beyá, M.; Rosiñol, L.; Martínez, C.; Fernández-Avilés, F.; Rovira, M. The Incidence of Veno-Occlusive Disease Following Allogeneic Hematopoietic Stem Cell Transplantation Has Diminished and the Outcome Improved over the Last Decade. Biol. Blood Marrow Transplant. 2011, 17, 1713–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazarbachi, A.H.; Al Hamed, R.; Labopin, M.; Halaburda, K.; Labussiere, H.; Bernasconi, P.; Schroyens, W.; Gandemer, V.; Schaap, N.P.M.; Loschi, M.; et al. Underdiagnosed veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) as a major cause of multi-organ failure in acute leukemia transplant patients: An analysis from the EBMT Acute Leukemia Working Party. Bone Marrow Transplant. 2020, 56, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Teefey, S.A.; Brink, J.A.; Borson, R.A.; Middleton, W.D. Diagnosis of venoocclusive disease of the liver after bone marrow transplantation: Value of duplex sonography. Am. J. Roentgenol. 1995, 164, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Schlaweck, S.; Bauer, C.J.; Schmitz, F.; Brossart, P.; Holderried, T.A.W.; Schäfer, V.S. Preliminary Report for the Development of a Multiparameter Protocol for the Identification of Sinusoidal Obstruction Syndrome including Abdominal Ultrasound before and after Allogeneic Stem Cell Transplantation. Appl. Sci. 2022, 12, 829. [Google Scholar] [CrossRef]
- Lassau, N.; Auperin, A.; Leclere, J.; Bennaceur, A.; Valteau-Couanet, D.; Hartmann, O. Prognostic value of doppler-ultrasonography in hepatic veno-occlusive disease. Transplantation 2002, 74, 60–66. [Google Scholar] [CrossRef]
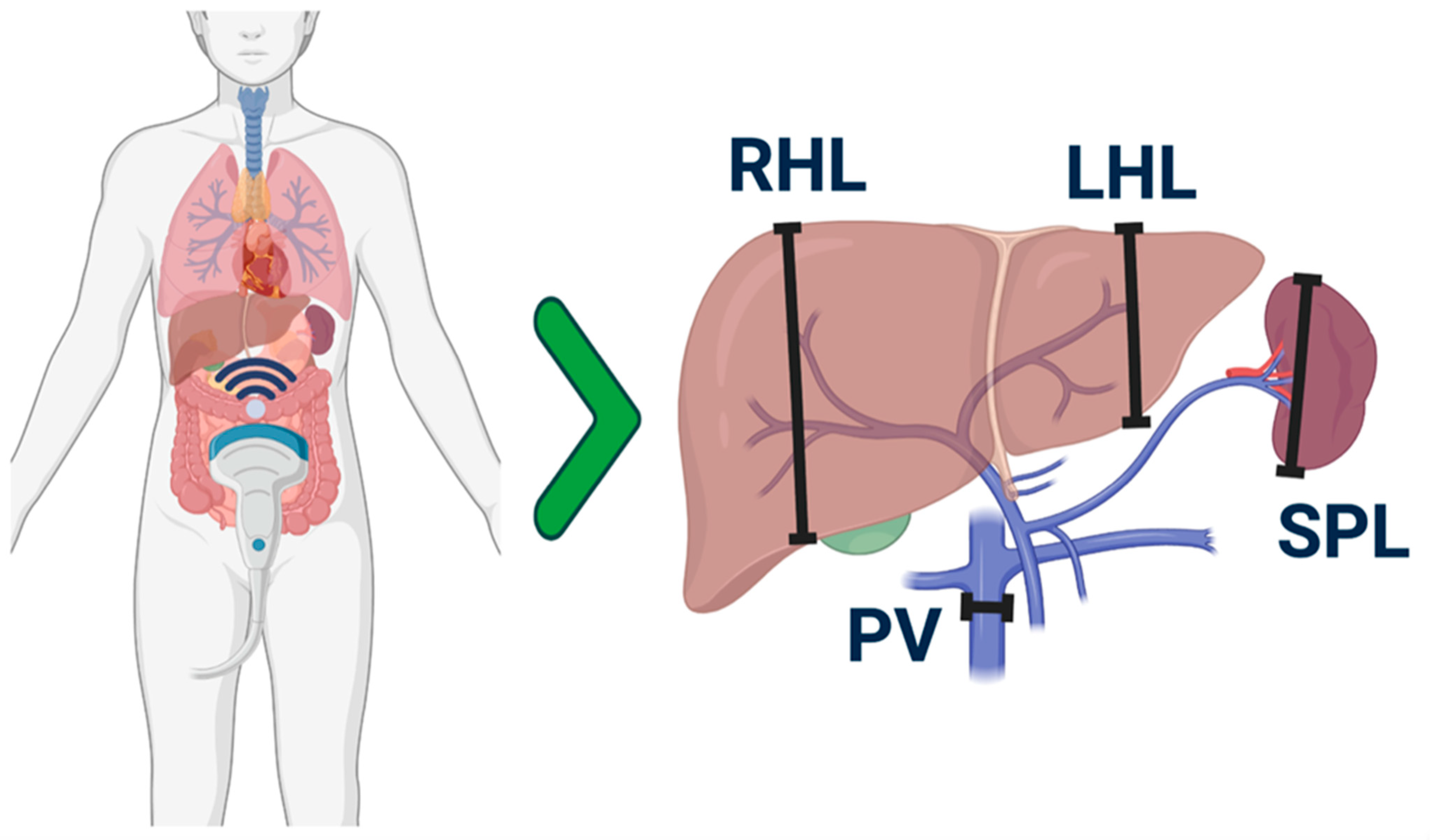
| HokUS-10 Parameters | Definition Threshold |
|---|---|
| Hepatic left lobe vertical diameter | ≥70 mm |
| Hepatic right lobe vertical diameter | ≥110 mm |
| Gallbladder wall thickening | ≥6 mm |
| PV diameter | ≥12 mm |
| PUV diameter | ≥2 mm |
| Amount of ascites | Moderate to severe |
| PV mean velocity | ≤2 mm |
| Direction of PV flow | Congestion or hepatofugal |
| Appearance of PUV blood flow signal | Yes |
| Hepatic artery Resistive Index | ≥7.5 |
| Further parameters analyzed in this study | |
| Spleen size | |
| Hepatic lesions | |
| Spleens lesions | |
| Steatosis’ grade | |
| Age (Years) | Mean (Range) | 52.5 (23–68) |
| Sex, males/females | N (%) | 7 (41.2)/10 (58.8) |
Hematological disease
| N (%) | 8 (47.1) 2 (11.7) 3 (17.7) 3 (17.7) 1 (5.8) |
Refined DRI
| N (%) | 10 (58.8) 4 (23.5) 3 (17.7) |
Status disease
| N (%) | 12 (70.6) 2 (11.7) 3 (17.7) |
Transplant type
| N (%) | 9 (52.9) 8 (47.1) |
Cells source
| N (%) | 14 (82.3) 3 (17.7) |
HLA match
| N (%) | 1 (5.8) 13 (76.5) 3 (17.7) |
Conditioning regimen
| N (%) | 17 (100) 0 (0) |
HCT-CI
| N (%) | 14 (82.3) 3 (17.7) |
| ATG use, yes/no | N (%) | 6 (35.3) /11 (64.7) |
Karnofsky Performance Score
| N (%) | 17 (100) 0 (0) |
| Basal Bilirubin (mg/dL) | Mean (range) | 0.56 (0.3–1.15) |
| Basal Creatinine (mg/dL) | Mean (range) | 0.72 (0.32–1.18) |
| HokUS-10 Parameters | Findings-Conventional US/Handheld US | p-Value | Kappa Statistic for Agreement between the Two Tests | |||
|---|---|---|---|---|---|---|
| Yes/Yes | Yes/No | No/Yes | No/No | |||
| Ascites | 7 | 1 | 1 | 71 | <0.0001 | 0.93 |
| Gallbladder wall thickening | 2 | 0 | 1 | 77 | <0.0009 | 0.79 |
| Appearance of PUV blood flow signal | 0 | 0 | 0 | 80 | <0.0001 | 1.0 |
| Hepatopetus conventional US/ handheld US | Hepatofuge conventional US/ handheld US | |||||
| Direction of PV flow | 80/80 | 0/0 | <0.0001 | 1.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duminuco, A.; Cupri, A.; Massimino, R.; Leotta, S.; Milone, G.A.; Garibaldi, B.; Giuffrida, G.; Garretto, O.; Milone, G. Handheld Ultrasound or Conventional Ultrasound Devices in Patients Undergoing HCT: A Validation Study. J. Clin. Med. 2023, 12, 520. https://doi.org/10.3390/jcm12020520
Duminuco A, Cupri A, Massimino R, Leotta S, Milone GA, Garibaldi B, Giuffrida G, Garretto O, Milone G. Handheld Ultrasound or Conventional Ultrasound Devices in Patients Undergoing HCT: A Validation Study. Journal of Clinical Medicine. 2023; 12(2):520. https://doi.org/10.3390/jcm12020520
Chicago/Turabian StyleDuminuco, Andrea, Alessandra Cupri, Rosario Massimino, Salvatore Leotta, Giulio Antonio Milone, Bruno Garibaldi, Giulia Giuffrida, Orazio Garretto, and Giuseppe Milone. 2023. "Handheld Ultrasound or Conventional Ultrasound Devices in Patients Undergoing HCT: A Validation Study" Journal of Clinical Medicine 12, no. 2: 520. https://doi.org/10.3390/jcm12020520






