Surgical Pharmacy for Optimizing Medication Therapy Management Services within Enhanced Recovery after Surgery (ERAS®) Programs
Abstract
1. Introduction
2. Perioperative Medication Therapy
2.1. Management of Antimicrobial Therapy
2.2. Thrombosis Prophylaxis and Antithrombotic Management
2.3. Pain Management
2.4. Nutrition Management
2.5. Glycemic Control
2.6. Management of Blood Pressure
2.7. Fluid Management
2.8. Management of Postoperative Nausea and Vomiting
2.9. Management of Postoperative Delirium
3. How Surgical Pharmacy Is Engaged in Perioperative Medication Management
3.1. Make an Estimate of Medication Deprescribing and Therapy-Related Problems before Surgery
3.2. Implement ERAS Standardized Medication Treatment Path during the Perioperative Period
3.3. Hospital Medication Education and Follow-Up
4. Workflow and Work Path of Medication Treatment Management
4.1. Stage I: Pre-Admission/Out-Patient Pharmaceutical Care
4.2. Stage II: Preoperative Pharmaceutical Assessment and Service
4.3. Stage III: Intraoperative Pharmaceutical Care
4.4. Stage IV: Postoperative Pharmacy Reassessment and Monitoring
4.5. Stage V: Medication Education and Follow-up after Discharge
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klek, S.; Salowka, J.; Choruz, R.; Cegielny, T.; Welanyk, J.; Wilczek, M.; Szczepanek, K.; Pisarska-Adamczyk, M.; Pedziwiatr, M. Enhanced Recovery after Surgery (ERAS) Protocol Is a Safe and Effective Approach in Patients with Gastrointestinal Fistulas Undergoing Reconstruction: Results from a Prospective Study. Nutrients 2021, 13, 1953. [Google Scholar] [CrossRef] [PubMed]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery after Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Z.; Zhou, J.; Yang, J.; Chen, X.; Chang, C.; Liu, C.; Li, K.; Hu, J. Barriers to implementation of enhanced recovery after surgery (ERAS) by a multidisciplinary team in China: A multicentre qualitative study. BMJ Open 2022, 12, e053687. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.K.; Walker, T.; Carter, F.; Hubner, M.; Balfour, A.; Jakobsen, D.H.; Burch, J.; Wasylak, T.; Demartines, N.; Lobo, D.N.; et al. Consensus on Training and Implementation of Enhanced Recovery after Surgery: A Delphi Study. World J. Surg. 2018, 42, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Roulin, D.; Najjar, P.; Demartines, N. Enhanced Recovery after Surgery Implementation: From Planning to Success. J. Laparoendosc. Adv. Surg. Tech. 2017, 27, 876–879. [Google Scholar] [CrossRef]
- Hoonhout, L.H.; de Bruijne, M.C.; Wagner, C.; Asscheman, H.; van der Wal, G.; van Tulder, M.W. Nature, occurrence and consequences of medication-related adverse events during hospitalization: A retrospective chart review in the Netherlands. Drug Saf. 2010, 33, 853–864. [Google Scholar] [CrossRef]
- Krahenbuhl-Melcher, A.; Schlienger, R.; Lampert, M.; Haschke, M.; Drewe, J.; Krahenbuhl, S. Drug-related problems in hospitals: A review of the recent literature. Drug Saf. 2007, 30, 379–407. [Google Scholar] [CrossRef]
- Mohammed, M.; Bayissa, B.; Getachew, M.; Adem, F. Drug-related problems and determinants among elective surgical patients: A prospective observational study. SAGE Open Med. 2022, 10, 20503121221122438. [Google Scholar] [CrossRef]
- Davies, E.C.; Green, C.F.; Taylor, S.; Williamson, P.R.; Mottram, D.R.; Pirmohamed, M. Adverse drug reactions in hospital in-patients: A prospective analysis of 3695 patient-episodes. PloS ONE 2009, 4, e4439. [Google Scholar] [CrossRef]
- Miguel, A.; Azevedo, L.F.; Araujo, M.; Pereira, A.C. Frequency of adverse drug reactions in hospitalized patients: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2012, 21, 1139–1154. [Google Scholar] [CrossRef]
- Barlow, A.; Prusak, E.S.; Barlow, B.; Nightingale, G. Interventions to reduce polypharmacy and optimize medication use in older adults with cancer. J. Geriatr. Oncol. 2021, 12, 863–871. [Google Scholar] [CrossRef]
- McIsaac, D.I.; Wong, C.A.; Bryson, G.L.; van Walraven, C. Association of Polypharmacy with Survival, Complications, and Healthcare Resource Use after Elective Noncardiac Surgery: A Population-based Cohort Study. Anesthesiology 2018, 128, 1140–1150. [Google Scholar] [CrossRef]
- Bos, J.M.; van den Bemt, P.M.; Kievit, W.; Pot, J.L.; Nagtegaal, J.E.; Wieringa, A.; van der Westerlaken, M.M.; van der Wilt, G.J.; de Smet, P.A.; Kramers, C. A multifaceted intervention to reduce drug-related complications in surgical patients. Br. J. Clin. Pharmacol. 2017, 83, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Dagnew, S.B.; Binega Mekonnen, G.; Gebeye Zeleke, E.; Agegnew Wondm, S.; Yimer Tadesse, T. Clinical Pharmacist Intervention on Drug-Related Problems among Elderly Patients Admitted to Medical Wards of Northwest Ethiopia Comprehensive Specialized Hospitals: A Multicenter Prospective, Observational Study. BioMed Res. Int. 2022, 2022, 8742998. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, J.; Zeng, Y.; Wang, R.; Wang, J.; Li, X.; Li, J.; Chen, W.; Wang, Y. Promoting the establishment of the position of “surgical pharmacist”. Pharm. Today 2020, 30, 259–260+271. (In Chinese) [Google Scholar]
- Zheng, Z.; Wu, J.; Zeng, Y.; Wang, R.; Wang, J.; Wang, Y. Creating the position of surgical pharmacist in China. Eur. J. Hosp. Pharm. 2020, 27, e99. [Google Scholar] [CrossRef]
- Guangdong Province Pharmaceutical Association. Consensus of medical experts on the management of perioperative medication therapy in ERAS. Pharm. Today 2020, 30, 361–371. (In Chinese) [Google Scholar]
- Wu, J.; Zhang, M.; Wang, R.; Wei, L.; Li, X.; Zeng, Y.; Chen, J.; Ji, B.; Wu, H.; Wang, J.; et al. Surgical pharmacy: Knowledge construction for surgical pharmacists. Pharm. Today 2021, 31, 1–8. (In Chinese) [Google Scholar]
- Zheng, Z.; Wu, J.; Wei, L.; Li, X.; Ji, B.; Wu, H. Surgical pharmacy: The knowledge system of surgical pharmacists. Eur. J. Hosp. Pharm. 2023, 30, e2. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Houck, P.M.; Surgical Infection Prevention Guideline Writers, W. Antimicrobial prophylaxis for surgery: An advisory statement from the National Surgical Infection Prevention Project. Am. J. Surg. 2005, 189, 395–404. [Google Scholar] [CrossRef]
- Kaiser, A.B. Surgical-wound infection. N. Engl. J. Med. 1991, 324, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Young, P.Y.; Khadaroo, R.G. Surgical site infections. Surg. Clin. N. Am. 2014, 94, 1245–1264. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S. Perioperative antibiotics: When, why? Thorac. Surg. Clin. 2005, 15, 229–235, vi. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Frere, C.; Connors, J.M.; Khorana, A.A.; Kakkar, A.; Ay, C.; Munoz, A.; Brenner, B.; Prata, P.H.; Brilhante, D.; et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet. Oncol. 2022, 23, e334–e347. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, G.; Li, Y.; Wang, H.; Liu, H.; Guo, N.; Han, C.; Peng, Y.; Yang, M.; Liu, Y.; et al. Efficacy and safety of thromboprophylaxis in cancer patients: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920907540. [Google Scholar] [CrossRef]
- Akl, E.A.; Vasireddi, S.R.; Gunukula, S.; Barba, M.; Sperati, F.; Terrenato, I.; Muti, P.; Schunemann, H. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst. Rev. 2011, CD006649. [Google Scholar] [CrossRef]
- Hornor, M.A.; Duane, T.M.; Ehlers, A.P.; Jensen, E.H.; Brown, P.S., Jr.; Pohl, D.; da Costa, P.M.; Ko, C.Y.; Laronga, C. American College of Surgeons’ Guidelines for the Perioperative Management of Antithrombotic Medication. J. Am. Coll. Surg. 2018, 227, 521–536.e1. [Google Scholar] [CrossRef]
- Kristensen, S.D.; Knuuti, J.; Saraste, A.; Anker, S.; Botker, H.E.; De Hert, S.; Ford, I.; Gonzalez Juanatey, J.R.; Gorenek, B.; Heyndrickx, G.R.; et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur. J. Anaesthesiol. 2014, 31, 517–573. [Google Scholar] [CrossRef]
- Narouze, S.; Benzon, H.T.; Provenzano, D.; Buvanendran, A.; De Andres, J.; Deer, T.; Rauck, R.; Huntoon, M.A. Interventional Spine and Pain Procedures in Patients on Antiplatelet and Anticoagulant Medications (Second Edition): Guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg. Anesth. Pain Med. 2018, 43, 225–262. [Google Scholar] [CrossRef]
- Diener, H.C.; Cunha, L.; Forbes, C.; Sivenius, J.; Smets, P.; Lowenthal, A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J. Neurol. Sci. 1996, 143, 1–13. [Google Scholar] [CrossRef]
- Mitra, S.; Carlyle, D.; Kodumudi, G.; Kodumudi, V.; Vadivelu, N. New Advances in Acute Postoperative Pain Management. Curr. Pain Headache Rep. 2018, 22, 35. [Google Scholar] [CrossRef]
- Joshi, G.P.; Kehlet, H. Postoperative pain management in the era of ERAS: An overview. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 259–267. [Google Scholar] [CrossRef]
- Tan, M.; Law, L.S.; Gan, T.J. Optimizing pain management to facilitate Enhanced Recovery after Surgery pathways. Can. J. Anaesth. 2015, 62, 203–218. [Google Scholar] [CrossRef]
- Amaechi, O.; Huffman, M.M.; Featherstone, K. Pharmacologic Therapy for Acute Pain. Am. Fam. Physician 2021, 104, 63–72. [Google Scholar]
- Blondell, R.D.; Azadfard, M.; Wisniewski, A.M. Pharmacologic therapy for acute pain. Am. Fam. Physician 2013, 87, 766–772. [Google Scholar]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hubner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Carli, F.; Evans, D.C.; Guilbert, S.; Kozar, R.; Pryor, A.; Thiele, R.H.; Everett, S.; Grocott, M.; Gan, T.J.; et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Nutrition Screening and Therapy within a Surgical Enhanced Recovery Pathway. Anesth. Analg. 2018, 126, 1883–1895. [Google Scholar] [CrossRef]
- Wobith, M.; Weimann, A. Oral Nutritional Supplements and Enteral Nutrition in Patients with Gastrointestinal Surgery. Nutrients 2021, 13, 2655. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hubner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- Elke, G.; van Zanten, A.R.; Lemieux, M.; McCall, M.; Jeejeebhoy, K.N.; Kott, M.; Jiang, X.; Day, A.G.; Heyland, D.K. Enteral versus parenteral nutrition in critically ill patients: An updated systematic review and meta-analysis of randomized controlled trials. Crit. Care 2016, 20, 117. [Google Scholar] [CrossRef]
- Abunnaja, S.; Cuviello, A.; Sanchez, J.A. Enteral and parenteral nutrition in the perioperative period: State of the art. Nutrients 2013, 5, 608–623. [Google Scholar] [CrossRef] [PubMed]
- Hellerman Itzhaki, M.; Singer, P. Advances in Medical Nutrition Therapy: Parenteral Nutrition. Nutrients 2020, 12, 717. [Google Scholar] [CrossRef] [PubMed]
- Cheisson, G.; Jacqueminet, S.; Cosson, E.; Ichai, C.; Leguerrier, A.M.; Nicolescu-Catargi, B.; Ouattara, A.; Tauveron, I.; Valensi, P.; Benhamou, D.; et al. Perioperative management of adult diabetic patients. Intraoperative period. Anaesth. Crit. Care Pain Med. 2018, 37 (Suppl. S1), S21–S25. [Google Scholar] [CrossRef]
- American Diabetes, A. 14. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S144–S151. [Google Scholar] [CrossRef] [PubMed]
- Dhatariya, K.; Levy, N.; Kilvert, A.; Watson, B.; Cousins, D.; Flanagan, D.; Hilton, L.; Jairam, C.; Leyden, K.; Lipp, A.; et al. NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet. Med. J. Br. Diabet. Assoc. 2012, 29, 420–433. [Google Scholar] [CrossRef]
- Palermo, N.E.; Garg, R. Perioperative Management of Diabetes Mellitus: Novel Approaches. Curr. Diabetes Rep. 2019, 19, 14. [Google Scholar] [CrossRef]
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee; Booth, G.; Cheng, A.Y. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Methods. Can. J. Diabetes 2013, 37 (Suppl. S1), S4–S7. [Google Scholar] [CrossRef]
- David, J.S.; Tavernier, B.; Amour, J.; Vivien, B.; Coriat, P.; Riou, B. Myocardial effects of halothane and sevoflurane in diabetic rats. Anesthesiology 2004, 100, 1179–1187. [Google Scholar] [CrossRef]
- Kadoi, Y. Blood glucose control in the perioperative period. Minerva Anestesiol. 2012, 78, 574–595. [Google Scholar]
- Deacon, S.P.; Karunanayake, A.; Barnett, D. Acebutolol, atenolol, and propranolol and metabolic responses to acute hypoglycaemia in diabetics. Br. Med. J. 1977, 2, 1255–1257. [Google Scholar] [CrossRef]
- Saugel, B.; Sessler, D.I. Perioperative Blood Pressure Management. Anesthesiology 2021, 134, 250–261. [Google Scholar] [CrossRef]
- Saugel, B.; Kouz, K.; Hoppe, P.; Maheshwari, K.; Scheeren, T.W.L. Predicting hypotension in perioperative and intensive care medicine. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 189–197. [Google Scholar] [CrossRef]
- Sousa-Uva, M.; Milojevic, M.; Head, S.J.; Jeppsson, A. The 2017 EACTS guidelines on perioperative medication in adult cardiac surgery and patient blood management. Eur. J. Cardio-Thorac. Surg. 2018, 53, 1–2. [Google Scholar] [CrossRef]
- San Roman, J.A.; Spanish Society of Cardiology Working Group for the the 2014 ESC/ESA Guidelines on Non-Cardiac Surgery; Expert Reviewers for the 2014 ESC/ESA Guidelines on Non-Cardiac Surgery; Clinical Practice Guidelines Committee of the Spanish Society of Cardiology. Comments on the 2014 ESC/ESA Guidelines on Noncardiac Surgery: Cardiovascular assessment and management. Rev. Esp. Cardiol. 2014, 67, 980–985. [Google Scholar] [CrossRef]
- Brandstrup, B.; Tønnesen, H.; Beier-Holgersen, R.; Hjortsø, E.; Ørding, H.; Lindorff-Larsen, K.; Rasmussen, M.S.; Lanng, C.; Wallin, L.; Iversen, L.H.; et al. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann. Surg. 2003, 238, 641–648. [Google Scholar] [CrossRef]
- Asklid, D.; Segelman, J.; Gedda, C.; Hjern, F.; Pekkari, K.; Gustafsson, U.O. The impact of perioperative fluid therapy on short-term outcomes and 5-year survival among patients undergoing colorectal cancer surgery—A prospective cohort study within an ERAS protocol. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2017, 43, 1433–1439. [Google Scholar] [CrossRef]
- Scheib, S.A.; Thomassee, M.; Kenner, J.L. Enhanced Recovery after Surgery in Gynecology: A Review of the Literature. J. Minim. Invasive Gynecol. 2019, 26, 327–343. [Google Scholar] [CrossRef]
- Navarro, L.H.; Bloomstone, J.A.; Auler, J.O., Jr.; Cannesson, M.; Rocca, G.D.; Gan, T.J.; Kinsky, M.; Magder, S.; Miller, T.E.; Mythen, M.; et al. Perioperative fluid therapy: A statement from the international Fluid Optimization Group. Perioper. Med. 2015, 4, 3. [Google Scholar] [CrossRef]
- Apfel, C.C.; Heidrich, F.M.; Jukar-Rao, S.; Jalota, L.; Hornuss, C.; Whelan, R.P.; Zhang, K.; Cakmakkaya, O.S. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br. J. Anaesth. 2012, 109, 742–753. [Google Scholar] [CrossRef]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef]
- Choi, Y.S.; Sohn, H.M.; Do, S.H.; Min, K.T.; Woo, J.H.; Baik, H.J. Comparison of ramosetron and ondansetron for the treatment of established postoperative nausea and vomiting after laparoscopic surgery: A prospective, randomized, double-blinded multicenter trial. Ther. Clin. Risk Manag. 2018, 14, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Deitrick, C.L.; Mick, D.J.; Lauffer, V.; Prostka, E.; Nowak, D.; Ingersoll, G. A comparison of two differing doses of promethazine for the treatment of postoperative nausea and vomiting. J. Perianesthesia Nurs. 2015, 30, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Kjellberg, F.; Henzi, I.; Tramèr, M.R. Treatment of established postoperative nausea and vomiting: A quantitative systematic review. BMC Anesthesiol. 2001, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Janssen, T.L.; Alberts, A.R.; Hooft, L.; Mattace-Raso, F.; Mosk, C.A.; van der Laan, L. Prevention of postoperative delirium in elderly patients planned for elective surgery: Systematic review and meta-analysis. Clin. Interv. Aging 2019, 14, 1095–1117. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R. Delirium in Hospitalized Older Adults. N. Engl. J. Med. 2018, 378, 96. [Google Scholar] [CrossRef]
- Allen, S.R.; Frankel, H.L. Postoperative complications: Delirium. Surg. Clin. N. Am. 2012, 92, 409–431. [Google Scholar] [CrossRef]
- Hughes, C.G.; Boncyk, C.S.; Culley, D.J.; Fleisher, L.A.; Leung, J.M.; McDonagh, D.L.; Gan, T.J.; McEvoy, M.D.; Miller, T.E.; Perioperative Quality Initiative, W. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Delirium Prevention. Anesth. Analg. 2020, 130, 1572–1590. [Google Scholar] [CrossRef]
- Santos, E.; Cardoso, D.; Neves, H.; Cunha, M.; Rodrigues, M.; Apostolo, J. Effectiveness of haloperidol prophylaxis in critically ill patients with a high risk of delirium: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 1440–1472. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Gao, M.; Guo, W.; Ma, Y. Dexmedetomidine reduces postoperative delirium after joint replacement in elderly patients with mild cognitive impairment. Aging Clin. Exp. Res. 2016, 28, 729–736. [Google Scholar] [CrossRef]
- Gamberini, M.; Bolliger, D.; Lurati Buse, G.A.; Burkhart, C.S.; Grapow, M.; Gagneux, A.; Filipovic, M.; Seeberger, M.D.; Pargger, H.; Siegemund, M.; et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery—A randomized controlled trial. Crit. Care Med. 2009, 37, 1762–1768. [Google Scholar] [CrossRef]
- Liptzin, B.; Laki, A.; Garb, J.L.; Fingeroth, R.; Krushell, R. Donepezil in the prevention and treatment of post-surgical delirium. Am. J. Geriatr. Psychiatry 2005, 13, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Rengel, K.F.; Pandharipande, P.P.; Hughes, C.G. Postoperative delirium. Presse Med. 2018, 47, e53–e64. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, G.; Baeza, N.; Cabre, L.; Portillo, E.; Gimeno, G.; Manzanedo, D.; Calizaya, M. Dexmedetomidine for the Treatment of Hyperactive Delirium Refractory to Haloperidol in Nonintubated ICU Patients: A Nonrandomized Controlled Trial. Crit. Care Med. 2016, 44, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, P.; Bordeianou, L. Implementation of an ERAS Pathway in Colorectal Surgery. Clin. Colon Rectal Surg. 2019, 32, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect. Control Hosp. Epidemiol. 1999, 20, 250–278. [Google Scholar] [CrossRef]
- National Health and Family Planning Commission of the People’s Republic of China; State Administration of Traditional Chinese Medicine of the People’s Republic of China; Medical Department of the People’s Liberation Army General Logistics Department. Guiding Principles for Clinical Use of Antibiotic (Version 2015) (In Chinese). 2015. Available online: https://www.nhc.gov.cn/yzygj/s3593/201508/c18e1014de6c45ed9f6f9d592b43db42.shtml (accessed on 27 August 2015).
- Beloeil, H.; Sulpice, L. Peri-operative pain and its consequences. J. Visc. Surg. 2016, 153, S15–S18. [Google Scholar] [CrossRef]
- Benoist, S.; Brouquet, A. Nutritional assessment and screening for malnutrition. J. Visc. Surg. 2015, 152 (Suppl. S1), S3–S7. [Google Scholar] [CrossRef]
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review [Formula: See text]. JPEN J. Parenter. Enter. Nutr. 2017, 41, 744–758. [Google Scholar] [CrossRef]
- Skeie, E.; Tangvik, R.J.; Nymo, L.S.; Harthug, S.; Lassen, K.; Viste, A. Weight loss and BMI criteria in GLIM’s definition of malnutrition is associated with postoperative complications following abdominal resections—Results from a National Quality Registry. Clin. Nutr. 2020, 39, 1593–1599. [Google Scholar] [CrossRef]
- Kakavas, S.; Karayiannis, D.; Bouloubasi, Z.; Poulia, K.A.; Kompogiorgas, S.; Konstantinou, D.; Vougas, V. Global Leadership Initiative on Malnutrition Criteria Predict Pulmonary Complications and 90-Day Mortality after Major Abdominal Surgery in Cancer Patients. Nutrients 2020, 12, 3726. [Google Scholar] [CrossRef]
- Beser, O.F.; Cokugras, F.C.; Erkan, T.; Kutlu, T.; Yagci, R.V. Evaluation of malnutrition development risk in hospitalized children. Nutrition 2018, 48, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Levetan, C.S.; Passaro, M.; Jablonski, K.; Kass, M.; Ratner, R.E. Unrecognized diabetes among hospitalized patients. Diabetes Care 1998, 21, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Frisch, A.; Chandra, P.; Smiley, D.; Peng, L.; Rizzo, M.; Gatcliffe, C.; Hudson, M.; Mendoza, J.; Johnson, R.; Lin, E.; et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010, 33, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Smiley, D.D.; Umpierrez, G.E. Perioperative glucose control in the diabetic or nondiabetic patient. South. Med. J. 2006, 99, 580–589, quiz 590–581. [Google Scholar] [CrossRef]
- Noordzij, P.G.; Boersma, E.; Schreiner, F.; Kertai, M.D.; Feringa, H.H.; Dunkelgrun, M.; Bax, J.J.; Klein, J.; Poldermans, D. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur. J. Endocrinol. 2007, 156, 137–142. [Google Scholar] [CrossRef]
- Kwon, S.; Thompson, R.; Dellinger, P.; Yanez, D.; Farrohki, E.; Flum, D. Importance of perioperative glycemic control in general surgery: A report from the Surgical Care and Outcomes Assessment Program. Ann. Surg. 2013, 257, 8–14. [Google Scholar] [CrossRef]
- Raju, T.A.; Torjman, M.C.; Goldberg, M.E. Perioperative blood glucose monitoring in the general surgical population. J. Diabetes Sci. Technol. 2009, 3, 1282–1287. [Google Scholar] [CrossRef]
- Gandhi, G.Y.; Nuttall, G.A.; Abel, M.D.; Mullany, C.J.; Schaff, H.V.; Williams, B.A.; Schrader, L.M.; Rizza, R.A.; McMahon, M.M. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin. Proc. 2005, 80, 862–866. [Google Scholar] [CrossRef]
- Himes, C.P.; Ganesh, R.; Wight, E.C.; Simha, V.; Liebow, M. Perioperative Evaluation and Management of Endocrine Disorders. Mayo Clin. Proc. 2020, 95, 2760–2774. [Google Scholar] [CrossRef]
- Sizemore, D.C.; Singh, A.; Dua, A.; Singh, K.; Grose, B.W. Postoperative Nausea. In StatPearls, © 2023; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- De Lange, E.; Verhaak, P.F.; van der Meer, K. Prevalence, presentation and prognosis of delirium in older people in the population, at home and in long term care: A review. Int. J. Geriatr. Psychiatry 2013, 28, 127–134. [Google Scholar] [CrossRef]
- Siddiqi, N.; House, A.O.; Holmes, J.D. Occurrence and outcome of delirium in medical in-patients: A systematic literature review. Age Ageing 2006, 35, 350–364. [Google Scholar] [CrossRef]
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Lovely, J.K.; Hyland, S.J.; Smith, A.N.; Nelson, G.; Ljungqvist, O.; Parrish, R.H., 2nd. Clinical pharmacist perspectives for optimizing pharmacotherapy within Enhanced Recovery after Surgery (ERAS((R))) programs. Int. J. Surg. 2019, 63, 58–62. [Google Scholar] [CrossRef]
- Ljungqvist, O.; Thanh, N.X.; Nelson, G. ERAS-Value based surgery. J. Surg. Oncol. 2017, 116, 608–612. [Google Scholar] [CrossRef]
- Lovely, J.K.; Larson, D.W.; Quast, J.M. A clinical practice agreement between pharmacists and surgeons streamlines medication management. Jt. Comm. J. Qual. Patient Saf. 2014, 40, 296–302. [Google Scholar] [CrossRef]
- Hammond, R.W.; Schwartz, A.H.; Campbell, M.J.; Remington, T.L.; Chuck, S.; Blair, M.M.; Vassey, A.M.; Rospond, R.M.; Herner, S.J.; Webb, C.E. Collaborative drug therapy management by pharmacists—2003. Pharmacotherapy 2003, 23, 1210–1225. [Google Scholar] [CrossRef]
- Dobesh, P.P.; Trujillo, T.C.; Finks, S.W. Role of the pharmacist in achieving performance measures to improve the prevention and treatment of venous thromboembolism. Pharmacotherapy 2013, 33, 650–664. [Google Scholar] [CrossRef]
- Louzon, P.; Jennings, H.; Ali, M.; Kraisinger, M. Impact of pharmacist management of pain, agitation, and delirium in the intensive care unit through participation in multidisciplinary bundle rounds. Am. J. Health Syst. Pharm. AJHP Off. J. Am. Soc. Health Syst. Pharm. 2017, 74, 253–262. [Google Scholar] [CrossRef]
- Neville, H.L.; Chevalier, B.; Daley, C.; Nodwell, L.; Harding, C.; Hiltz, A.; MacDonald, T.; Skedgel, C.; MacKinnon, N.J.; Slayter, K. Clinical benefits and economic impact of post-surgical care provided by pharmacists in a Canadian hospital. Int. J. Pharm. Pract. 2014, 22, 216–222. [Google Scholar] [CrossRef]
- Charpiat, B.; Goutelle, S.; Schoeffler, M.; Aubrun, F.; Viale, J.P.; Ducerf, C.; Leboucher, G.; Allenet, B. Prescriptions analysis by clinical pharmacists in the post-operative period: A 4-year prospective study. Acta Anaesthesiol. Scand. 2012, 56, 1047–1051. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Lam, A.; Banakh, I.; Lam, S.; Crofts, T. Improved Medication Management with Introduction of a Perioperative and Prescribing Pharmacist Service. J. Pharm. Pract. 2020, 33, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Beckman, E.J.; Hovey, S.; Bondi, D.S.; Patel, G.; Parrish, R.H., 2nd. Pediatric Perioperative Clinical Pharmacy Practice: Clinical Considerations and Management: An Opinion of the Pediatrics and Perioperative Care Practice and Research Networks of the American College of Clinical Pharmacy. J. Pediatr. Pharmacol. Ther. JPPT Off. J. PPAG 2022, 27, 490–505. [Google Scholar] [CrossRef]
- Rove, K.O.; Edney, J.C.; Brockel, M.A. Enhanced recovery after surgery in children: Promising, evidence-based multidisciplinary care. Pediatr. Anesth. 2018, 28, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.D.; Ribeiro, L.C.; Pereira dos Santos, J.C.; Ayres, L.R.; Chemello, C. Medication Reconciliation at hospital admission and discharge: Evaluation of fidelity and process outcomes in a real-world setting. Int. J. Clin. Pract. 2021, 75, e14656. [Google Scholar] [CrossRef]
- Zheng, X.; Xiao, L.; Li, Y.; Qiu, F.; Huang, W.; Li, X. Improving safety and efficacy with pharmacist medication reconciliation in orthopedic joint surgery within an enhanced recovery after surgery program. BMC Health Serv. Res. 2022, 22, 448. [Google Scholar] [CrossRef]
- Guisado-Gil, A.B.; Ramirez-Duque, N.; Baron-Franco, B.; Sanchez-Hidalgo, M.; De la Portilla, F.; Santos-Rubio, M.D. Impact of a multidisciplinary medication reconciliation program on clinical outcomes: A pre-post intervention study in surgical patients. Res. Soc. Adm. Pharm. RSAP 2021, 17, 1306–1312. [Google Scholar] [CrossRef]
- Akamine, A.; Nagasaki, Y.; Tomizawa, A.; Arai, M.; Atsuda, K. Risk Factors for Non-Adherence to Medications That Affect Surgery: A Retrospective Study in Japan. Patient Prefer. Adherence 2022, 16, 1623–1635. [Google Scholar] [CrossRef]
- Wang, R.; Dong, X.; Zhang, X.; Gan, S.; Kong, L.; Lu, X.; Rao, Y. Pharmacist-driven multidisciplinary initiative continuously improves postoperative nausea and vomiting in female patients undergoing abdominal surgery. J. Clin. Pharm. Ther. 2020, 45, 959–967. [Google Scholar] [CrossRef]
- Khan, Y.H.; Alzarea, A.I.; Alotaibi, N.H.; Alatawi, A.D.; Khokhar, A.; Alanazi, A.S.; Butt, M.H.; Alshehri, A.A.; Alshehri, S.; Alatawi, Y.; et al. Evaluation of Impact of a Pharmacist-Led Educational Campaign on Disease Knowledge, Practices and Medication Adherence for Type-2 Diabetic Patients: A Prospective Pre- and Post-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 10060. [Google Scholar] [CrossRef]
- Zhuo, Y.; Pan, Y.; Lin, K.; Yin, G.; Wu, Y.; Xu, J.; Cai, D.; Xu, L. Effectiveness of clinical pharmacist-led smartphone application on medication adherence, insulin injection technique and glycemic control for women with gestational diabetes receiving multiple daily insulin injection: A randomized clinical trial. Prim. Care Diabetes 2022, 16, 264–270. [Google Scholar] [CrossRef]
- Poonprapai, P.; Lerkiatbundit, S.; Saengcharoen, W. Family support-based intervention using a mobile application provided by pharmacists for older adults with diabetes to improve glycaemic control: A randomised controlled trial. Int. J. Clin. Pharm. 2022, 44, 680–688. [Google Scholar] [CrossRef]
- Shahrami, B.; Sefidani Forough, A.; Najmeddin, F.; Hadidi, E.; Toomaj, S.; Javadi, M.R.; Gholami, K.; Sadeghi, K. Identification of drug-related problems followed by clinical pharmacist interventions in an outpatient pharmacotherapy clinic. J. Clin. Pharm. Ther. 2022, 47, 964–972. [Google Scholar] [CrossRef]
- Peasah, S.K.; Hammond, T.; Campbell, V.; Liu, Y.; Morgan, M.; Kearney, S.; Good, C.B. Assessing the impact of adding pharmacist management services to an existing discharge planning program on 30-day readmissions. J. Am. Pharm. Assoc. JAPhA 2022, 62, 734–739. [Google Scholar] [CrossRef]
- Van Lieshout, J.; Lacroix, J.; van Halteren, A.; Teichert, M. Effectiveness of a Pharmacist-Led Web-Based Medication Adherence Tool with Patient-Centered Communication: Results of a Clustered Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, e16141. [Google Scholar] [CrossRef]
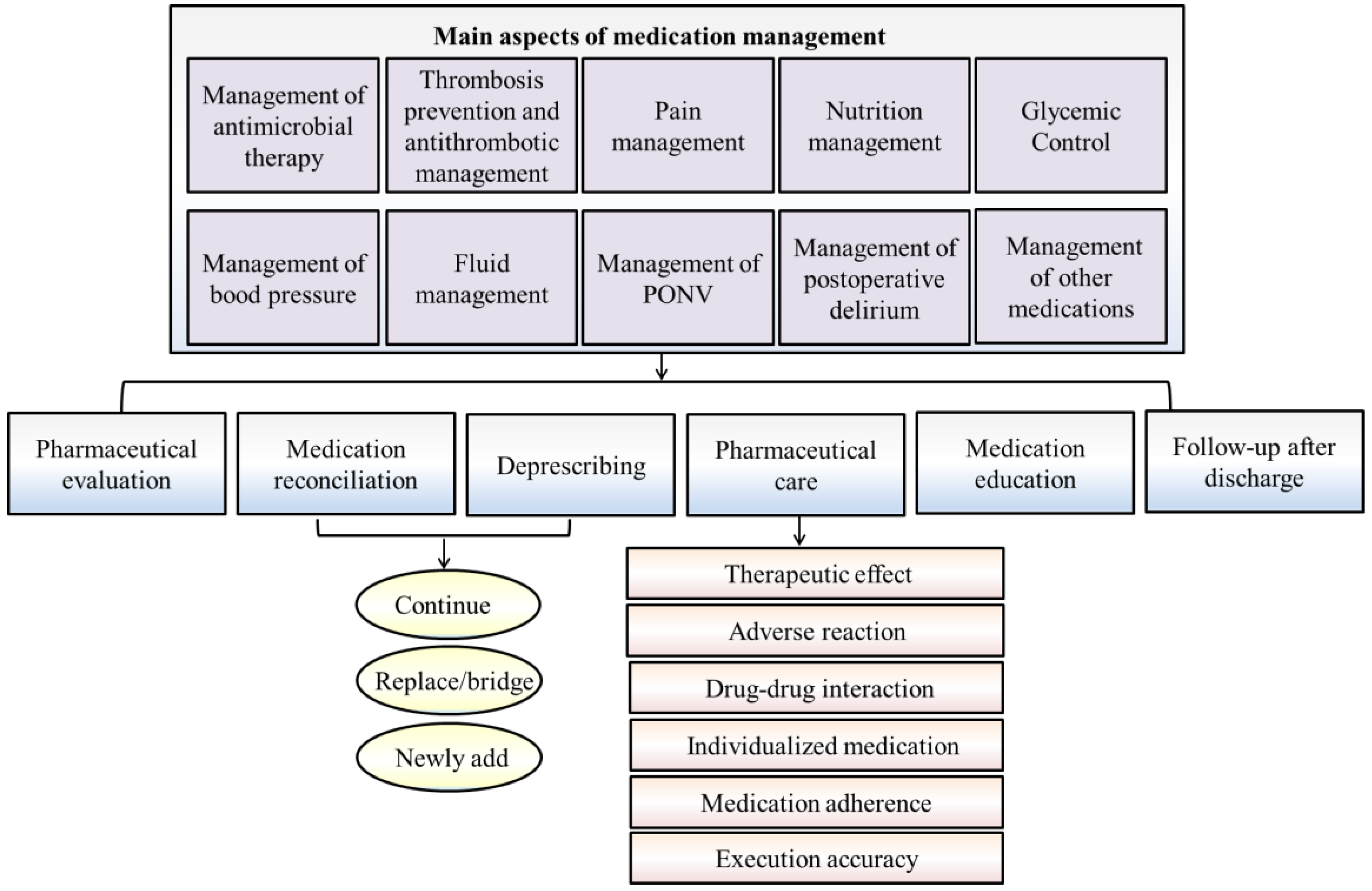

| Main Aspects | Key Points of Medication Monitoring |
|---|---|
| Management of antimicrobial therapy [20,21,22,23] |
|
| Thrombosis prevention and antithrombotic management [24,25,26,27,28,29,30] |
|
| Pain management [31,32,33,34,35] |
(2) Opioid conversion, evaluation of evidence-based medical strategy and other pharmacy services; (3) Pain health education and analgesic medication advice; (4) Quality control of pain assessment.
|
| Nutrition management [36,37,38,39,40,41,42] |
|
| Glycemic control [43,44,45,46,47,48,49,50] |
|
| Management of blood pressure [51,52,53,54] |
|
| Fluid management [55,56,57,58] | 1. According to different therapeutic purposes, disease states and stages, a reasonable fluid treatment plan is formulated and implemented individually; 2. Maintain the homeostasis of body fluids and avoid postoperative complications and gastrointestinal dysfunction due to fluid overload or organ insufficiency; 3. For patients who have insufficient blood volume and need a large amount of fluid replacement, it is recommended to supplement the crystalloid solution and infuse the colloidal solution appropriately to control the infusion volume and reduce tissue edema; 4. For patients who do not have hypovolemia (only extracellular fluid or functional extracellular fluid), it is recommended to supplement the physiological requirement with a crystalloid solution; 5. For critically ill patients who require a large amount of fluid resuscitation, especially when complicated with acute lung injury, it is recommended to choose albumin for goal-directed restrictive fluid therapy. |
| Management of postoperative nausea and vomiting (PONV) [59,60,61,62,63] | 1. Identify the patient’s risk for PONV and take corresponding preventive measures according to the patient’s risk grade; 2. Use multimodal prophylaxis in patients with one or more risk factors; 3. Administer PONV prophylaxis Using two agents in adults at 1–2 risks for PONV; 4. Administer PONV prophylaxis Using four agents in adults at > 2 risks for PONV; 5. For patients who do not receive PONV prophylaxis, low-dose 5-HT3 receptor antagonist therapy remains the first line for dealing with the occurrence of PONV; 6. If PONV prophylaxis fails, drugs with different mechanisms of action may be used for prophylaxis. |
| Management of postoperative delirium (POD) [64,65,66,67,68,69,70,71,72,73,74] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Huang, X.; Gao, M.; Wei, L.; Wang, R.; Chen, J.; Zeng, Y.; Ji, B.; Liu, T.; Wang, J.; et al. Surgical Pharmacy for Optimizing Medication Therapy Management Services within Enhanced Recovery after Surgery (ERAS®) Programs. J. Clin. Med. 2023, 12, 631. https://doi.org/10.3390/jcm12020631
Xie J, Huang X, Gao M, Wei L, Wang R, Chen J, Zeng Y, Ji B, Liu T, Wang J, et al. Surgical Pharmacy for Optimizing Medication Therapy Management Services within Enhanced Recovery after Surgery (ERAS®) Programs. Journal of Clinical Medicine. 2023; 12(2):631. https://doi.org/10.3390/jcm12020631
Chicago/Turabian StyleXie, Jingwen, Xiaoyan Huang, Min Gao, Li Wei, Ruolun Wang, Jisheng Chen, Yingtong Zeng, Bo Ji, Tao Liu, Jinghao Wang, and et al. 2023. "Surgical Pharmacy for Optimizing Medication Therapy Management Services within Enhanced Recovery after Surgery (ERAS®) Programs" Journal of Clinical Medicine 12, no. 2: 631. https://doi.org/10.3390/jcm12020631
APA StyleXie, J., Huang, X., Gao, M., Wei, L., Wang, R., Chen, J., Zeng, Y., Ji, B., Liu, T., Wang, J., Wu, H., Wang, Y., Qin, L., Wang, Y., Zheng, Z., Xue, J., Wu, J., Chen, X., Zheng, Z., & Li, X. (2023). Surgical Pharmacy for Optimizing Medication Therapy Management Services within Enhanced Recovery after Surgery (ERAS®) Programs. Journal of Clinical Medicine, 12(2), 631. https://doi.org/10.3390/jcm12020631






