Abstract
Patients with cancer are presumed to be vulnerable to an increased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe clinical outcomes due to the immunocompromised state mediated by their underlying malignancies and therapy. The aim of this study was to estimate the SARS-CoV-2 seroprevalence, following second to fourth waves in solid tumour patients attending the Steve Biko Academic Hospital (SBAH) for diagnosis and treatment of cancer. We used the single-prick COVID-19 IgG/IgM Rapid Test Cassettes to detect SARS-CoV-2 IgG/IgM antibodies in 760 patients with solid tumours who were asymptomatic and who had never tested positive for coronavirus disease 2019 (COVID-19). Out of the 760 patients, 277 were male (36.4%), 483 were female (63.6%), and the mean age was 55 years (range 18–92). The estimated total seroprevalence was 33.2%. The seroprevalence status of the COVID-19 IgG/IgM antibodies rose significantly from the second wave (11.3%) to the third (67.38%) and then the fourth (69.81%) waves with roughly similar counts. A significant number of the seropositive patients were asymptomatic to COVID-19 (96%). There was a higher rate of seropositivity in cancer patients with hypertension (p < 0.05). Patients with breast, gynaecologic, and prostate cancers exhibited increased SARS-CoV-2 seropositivity. Although oncology patients may be susceptible to SARS-CoV-2 infection, our data indicate that these patients remained asymptomatic throughout various waves with an overall COVID-19 IgG/IgM antibody seropositivity of 33.16%, suggesting no risk of severe or fatal cases of COVID-19.
1. Introduction
The coronavirus disease of 2019 (COVID-19) is a highly infectious disease that is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. It has caused the current epidemic, and the World Health Organisation (WHO) reports that it is responsible for over 6 million fatalities globally. The initial detection of this virus was in Wuhan, China, around December 2019, and it is now present in approximately 200 countries [1]. SARS-CoV-2 has been reported to have phylogenetic similarities to SARS-CoV-1 which was responsible for the SARS outbreak of 2002–2004 [2]. Infection by SARS-CoV-2 is characterized by rapid human-to-human transmission, and the host immune response plays a crucial role in disease pathogenesis and symptoms [3].
Following the declaration of COVID-19 as a pandemic by the WHO [4], numerous reports have indicated the need for a focus on the impact of this disease on cancer patients. These reports from clinical studies have shown that patients with cancer are more susceptible to COVID-19 than individuals without cancer, due to malignancy and treatments which lead to an immunosuppressive state [5,6]. South Africa was severely affected by the SARS-CoV-2 pandemic waves caused by the original strain of SARS-CoV-2 and three variants of concern (VOCs) (beta, delta, and omicron). Distinctively, South Africa had the initial surge for two of the five VOCs identified to date—namely, beta and omicron [7]. Furthermore, several cancer surveillance systems have been implemented for the collection of cancer data, but underreporting of prevalence and mortalities is still a challenge in South Africa [8]. Some individuals, including cancer patients, may remain asymptomatic following SARS-CoV-2 infection, and they may continue to further spread the infection unknowingly. It is therefore important to estimate the prevalence of SARS-CoV-2 exposure in cancer patients, and its effects on their clinical outcome. This will provide a better understanding of the impact of SARS-CoV-2 on cancer patients, and enable the implementation of effective strategies to lower the risk of becoming infected. Additionally, this will mitigate negative effects of the COVID-19 pandemic on the diagnosis, and offer better cancer care delivery. IgG and IgM antibodies to COVID-19 are usually produced by the body’s immune system to fight infection, and can be detected within 1-3 weeks after exposure. Serologic tests are effective tools for the rapid monitoring of previous infection, by detecting antibodies specific to the virus in individuals who may have been exposed to the virus and who remained asymptomatic or mildly infected. In this study, we employed single-prick COVID-19 IgG/IgM Rapid Test Cassettes (Zheijiang Orient Gene Biotech, China) to trace SARS-CoV-2 previous infection in cancer patients treated at the Steve Biko Academic Hospital (SBAH), South Africa, where no specific study of this kind had been performed previously, according to our knowledge. The sensitivity of the single-prick COVID-19 IgG/IgM Rapid Test is 87.9% for IgM and 97.2 for IgG, as compared to RT-PCR. The specificity for both IgM and IgG antibodies is 100%.
2. Materials and Methods
2.1. The Characterisation and Location of the Study
This is a single-centred prospective and exploratory study conducted in patients visiting Radiation and Medical Oncology departments at the SBAH. The SBAH is a 900-bed quaternary academic hospital that serves as the referral centre providing oncology care for patients from four out of nine provinces in South Africa. Radiation and Medical Oncology departments are responsible for the management of both in- and out-patients with various cancers. They are about a 500 m bridge away from the SBAH complex, a combination of the SBAH and Tshwane District Hospital (TDH) bridge, that was repurposed for the management of COVID-19 patients.
2.2. Patient Population
Cancer patients who are newly diagnosed or receiving treatment for solid tumours at the Radiation and Medical Oncology departments of SBAH were prospectively recruited for this study. From 24 March 2021 to 30 March 2022, following the second to fourth waves of COVID-19, 760 tests were performed in the Radiation and Medical Oncology departments using the single-prick COVID-19 IgG/IgM Rapid Test Cassettes (Zheijiang Orient Gene Biotech, Huzhou, China). The second wave occurred between November 2020 and January 2021, the third wave occurred between May 2021 and September 2021 and the fourth wave occurred between November 2021 and present day. The participants recruited were 760 patients with various solid tumours who were admitted or who visited the hospital for their cancer management. To validate the accuracy of the test, 150 non-cancer patients hospitalised due to COVID-19, with similar underlying co-morbidities as cancer patients, were also tested, and these tests were all seropositive as expected. These patients were recruited from the same hospital for another unpublished study. The study was also approved by the University of Pretoria (Ethics Ref No: 28/2021).
2.3. Protocol for IgG/IgM Antibody Detection
The detection of SARS-CoV-2 IgG/IgM antibodies was carried out using the single-prick COVID-19 IgG/IgM Rapid Test Cassettes according to the manufacturer’s instructions (Zheijiang Orient Gene Biotech, China). This test is specific for detecting SARS-CoV-2 anti-nucleocapsid antibody. It does not detect anti-spike antibody, hence patients who have been vaccinated for COVID-19, who have not been previously infected by the wild virus, do not test positive on the rapid test kit. A test cassette was removed from the sealed foil pouch and used immediately. Briefly, a test cassette was placed on a clean and level surface. The participant’s finger was pricked with a needle and a drop of blood was drawn with a plastic dropper and transferred to the specimen well of a test cassette. Two drops of sample buffer, provided with the kit, were added immediately into the buffer well on a test cassette. The result was ready within 10 min. In the vast majority of tests, positive results became visible within 2 min.
2.4. Statistical Analysis
Statistical calculations were performed using the IBM® SPSS® Statistical Package for the Social Sciences (SPSS) version 28 (IBM, Armonk, NY, USA). A comparison between categorical variables was computed using Fisher’s exact test or Pearson Chi-squared test. A p-value of <0.05 was considered statistically significant. To calculate the seroprevalence, the following formula was used:
The percentage of the total cumulative number of COVID-19 cases and number of people vaccinated with either Johnson & Johnson or Pfizer vaccines, that were reported for both Gauteng Province and nationally (South Africa), was obtained from the daily reports of South African coronavirus latest statistics website on the 5 August 2022 [9]. These data were used to compare seropositivity and vaccination percentage among the population of South Africa, Gauteng Province, and our cancer patients’ data.
Age (<40, 40–69, ≥70 years), gender (female and male), smoking status (yes, no, ex-smoker), co-morbidities such as HIV, hypertension, diabetes, cancer type (breast cancer, colon and rectal, gynaecologic cancer, prostate cancer, and others), and COVID-19 symptoms (yes/no) were evaluated in order to include them in a binary logistic regression analysis model. Because seropositivity is a dependent variable that is dichotomous/binary in nature, basic linear regression could not be applied. Instead, binary logistic regression was used for dichotomous or binary dependent variables. The factors were found to be significant at a relaxed level (p < 0.6). The model’s goodness-of-fit was assessed using the Hosmer–Lemeshow test. Odds ratios (OR) and associated 95% confidence intervals (CI) were generated to determine the degree of association.
3. Results
3.1. General Characteristics
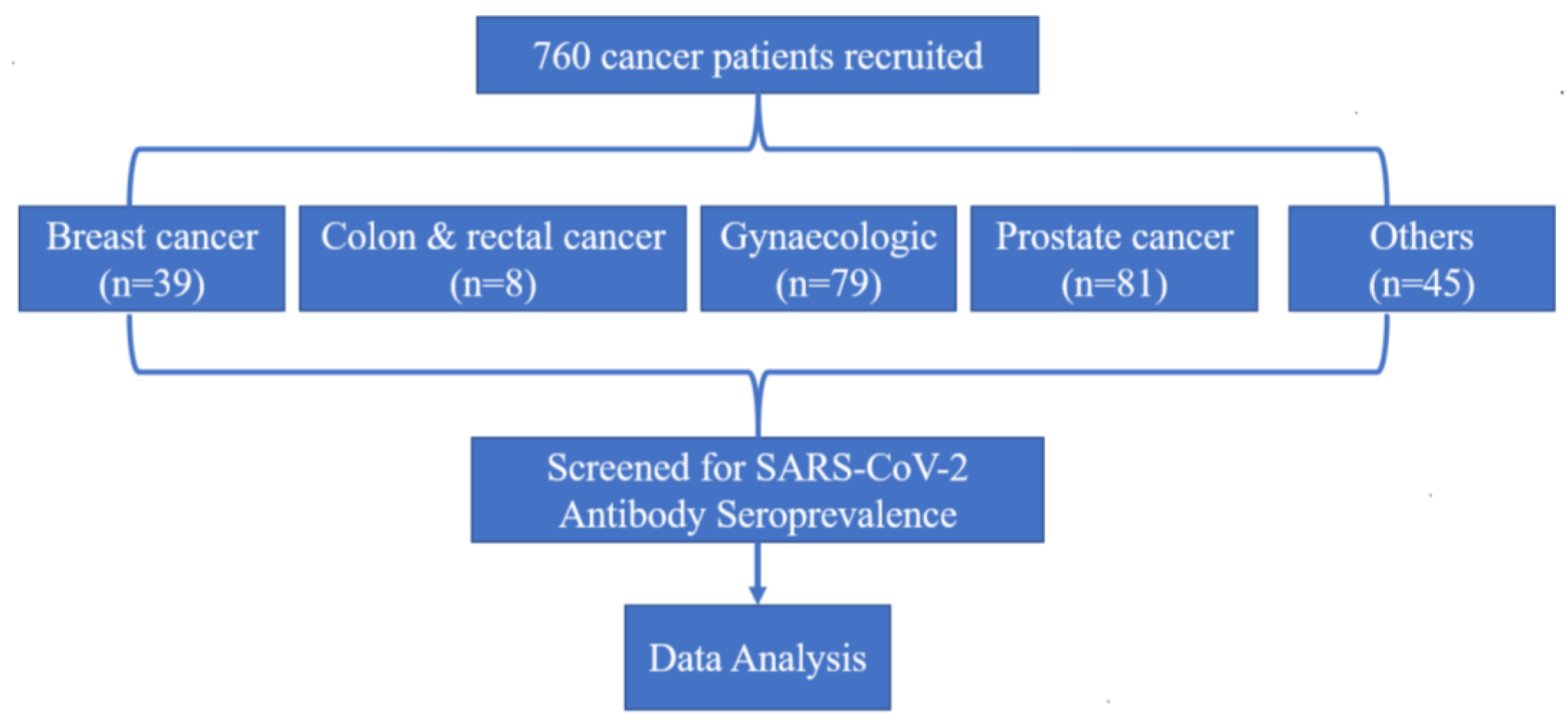
In this study, a total of 760 cancer patients were included (Figure 1). A total of 150 non-cancer patients hospitalised with COVID-19 were also included for the validation of the test, and all of them were COVID-19 IgG/IgM seropositive as expected (data not shown). The ratio of male to female, favoured females, and the average age of the study group was 55 years (range: 18 to 92). Of the 760 patients, 277 (36.4%) of them were male, and 483 (63.6%) were female. There was greater participation of non-smokers (84.1%) and frequencies of patients with co-morbidities such as HIV (19.6%), hypertension (20.4%), and diabetes (20.8%).
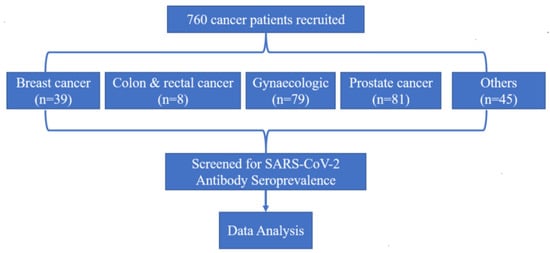
Figure 1.
Study profile.
Almost three-quarters of the treatments were radical treatment (72%); the rest was palliative (17.5%) or other (10.5%). Overall, 322 (42.4%) out of 760 patients were previously under cancer treatment (13.3% chemotherapy, 11.3% radiotherapy, 28.7% surgery, 0.7% Zoladex, and 57.8% either completed or other therapy). A total of 386 (50.8%) patients were on active cancer treatment (34.9% chemotherapy, 28.8% radiotherapy, 0.3% surgery, 1.2% Zoladex, and 39.3% either completed or had other therapy).
3.2. Seroprevalence of COVID-19
A more significant number of the seropositive patients were asymptomatic to COVID-19 (96%). Out of 760 patients, 153 (20.1%) tested positive for SARS-CoV-2 IgG antibodies, 4 (0.53%) for SARS-CoV-2 IgM antibodies, and 94 (12.5%) for both IgG and IgM antibodies. The estimated total seroprevalence (i.e., IgG or IgM positive) was 33.2%. A summary regarding the count of antibody test status is given in Table 1. The cancer patient’s age group, HIV status, and diabetes status were not significantly related to SARS-CoV-2 seropositivity (p > 0.05), as opposed to gender, smoking, different cancers, hypertension, and symptoms that exhibited a significant relationship with SARS-CoV-2 seropositivity (p < 0.05) (Table 2). From our analysis, we have observed that the percentage of female cancer patients is higher than of males, irrespective of the seroprevalence status (Table 2). Among the seropositive patients, it is observed that 57.94% of the cohort are females. Furthermore, 66.34% of the screened seronegative cancer patients were females. In the case of treatment, previous cancer treatment, radiotherapy, and Zoladex monotherapy did not have significance in the pandemic. Again, in the current cancer treatments, Zoladex monotherapy with surgery did not have a statistical significance on seropositivity (Table 3).

Table 1.
Count and percentage of SARS-CoV-2 seroprevalence in cancer patients.

Table 2.
General characteristics of patients, tumour type, underlying co-morbidities, and SARS-CoV-2 seroprevalence.

Table 3.
Characteristics of cancer treatment for the screened patients and SARS-CoV-2 seroprevalence.
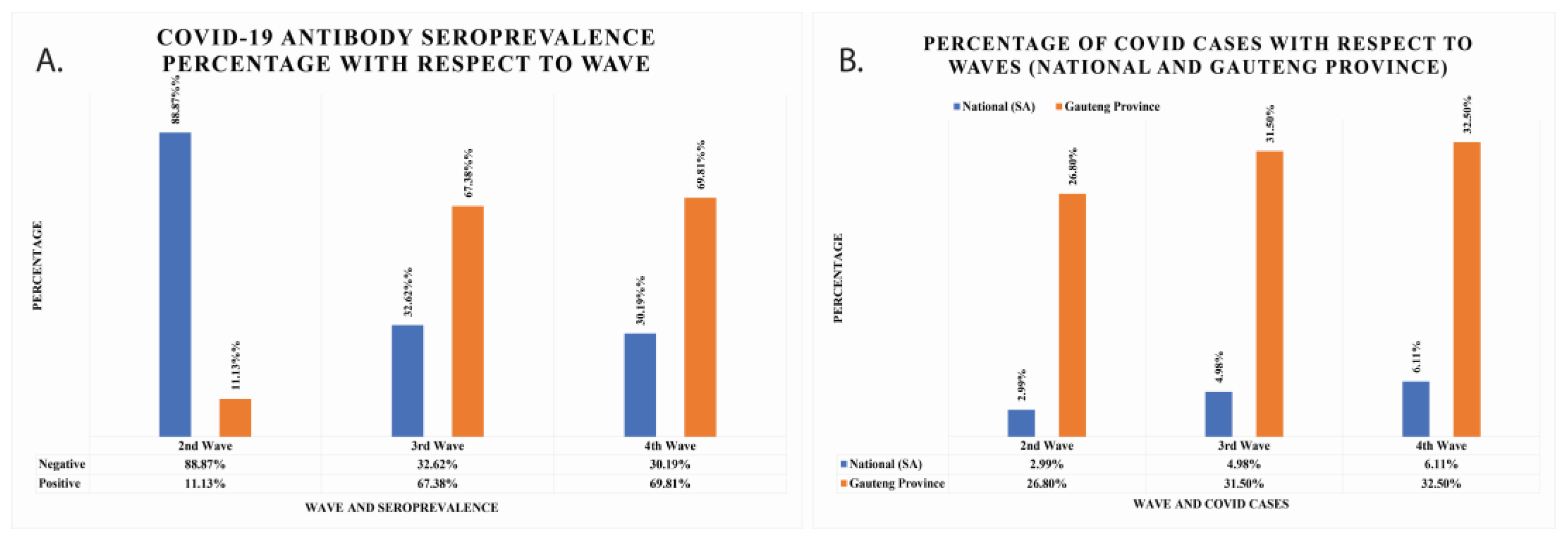
With respect to the second wave, the seropositivity increased by almost six-fold to 67.38% and 69.81% for the third and fourth waves, respectively (Figure 2A). The seropositivity was 11.13% for the second wave. This information means that the negativity rate was higher in the second wave, while the positivity rate increased in the third and fourth waves. The percentage population infected with COVID-19 in South African national data and Gauteng Province data has also been calculated and is shown in Figure 2B. In national data, the seropositivity was found to be 2.99%, 4.98%, and 6.11% for second, third, and fourth waves, respectively. In Gauteng Province, the total cumulative cases were 26.8%, 31.5%, and 32.5% for second, third, and fourth waves, respectively.
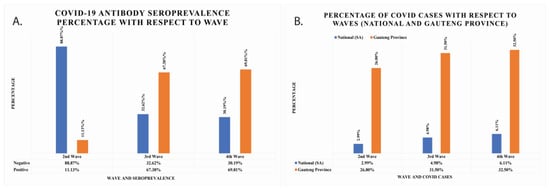
Figure 2.
(A) The COVID-19 IgG/IgM seroprevalence percentage of cancer patients per wave represented with grey colours. The positive seroprevalence of COVID-19 IgG/IgM antibodies rises with waves. (B) The total number of COVID-19 cumulative cases per waves reported nationally and in Gauteng Province.
3.3. Vaccination in the Population
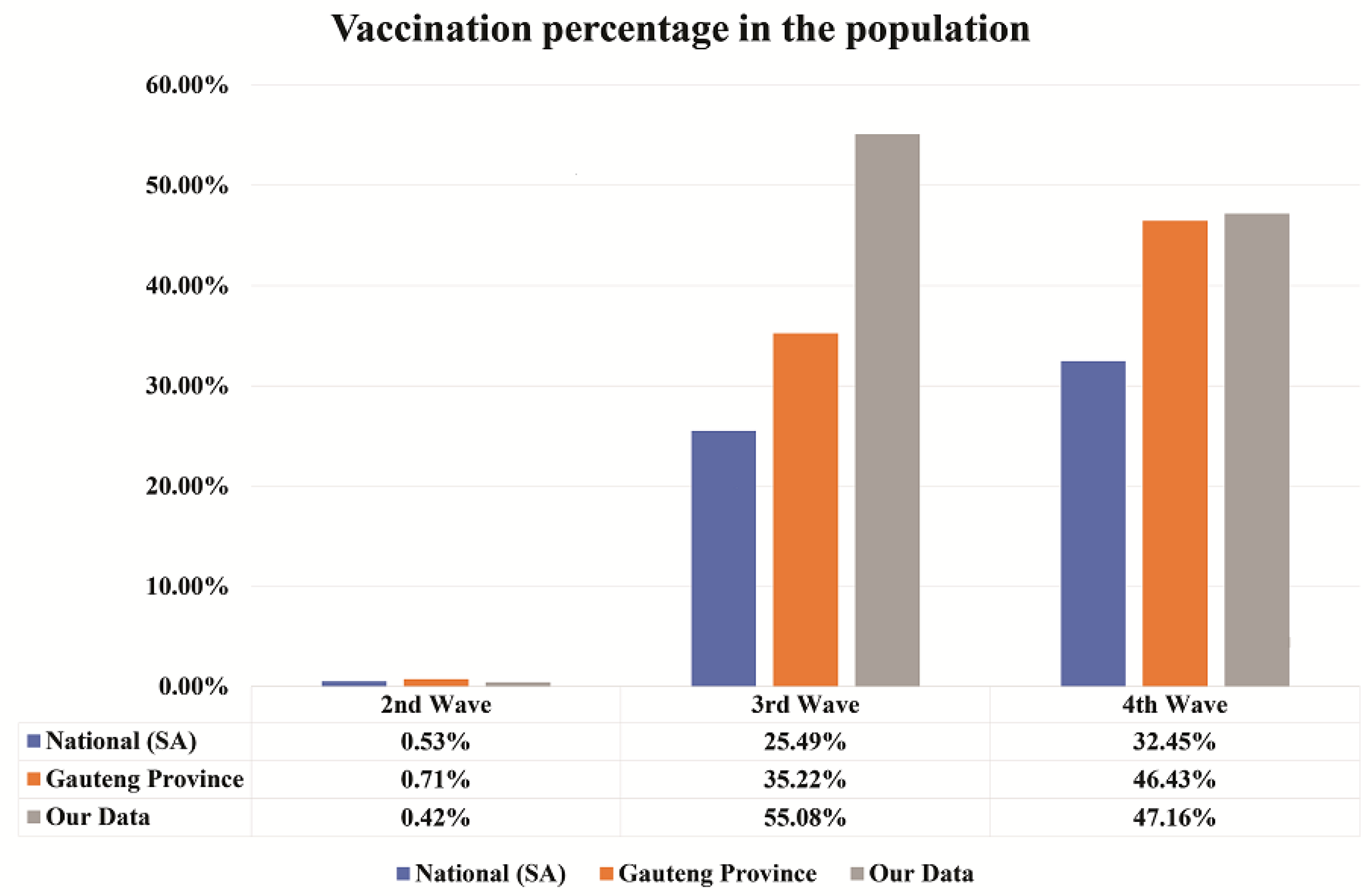
The information for Johnson & Johnson and Pfizer vaccination was collected for national (South Africa) and Gauteng Province from the daily reports of South African coronavirus latest statistics website on the 5 August 2022 [9] and this was compared with our data from cancer patients. The comparison of vaccination percentage can be seen in Figure 3.
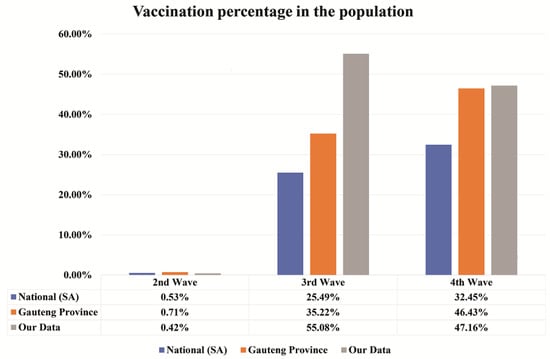
Figure 3.
The vaccination percentage per wave. A comparison of vaccination percentage in the South African population, Gauteng Province, and our cancer patient’s data represented in blue, orange, and grey colours, respectively. Vaccination percentage is seen to be increased with waves. Our test is specific for detecting SARS-CoV-2 anti-nucleocapsid than anti-spike antibodies associated with vaccines.
In the second wave, the percentage of vaccination is comparatively less with respect to third and fourth waves. In the second wave, the vaccination percentage was 0.53%, 0.71%, and 0.42% for national, Gauteng Province, and our cancer patients’ data, respectively. In the third wave, 25.49%, 35.22%, and 55.08% of the people were vaccinated for national, Gauteng Province, and our cancer patients’ data, respectively. Finally, in the fourth wave, vaccination percentage was 32.45%, 46.43%, and 47.16% for national, Gauteng Province, and our cancer patients’ data, respectively. It appears that the vaccination percentage increased with waves, especially for the national and Gauteng Province, as compared to our cancer patients’ data.
3.4. The Regression Model
The omnibus test showed a p-value of <0.001, indicating that the regression model was a good fit compared to the null model. The goodness-of-fit test showed low significance (p > 0.4). There was no difference between the observed and predicted models. The Nagelkerke R Square value for the model was 0.311, suggesting that 31.1% of the dependent variable could be accounted for by the dependent variables of the model. The classification table indicates that the independent variables correctly predicted the seropositivity of the patients with a rate of 75.8%, which may be considered to measure the prediction accuracy of the model.
The odds ratio of every variable indicates the probability of falling into the target group and the non-target group. The odds ratio and 95% CI of the factors associated with seropositivity are presented in Table 4. Patients aged less than 40 years were more prone to COVID-19 than the age group of between 40–69 (95% CI; 0.419–1.19) and ≥70 (95% CI; 0.37–1.46). Female patients (95% CI; 0.40–1.48) were less likely to be seropositive than males. The number of non-smokers was higher in the population. Smokers (95% CI; 0.57–1.70) and non-smokers had an almost equal probability of being SARS-CoV-2 seropositive. According to the smoking history of the patients, ex-smokers (95% CI; 0.70–6.60) had 2.15 times higher chance of being infected with coronavirus, when compared to non-smokers. COVID-19 seropositivity prevalence was 1.25 times among HIV patients (95% CI; 0.78–2.01) compared to non-HIV patients. Patients with hypertension (95% CI; 1.18–2.80) had 1.8 times higher seroprevalence than patients without hypertension. Again, patients with diabetes mellitus (95% CI; 0.86–2.20) had approximately 1.4 times higher seroprevalence than those with no diabetes mellitus.

Table 4.
Binary logistic regression analysis of factors associated with seropositivity.
Cancer patients who were symptomatic (95% CI; 0.27–1.41) of SARS-CoV-2 had less seroprevalence than asymptomatic patients. The cancer types such as breast, colon and rectal, gynaecologic, and prostate cancers had higher seroprevalence when compared to other cancer types. Breast (95% CI; 0.99–3.68), gynaecologic (95% CI; 1.19–4.17), and prostatic cancers (95% CI; 1.29–4.84) had almost two times, or higher, seroprevalence than the other cancer types. Colon and rectal cancer (95% CI; 0.57–3.94) had 1.5 times higher seroprevalence than other cancer types. Radical (95% CI; 0.03–0.21) and palliative (95% CI; 0.16–0.57) treatments were negatively associated with COVID seroprevalence. There were two stages of treatments, namely previous and current cancer treatment. None of the previous cancer treatments showed higher seroprevalence. On the contrary, radiotherapy (95% CI; 0.46–3.35) and surgery (95% CI; 0.12–51.69) in current cancer treatments showed 1.2 and 2.5 times higher seroprevalence, respectively.
4. Discussion
Our study focused on tracking the seroprevalence of anti-SARS-CoV-2 IgG and IgM antibodies in patients with solid tumours following three successive COVID-19 waves with different VOCs including beta, delta, and omicron that occurred from November 2020 to January 2021, May 2021 to September 2021 and November 2021 to present, respectively, in South Africa [10]. Our data show that SARS-CoV-2 exposure in cancer patients rose significantly with the waves, and yet these patients remained asymptomatic. Prostatic, gynaecologic, and breast cancers exhibited higher seroprevalence than their reference category. These cancers are common in the South African population and clinical settings [11,12]. Approximately 50% of the total cancer patients tested for SARS-CoV-2 seroprevalence had breast and gynaecologic cancers, explaining the increased seroprevalence and favouritisms towards females.
Gender, smoking, different cancers, hypertension, and symptoms also exhibited significant relationships with SARS-CoV-2 seropositivity, and this may be attributable to various mechanisms previously postulated. Transmembrane protease serine 2 (TMPRSS2), angiotensin-converting enzyme 2 (ACE-2), and other host cell proteases including cathepsin L, which is frequently expressed in cancer patients, are known to enhance COVID-19 infection [13,14]. The immunosuppressive conditions of cancer patients render them more susceptible to serious COVID-19 consequences than the general population, which may have an impact on the disease’s prognosis. Several studies reported a consistent relationship between past smoking and elevated ACE-2 expression in a series of seminal in vitro experiments [6,15,16]. The association was dose-dependent, and also detectable in both bulk and single-cell analyses, which remained significant after multivariate linear regression controlling for factors such as age, sex, race, and body mass index [16]. Since smoking increases the expression of ACE-2, it is conceivable that smokers are exposed to a larger SARS-CoV-2 load. This could offer a mechanistic explanation for the increased risk of infection, as well as fatal illness and mortality linked to smoking in COVID-19 patients. According to Liang et al. [17], cancer patients are more likely to experience adverse outcomes such as the need for an intense level of care, mechanical breathing, and mortality (39% vs. 8%, p = 0.0003) than non-cancer patients. It is also noted that 70% of stage 4 cancer patients experienced serious events [18]. If any harsh immunosuppressive chemotherapy treatment is recommended [18], we suggest that the dose be reduced or postponed for the patients who are generally in poor condition.
The beta variant drove the second wave of SARS-CoV-2 infections occurring from November 2020, and it exited in January 2021 [10]. Our analysis demonstrated that this variant contributed the highest SARS-CoV-2 infection rates and was 57% more likely to cause severe to fatal cases than the earlier variant that contributed only 25%. During the early days of the COVID-19 pandemic, SBAH delayed treatments, and reduced the number of visits and appointments for oncology outpatients as part of COVID-19, prioritizing management and protective measures to contain the infection and protect vulnerable patients [10]. Additionally, strict adherence to social distancing and other measures were also put in place. This may explain an increased percentage of SARS-CoV-2 negative seroprevalence by oncology patients in the second wave, as observed in our data. Perceived increased SARS-CoV-2 infection susceptibility and associated aggressiveness of COVID-19 in oncology patients may have also encouraged these patients to avoid crowds and public spaces that may have put them at high risk to exposure. Indeed, many patients reported that they avoided unnecessary outings to protect themselves. Additionally, it was rather surprising to those who had tested positive for anti-SARS-CoV-2 antibodies because they were unaware that they had been exposed. Although we did not collect the data for the first wave, we believe that the trajectory may have been similar because of the early onset of the pandemic and the above protective measures.
South Africa started its national vaccination program on 21 February 2021 prioritizing healthcare/frontline workers and those over the age of 60 [19]. This was followed by the easing of some of the restrictions introduced above, explaining the shifted prevalence trajectory with a significant rise in seropositivity (67.4%) following the third wave. The third wave was dominated by the delta variant that occurred between May 2021 and September 2021, and it was associated with a surge of SARS-CoV-2 infections accompanied by higher numbers of fatalities [10]. This continued with the fourth wave that was dominated by the omicron variant, which was spreading twice as fast as the delta. The increased seropositivity prevalence trajectory may have been attributable to the biopsychosocial effects of vaccination. The subsequent ease of some protective measures initially put in place by the hospital, and established knowledge of COVID-19, may have encouraged less concern about exposure.
Most patients never tested positive for COVID-19, and many were unvaccinated at the time when they were recruited to participate in this study. About 35.22% of people in the Gauteng province had been vaccinated with at least one dose by the time of the third wave, and this increased to 46.4% during the fourth wave. This suggested an increase in waves as observed with the whole South African data, partly attributable to ongoing vaccination promotion campaigns. Although the same trajectory was observed from the second to third waves (0.4 to 55.1%), the percentage dropped down to 47.2% with the cancer patients’ data. This may have just been by chance or partially due to COVID-19 vaccine hesitancy, which has been a big challenge in South Africa [20]. Common reasons for this hesitancy included the perception that these vaccines were a government or global plot for making money, or the concern that these vaccines were unsafe or harmful as their production and roll-out were rushed without considering the profound testing of safety and side effects reported by early-on studies [21,22].
Interestingly, our data also show that most patients, regardless of vaccination status, were unaware and only found out through our screening that they were silently developing SARS-CoV-2, i.e., reporting that they had never experienced any symptoms or sickness suggesting any exposure to SARS-CoV-2 infection. This is similar to the findings obtained by other studies that also found their cancer populations to be asymptomatic to COVID-19 [23,24,25]. This suggests that, although these patients may be susceptible to SARS-CoV-2 infection, they remained asymptomatic throughout the pandemic, and thus they were not at risk of severe illness necessitating invasive ventilation and intensive care unit (ICU) hospitalisation, as several reports initially assumed [26,27,28,29,30]. It has been reported that possible pre-existing and de novo humoral immunity, that stemmed from previous coronavirus exposure, rendered some protection to children and individuals who are supposedly at risk due to human immunodeficiency virus and tuberculosis infection [31,32]. Ng et al.’s [31] study has demonstrated that 5% and 62% of SARS-CoV-2 unexposed adults and children, respectively, had antibodies that also recognise SARS-CoV-2. These antibodies appear to be produced through previous coronaviruses that share similar genome features with the SARS-CoV-2 virus. Indeed, the S2 subunit of SARS-CoV-2 is similar across other coronaviruses, and can stop SARS-CoV-2 virus from entering the cells of previously exposed individuals, and thus protecting them against the new exposure with SARS-CoV-2. This may also be the same case with the vast majority of oncology patients. Due to the immunocompromised status of oncology patients, it is possible that some of these patients have also been previously exposed to other coronaviruses, and this rendered them protection, explaining less exposure to SARS-CoV-2 or maintained asymptomatic or mild status when infected.
Our study has some limitations. For instance, due to the boundaries of the ethics approval and the single centre prospective and exploratory study method employed, our findings should not be generalized to the entire population, but only applied to the current population due to inadequate thoroughness. Furthermore, diagnosis was only based on the result of a single test, not in conjunction with SARS-CoV-2 PCR tests or complete clinical assays.
5. Conclusions
The asymptomatic presentation of SARS-CoV-2 viral infection in cancer patients is possible but uncommon. This occurrence presents a challenge to the health sector for both diagnosis and treatment of these patients. Our study supplements current information on the presentation of SARS-CoV-2 viral infection in cancer patients, which may be used by policy makers and healthcare providers to improve the management of these patients.
Author Contributions
Conceptualization, M.K., Y.M. (Yunus Munga), T.E., J.R.Z. and M.S.; data curation, M.K.; formal analysis, M.K., R.D. and A.V.; funding acquisition, J.R.Z. and M.S.; investigation, M.K., C.G., L.M., N.N., V.G., H.N., Y.M. (Yonwaba Mzizi), J.C., J.D., D.M., N.G., L.Z., T.M., S.B., R.K. and M.S.; methodology, M.K., I.L., T.B., K.M., J.C., M.V., A.V., R.K., Y.M. (Yunus Munga), T.E., J.R.Z. and M.S.; project administration, M.K. and M.S.; validation, M.K. and R.D.; writing—original draft, M.K. and M.S.; writing—review and editing, M.K., R.D., I.L., T.B., K.M., C.G., L.M., N.N., V.G., H.N., Y.M. (Yonwaba Mzizi), J.C., J.D., D.M., N.G., L.Z., T.M., M.V., S.B., A.V., R.K., Y.M. (Yunus Munga), T.E., J.R.Z. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, and the APC was self-funded by NuMeRI.
Institutional Review Board Statement
The study was approved by the University of Pretoria Research Ethics Committee and all patients signed informed consent forms (Ethics Ref No: 787/2020 and 15 October 2020 until 15 October 2022).
Informed Consent Statement
Informed consent to participate in and to publish this paper was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results can be requested by email from both corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.; Wang, C.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Junejo, Y.; Ozaslan, M.; Safdar, M.; Khailany, R.A.; Rehman, S.; Yousaf, W.; Khan, M.A. Novel SARS-CoV-2/COVID-19: Origin, pathogenesis, genes and genetic variations, immune responses and phylogenetic analysis. Gene Rep. 2020, 20, 100752. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Hong, N.; Zhou, X.; He, J.; Ma, Y.; Jiang, H.; Han, L.; Chang, F.; Shan, G.; Zhu, W. Evaluation of the secondary transmission pattern and epidemic prediction of COVID-19 in the four metropolitan areas of China. Front. Med. 2020, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar]
- Sinha, S.; Kundu, C.N. Cancer and COVID-19: Why are cancer patients more susceptible to COVID-19? Med. Oncol. 2021, 38, 101. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 OutbreakPatients with Cancer in SARS-COV-2 Infection. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shaman, J. COVID-19 pandemic dynamics in India, the SARS-CoV-2 Delta variant and implications for vaccination. J. R. Soc. Interface 2022, 19, 20210900. [Google Scholar] [CrossRef]
- Made, F.; Wilson, K.; Jina, R.; Tlotleng, N.; Jack, S.; Ntlebi, V.; Kootbodien, T. Distribution of cancer mortality rates by province in South Africa. Cancer Epidemiol. 2017, 51, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Sacoronavirus Latest Vaccine Statistics. SA Corona Virus Online Portal. Available online: https://sacoronavirus.co.za/latest-vaccine-statistics/ (accessed on 5 August 2022).
- Maslo, C.; Friedland, R.; Toubkin, M.; Laubscher, A.; Akaloo, T.; Kama, B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA 2022, 327, 583–584. [Google Scholar] [CrossRef]
- Somdyala, N.I.; Bradshaw, D.; Gelderblom, W.C.; Parkin, D.M. Cancer incidence in a rural population of South Africa, 1998–2002. Int. J. Cancer 2010, 127, 2420–2429. [Google Scholar] [CrossRef]
- Moodley, J.; Constant, D.; Mwaka, A.D.; Scott, S.E.; Walter, F.M. Mapping awareness of breast and cervical cancer risk factors, symptoms and lay beliefs in Uganda and South Africa. PLoS ONE 2020, 15, e0240788. [Google Scholar] [CrossRef]
- Chai, P.; Yu, J.; Ge, S.; Jia, R.; Fan, X. Genetic alteration, RNA expression, and DNA methylation profiling of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignancies: A pan-cancer analysis. J. Hematol. Oncol. 2020, 13, 43. [Google Scholar] [CrossRef]
- Yu, J.; Chai, P.; Ge, S.; Fan, X. Recent Understandings Toward Coronavirus Disease 2019 (COVID-19): From Bench to Bedside. Front. Cell. Dev. Biol. 2020, 8, 476. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Mikami, T.; Chopra, N.; Yamada, T.; Chernyavsky, S.; Rizk, D.; Cruz, C. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Sausville, E.L.; Girish, V.; Yuan, M.L.; Vasudevan, A.; John, K.M.; Sheltzer, J.M. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev. Cell. 2020, 53, 514–529.e3. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef] [PubMed]
- SA’s COVID-19 Vaccination Rollout Kicks Off in Khayelitsha—SA Corona Virus Online Portal. Available online: https://sacoronavirus.co.za/2021/02/18/sas-covid-19-vaccination-rollout-kicks-off-in-khayelitsha/ (accessed on 8 August 2022).
- Burger, R.; Köhler, T.; Golos, A.M.; Buttenheim, A.M.; English, R.; Tameris, M.; Maughan-Brown, B. Longitudinal changes in COVID-19 vaccination intent among South African adults: Evidence from the NIDS-CRAM panel survey, February to May 2021. BMC Public Health 2022, 22, 422. [Google Scholar] [CrossRef]
- Ostergaard, S.D.; Schmidt, M.; Horvath-Puho, E.; Thomsen, R.W.; Sorensen, H.T. Thromboembolism and the Oxford-AstraZeneca COVID-19 vaccine: Side-effect or coincidence? Lancet 2021, 397, 1441–1443. [Google Scholar] [CrossRef]
- Wise, J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 2021, 372, 699. [Google Scholar] [CrossRef]
- Chen, N.; Jotwani, A.; Li, A. Care Delivery in Cancer Patients with Asymptomatic COVID-19 Infection in a Tertiary, Safety-Net Hospital in Houston, Texas. Am. J. Clin. Oncol. 2021, 44, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Natarajan, V.; Murthy, P.; Meghal, T.; Xu, Y.; Wiesel, O. The prevalence of asymptomatic COVID-19 infection in cancer patients. A cross-sectional study at a tertiary cancer center in New York City. Cancer Treat Res. Commun. 2021, 27, 100346. [Google Scholar] [CrossRef] [PubMed]
- Piciu, A.; Manole, S.; Piciu, D.; Dreve, T.; Roman, A. Asymptomatic COVID-19 cancer patients incidentally discovered during F18-FDG PET/CT monitoring. Med. Pharm. Rep. 2021, 94, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Lidenge, S.J.; Ngowi, J.R.; Peña, P.B.; Clegg, A.A.; Ngalamika, O.; Mwita, C.J.; Mwaiselage, J.; Wood, C. Lower SARS-CoV-2 Seroprevalence among Cancer Patients in Sub-Saharan Africa. J. Clin. Med. 2022, 11, 4428. [Google Scholar]
- Ofori-Asenso, R.; Ogundipe, O.; Agyeman, A.A.; Chin, K.L.; Mazidi, M.; Ademi, Z.; De Bruin, M.L.; Liew, D. Cancer is associated with severe disease in COVID-19 patients: A systematic review and meta-analysis. Ecancermedicalscience 2020, 14, 1047. [Google Scholar] [CrossRef]
- Tian, Y.; Qiu, X.; Wang, C.; Zhao, J.; Jiang, X.; Niu, W.; Huang, J.; Zhang, F. Cancer associates with risk and severe events of COVID-19: A systematic review and meta-analysis. Int. J. Cancer 2021, 148, 363–374. [Google Scholar] [CrossRef]
- Robilotti, E.V.; Babady, N.E.; Mead, P.A.; Rolling, T.; Perez-Johnston, R.; Bernardes, M.; Bogler, Y.; Caldararo, M.; Figueroa, C.J.; Glickman, M.S. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020, 26, 1218–1223. [Google Scholar] [CrossRef]
- El-Sharkawi, D.; Iyengar, S. Haematological cancers and the risk of severe COVID-19: Exploration and critical evaluation of the evidence to date. Br. J. Haematol. 2020, 190, 336–345. [Google Scholar] [CrossRef]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef]
- Ng, K.W.; Faulkner, N.; Wrobel, A.G.; Gamblin, S.J.; Kassiotis, G. Heterologous humoral immunity to human and zoonotic coronaviruses: Aiming for the achilles heel. Semin. Immunol. 2021, 55, 101507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).