Analysis of Pulp Tissue Viability and Cytotoxicity of Pulp Capping Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Culturing of hDPSC
Culturing of hDPSC [7]
2.2. Sample Preparation
2.3. Cytotoxicity Assay
MTT Assay
3. Results
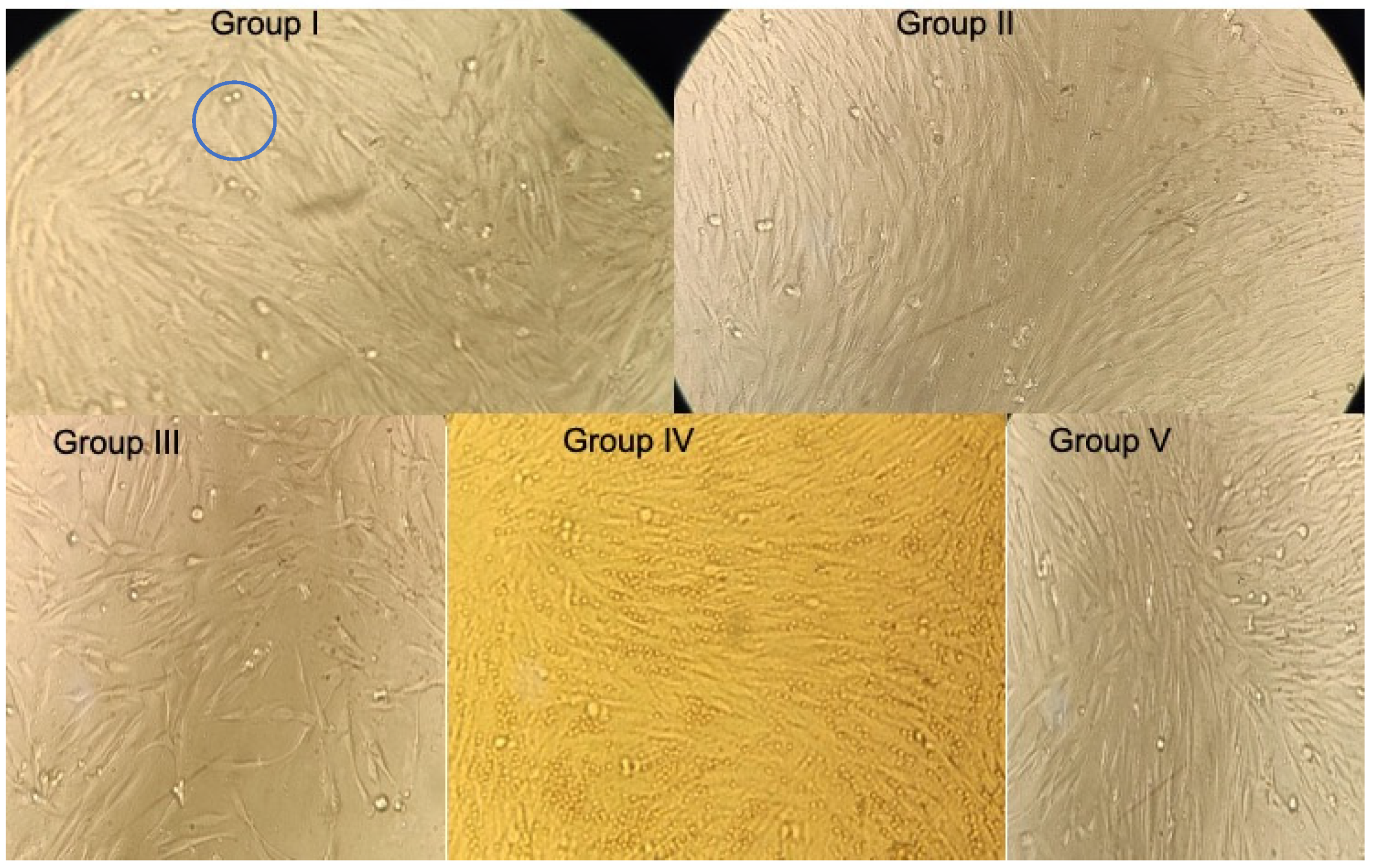
3.1. Comparison of MTT Assay Results at 24 h
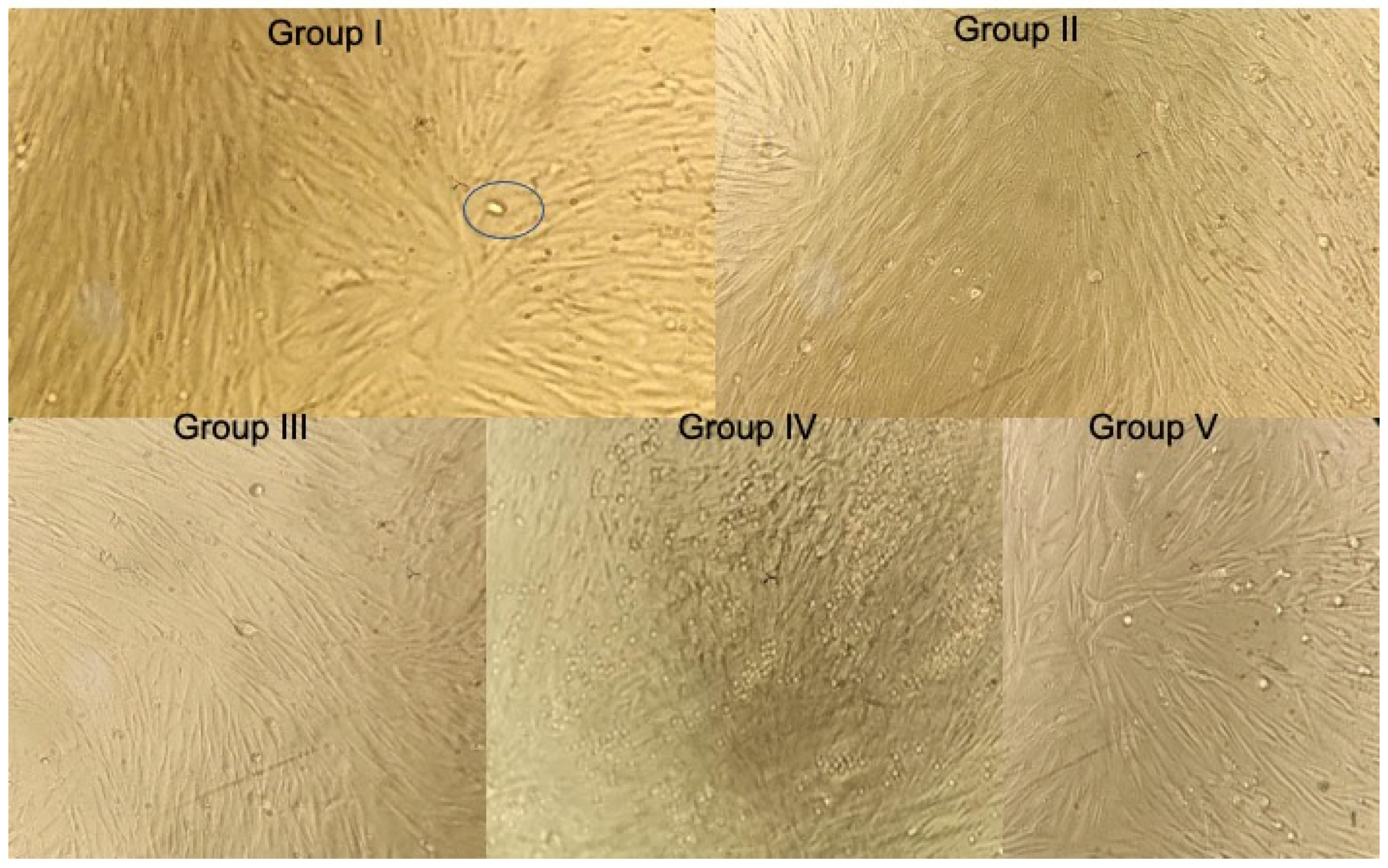
3.1.1. At 48 h
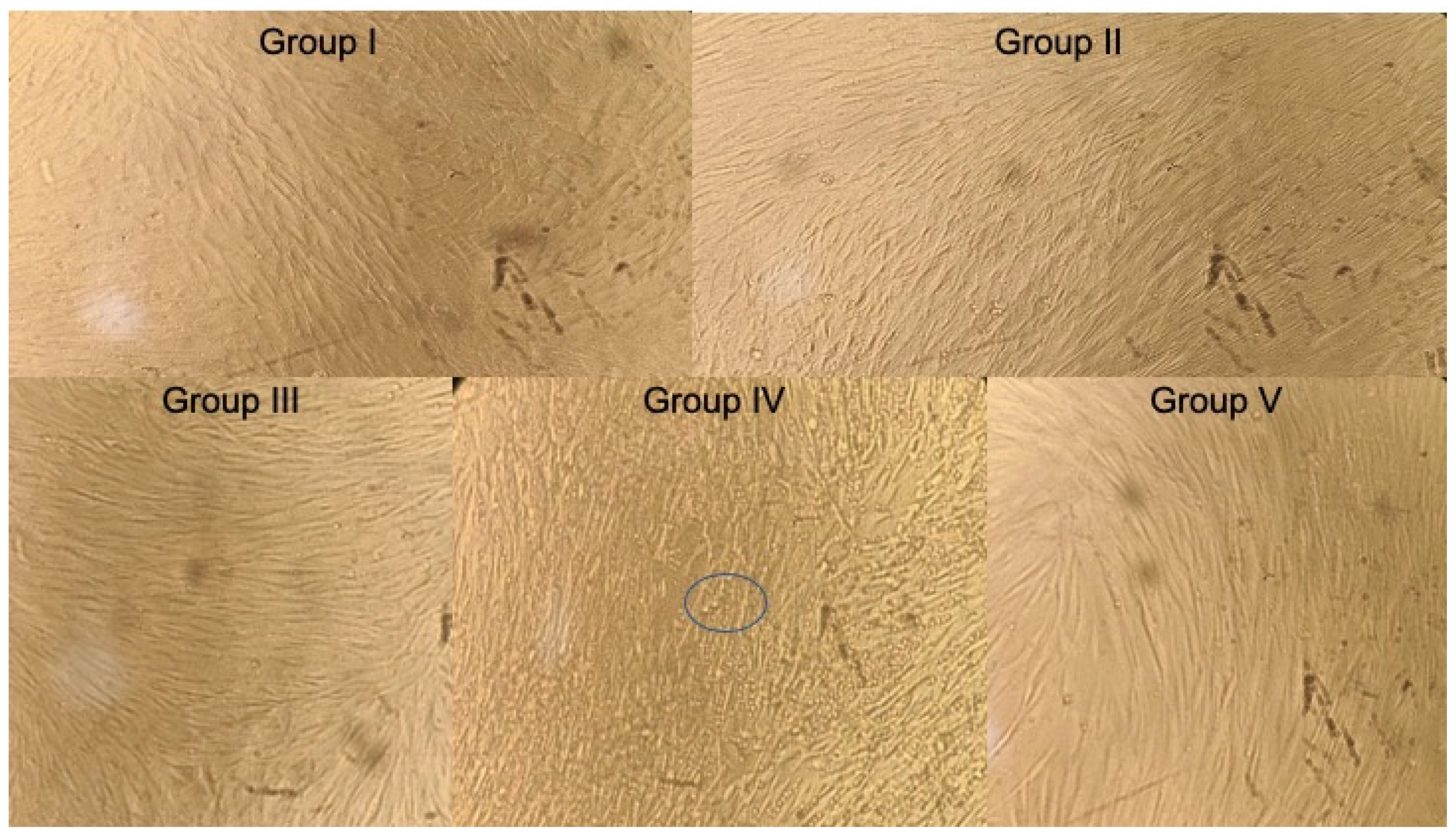
3.1.2. At 72 h
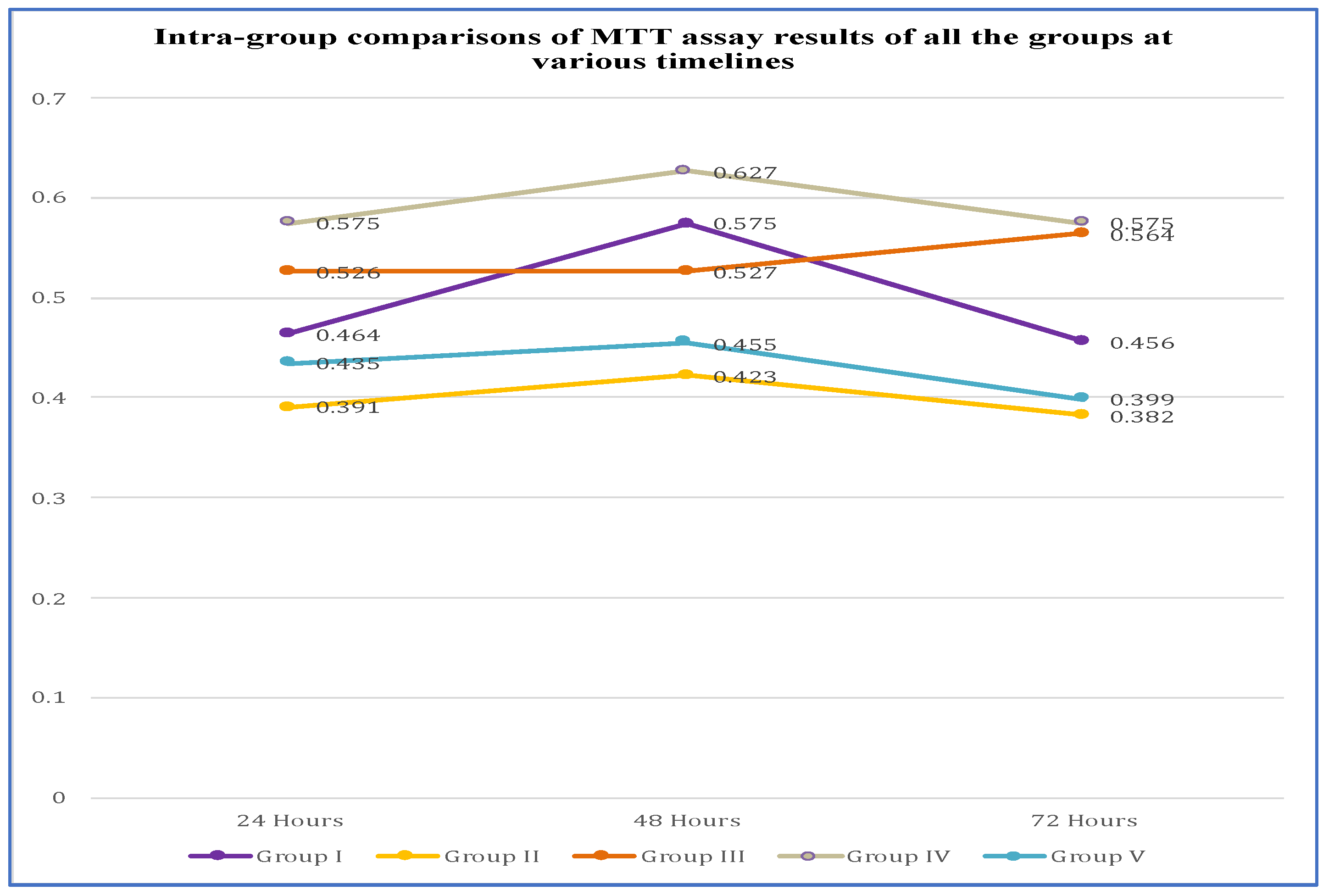
3.2. Intra Group Comparison of MTT Assay Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, M.; Yu, B.; Kim, S.; Hayashi, M.; Smith, C.; Sohn, S.; Kim, E.; Lim, J.; Stevenson, R.G.; Kim, R.H. Clinical and Molecular Perspectives of Reparative Dentin Formation: Lessons Learned from Pulp-Capping Materials and the Emerging Roles of Calcium. Dent. Clin. N. Am. 2017, 61, 93–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sequeira, D.B.; Oliveira, A.R.; Seabra, C.M.; Palma, P.J.; Ramos, C.; Figueiredo, M.H.; Santos, A.C.; Cardoso, A.L.; Peça, J.; Santos, J.M. Regeneration of pulp-dentin complex using human stem cells of the apical papilla: In vivo interaction with two bioactive materials. Clin. Oral Investig. 2021, 25, 5317–5329. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-W.; Lee, S.-Y.; Kum, K.-Y.; Kim, E.-C. Effects of ProRoot MTA, Bioaggregate, and Micromega MTA on odontoblastic differentiation in human dental pulp cells. J. Endod. 2014, 40, 113–118. [Google Scholar] [CrossRef]
- Paula, A.B.; Laranjo, M.; Marto, C.-M.; Paulo, S.; Abrantes, A.M.; Fernandes, B.; Casalta-Lopes, J.; Marques-Ferreira, M.; Botelho, M.F.; Carrilho, E. Evaluation of dentinogenesis inducer biomaterials: An in vivo study. J. Appl. Oral Sci. 2019, 28, e20190023. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-W.; Lee, S.-Y.; Ann, H.-J.; Kum, K.-Y.; Kim, E.-C. Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. J. Endod. 2014, 40, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, N.; Khademi, A. Outcomes of vital pulp therapy in permanent teeth with different medicaments based on review of the literature. Dent. Res. J. 2015, 12, 406–417. [Google Scholar]
- Bulbule, A.M.; Mandroli, P.S.; Bhat, K.G.; Bogar, C.M. In vitro evaluation of cytotoxicity of Emblica officinalis (amla) on cultured human primary dental pulp fibroblasts. J. Indian Soc. Pedod. Prev. Dent. 2019, 37, 251–257. [Google Scholar] [PubMed]
- Ha, W.N.; Nicholson, T.; Kahler, B.; Walsh, L.J. Mineral trioxide aggregate—A review of properties and testing methodologies. Materials 2017, 10, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.-L.; Ke, M.-C.; Chen, Y.-H.; Kuo, H.-K.; Yu, H.-J.; Chen, C.-T.; Tseng, Y.-C.; Chuang, P.-C.; Wu, P.-C. Mineral trioxide aggregate affects cell viability and induces apoptosis of stem cells from human exfoliated deciduous teeth. BMC Pharmacol. Toxicol. 2018, 19, 21. [Google Scholar] [CrossRef]
- Huang, F.M.; Chang, Y.C. Cytotoxicity of dentine-bonding agents on human pulp cells in vitro. Int. Endod. J. 2002, 35, 905–909. [Google Scholar] [CrossRef]
- Silva, G.A.B.; Gava, E.; Lanza, L.D.; Estrela, C.; Alves, J.B. Subclinical failures of direct pulp capping of human teeth by using a dentin bonding system. J. Endod. 2013, 39, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Tayal, A.; Niyogi, A.; Adhikari, H.D.; Adhya, P.; Ghosh, A. Comparative evaluation of effect of One Coat 7 Universal and Tetric N-Bond Universal adhesives on shear bond strength at resin–zirconia interface: An in vitro study. J. Conserv. Dent. JCD 2021, 24, 336. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Torii, Y.; Ogawa, T.; Osaka, A.; Meerbeek, B.V. Self-assembled nano-layering at the adhesive interface. J. Dent. Res. 2012, 91, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Sezinando, A.; Luque-Martinez, I.; Muñoz, M.A.; Reis, A.; Loguercio, A.D.; Perdigão, J. Influence of a hydrophobic resin coating on the immediate and 6-month dentin bonding of three universal adhesives. Dent. Mater. 2015, 31, e236–e246. [Google Scholar] [CrossRef]
- TheraCal PT|BISCO Dental. Available online: https://www.bisco.com/theracal-pt-/ (accessed on 20 December 2022).
- Hanna, S.N.; Perez Alfayate, R.; Prichard, J. Vital Pulp Therapy an Insight Over the Available Literature and Future Expectations. Eur. Endod. J. 2020, 5, 46–53. [Google Scholar]
- Huang, F.-M.; Yang, S.-F.; Zhao, J.-H.; Chang, Y.-C. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J. Endod. 2010, 36, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Han, Q.; Chen, W.; Song, J.; Zhao, X.; Ouyang, Y.; Yuan, W.; Fan, C. Platelet-Rich Plasma Derived Growth Factors Contribute to Stem Cell Differentiation in Musculoskeletal Regeneration. Front. Chem. 2017, 5, 89. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, K.M.; Giesler, T.; Henry, M.; Wang, Y. Regeneration potential of the young permanent tooth: What does the future hold? Pediatr. Dent. 2008, 30, 253–260. [Google Scholar] [CrossRef]
- Murray, P.E.; García Godoy, C.; García Godoy, F. How is the biocompatibilty of dental biomaterials evaluated? Med. Oral Patol. Oral Cirugia Bucal 2007, 12, E258–E266. [Google Scholar]
- Doyle, A.; Griffiths, J.B. Cell and Tissue Culture for Medical Research; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Goorha, S.; Reiter, L.T. Culturing and Neuronal Differentiation of Human Dental Pulp Stem Cells. Curr. Protoc. Hum. Genet. 2017, 92, 21.6.1–21.6.10. [Google Scholar] [CrossRef] [Green Version]
- Mandroli, P.S.; Prabhakar, A.R.; Bhat, K.; Krishnamurthy, S.; Bogar, C. An in vitro evaluation of cytotoxicity of curcumin against human periodontal ligament fibroblasts. Ayu 2019, 40, 192. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Zohaib, S.; Siddiqui, F. Efficacy of enamel matrix derivative in vital pulp therapy: A review of literature. Iran. Endod. J. 2017, 12, 269. [Google Scholar] [PubMed]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Dahake, P.T.; Panpaliya, N.P.; Kale, Y.J.; Dadpe, M.V.; Kendre, S.B.; Bogar, C. Response of stem cells from human exfoliated deciduous teeth (SHED) to three bioinductive materials–An in vitro experimental study. Saudi Dent. J. 2020, 32, 43–51. [Google Scholar] [CrossRef]
- Vinken, M.; Blaauboer, B.J. In vitro testing of basal cytotoxicity: Establishment of an adverse outcome pathway from chemical insult to cell death. Toxicol. In Vitro 2017, 39, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iohara, K.; Nakashima, M.; Ito, M.; Ishikawa, M.; Nakasima, A.; Akamine, A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J. Dent. Res. 2004, 83, 590–595. [Google Scholar] [CrossRef]
- Gandhi, A.; Gandhi, T.; Madan, N. Dental pulp stem cells in endodontic research: A promising tool for tooth tissue engineering. RSBO Rev. Sul-Bras. Odontol. 2011, 8, 335–340. [Google Scholar]
- Rai, S.; Kaur, M.; Kaur, S. Applications of stem cells in interdisciplinary dentistry and beyond: An overview. Ann. Med. Health Sci. Res. 2013, 3, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, T.; Suzuki, A.; Yuzawa, S.; Baba, Y.; Kimura, Y.; Kato, Y. Mineral trioxide aggregate induces osteoblastogenesis via Atf6. Bone Rep. 2015, 2, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Vishnubalaji, R.; Al-Nbaheen, M.; Kadalmani, B.; Aldahmash, A.; Ramesh, T. Comparative investigation of the differentiation capability of bone-marrow-and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 2012, 347, 419–427. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, F.J.; López-García, S.; Garcia-Bernal, D.; Sanz, J.L.; Lozano, A.; Pecci-Lloret, M.P.; Melo, M.; López-Ginés, C.; Forner, L. Cytocompatibility and bioactive properties of the new dual-curing resin-modified calcium silicate-based material for vital pulp therapy. Clin. Oral Investig. 2021, 25, 5009–5024. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; Soler-Doria, A.; López-García, S.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Lozano, A.; Llena, C.; Forner, L.; Guerrero-Gironés, J.; Melo, M. Comparative biological properties and mineralization potential of 3 endodontic materials for vital pulp therapy: Theracal PT, Theracal LC, and biodentine on human dental pulp stem cells. J. Endod. 2021, 47, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Silva, G.A.; Lopes Jr, N.; Napimoga, M.H.; Benatti, B.B.; Alves, J.B. Direct capping of human pulps with a dentin bonding system and calcium hydroxide: An immunohistochemical analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, S.R. Comparative Performance of Mineral Trioxide Aggregate Versus Calcium Hydroxide as a Direct Pulp Capping Agent. J. Esthet. Restor. Dent. 2016, 28, 131–135. [Google Scholar] [CrossRef]
- Grech, L.; Mallia, B.; Camilleri, J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent. Mater. 2013, 29, e20–e28. [Google Scholar] [CrossRef]
- Pawlak, J.; Trzcionka, A.; Mertas, A.; Dziedzic, A.; Hildebrandt, T.; Tanasiewicz, M. The Cytotoxicity Assessment of Novel Formulation Developed to Reduce Dentin Hypersensitivity Utilizing Dehydrogenase Assay. Coatings 2021, 11, 217. [Google Scholar] [CrossRef]
- Campos, M.D.F.T.; Moura, D.M.D.; Borges, B.C.D.; Assuncao, I.V.D.; Caldas, M.R.G.R.; Platt, J.A.; Özcan, M.; Souza, R.O.D.A. Influence of Acid Etching and Universal Adhesives on the Bond Strength to Dentin. Braz. Dent. J. 2020, 31, 272–280. [Google Scholar] [CrossRef]
- Silva, G.A.; Lanza, L.D.; Lopes-Júnior, N.; Moreira, A.; Alves, J.B. Direct pulp capping with a dentin bonding system in human teeth: A clinical and histological evaluation. Oper. Dent. 2006, 31, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, A.; Łagocka, R.; Lipski, M.; Parafiniuk, M.; Grocholewicz, K.; Sobolewska, E.; Witek, A.; Buczkowska-Radlińska, J. Clinical and histological evaluation of direct pulp capping on human pulp tissue using a dentin adhesive system. BioMed Res. Int. 2016, 2016, 2591273. [Google Scholar] [CrossRef] [Green Version]
- Prakash, S.; Thakur, A. Platelet concentrates: Past, present and future. J. Maxillofac. Oral Surg. 2011, 10, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Bartold, P.M.; Miura, M.; Seo, B.M.; Robey, P.G.; Gronthos, S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod. Craniofac. Res. 2005, 8, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Hotwani, K.; Sharma, K. Platelet rich fibrin-a novel acumen into regenerative endodontic therapy. Restor. Dent. Endod. 2014, 39, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, L.; Yan, Q.; Yang, D. Effect of five dental pulp capping agents on cell proliferation, viability, apoptosis and mineralization of human dental pulp cells. Exp. Ther. Med. 2020, 19, 2377–2383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-J.; Zhao, Y.-H.; Zhao, Y.-J.; Liu, N.-X.; Lv, X.; Li, Q.; Chen, F.-M.; Zhang, M. Potential dental pulp revascularization and odonto-/osteogenic capacity of a novel transplant combined with dental pulp stem cells and platelet-rich fibrin. Cell Tissue Res. 2015, 361, 439–455. [Google Scholar] [CrossRef] [PubMed]
| 24 h | 48 h | 72 h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | OD Values | Mean OD | Cell Proliferation (%) | Cell Death (%) | OD Values | Mean OD | Cell Proliferation (%) | Cell Death (%) | OD Values | Mean OD | Cell Proliferation (%) | Cell Death (%) |
| Group I | 0.464 | 0.464 | 106% | 00 | 0.577 | 0.576 | 126% | 00 | 0.459 | 0.456 | 114% | 00 |
| 0.462 | 0.579 | 0.458 | ||||||||||
| 0.468 | 0.571 | 0.451 | ||||||||||
| Group II | 0.398 | 0.391 | 90% | 10% | 0.420 | 0.423 | 92% | 8% | 0.382 | 0.382 | 95% | 5% |
| 0.392 | 0.422 | 0.381 | ||||||||||
| 0.384 | 0.428 | 0.385 | ||||||||||
| Group III | 0.525 | 0.526 | 120% | 00 | 0.529 | 0.527 | 115% | 00 | 0.561 | 0.564 | 141% | 00 |
| 0.529 | 0.528 | 0.565 | ||||||||||
| 0.526 | 0.526 | 0.566 | ||||||||||
| Group IV | 0.579 | 0.575 | 132% | 00 | 0.620 | 0.627 | 137% | 00 | 0.575 | 0.575 | 144% | 00 |
| 0.575 | 0.629 | 0.579 | ||||||||||
| 0.572 | 0.632 | 0.572 | ||||||||||
| Group V | 0.431 | 0.435 | 100% | 00 | 0.452 | 0.455 | 100% | 00 | 0.399 | 0.399 | 100% | 00 |
| 0.436 | 0.459 | 0.401 | ||||||||||
| 0.439 | 0.454 | 0.398 | ||||||||||
| 24 h | 48 h | 72 h | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | N | Mean | S.D. | F-Value | p-Value | Mean | S.D. | F-Value | p-Value | Mean | S.D. | F-Value | p-Value |
| Group I | 3 | 0.464 | 0.003 | 873.382 | <0.001 † | 0.576 | 0.004 | 1186.55 | <0.001 † | 0.456 | 0.004 | 2716.54 | <0.001 † |
| Group II | 3 | 0.391 | 0.007 | 0.423 | 0.004 | 0.382 | 0.002 | ||||||
| Group III | 3 | 0.526 | 0.002 | 0.527 | 0.001 | 0.564 | 0.002 | ||||||
| Group IV | 3 | 0.575 | 0.003 | 0.627 | 0.006 | 0.575 | 0.003 | ||||||
| Group V | 3 | 0.435 | 0.004 | 0.455 | 0.003 | 0.399 | 0.001 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, P.; Govind, S.; Sahoo, S.K.; Pattanaik, S.; Mallikarjuna, R.M.; Nalawade, T.; Saraf, S.; Khaldi, N.A.; Jahdhami, S.A.; Shivagange, V.; et al. Analysis of Pulp Tissue Viability and Cytotoxicity of Pulp Capping Agents. J. Clin. Med. 2023, 12, 539. https://doi.org/10.3390/jcm12020539
Panda P, Govind S, Sahoo SK, Pattanaik S, Mallikarjuna RM, Nalawade T, Saraf S, Khaldi NA, Jahdhami SA, Shivagange V, et al. Analysis of Pulp Tissue Viability and Cytotoxicity of Pulp Capping Agents. Journal of Clinical Medicine. 2023; 12(2):539. https://doi.org/10.3390/jcm12020539
Chicago/Turabian StylePanda, Pratima, Shashirekha Govind, Sanjit Kumar Sahoo, Satabdi Pattanaik, Rachappa M. Mallikarjuna, Triveni Nalawade, Sanjay Saraf, Naseer Al Khaldi, Salma Al Jahdhami, Vinay Shivagange, and et al. 2023. "Analysis of Pulp Tissue Viability and Cytotoxicity of Pulp Capping Agents" Journal of Clinical Medicine 12, no. 2: 539. https://doi.org/10.3390/jcm12020539
APA StylePanda, P., Govind, S., Sahoo, S. K., Pattanaik, S., Mallikarjuna, R. M., Nalawade, T., Saraf, S., Khaldi, N. A., Jahdhami, S. A., Shivagange, V., & Jena, A. (2023). Analysis of Pulp Tissue Viability and Cytotoxicity of Pulp Capping Agents. Journal of Clinical Medicine, 12(2), 539. https://doi.org/10.3390/jcm12020539






