Association between Serum Soluble α-Klotho and Urinary Albumin Excretion in Middle-Aged and Older US Adults: NHANES 2007–2016
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Population
2.2. Assessment of Increased UAE
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Baseline Features
3.2. The Association between α-Klotho and ACR
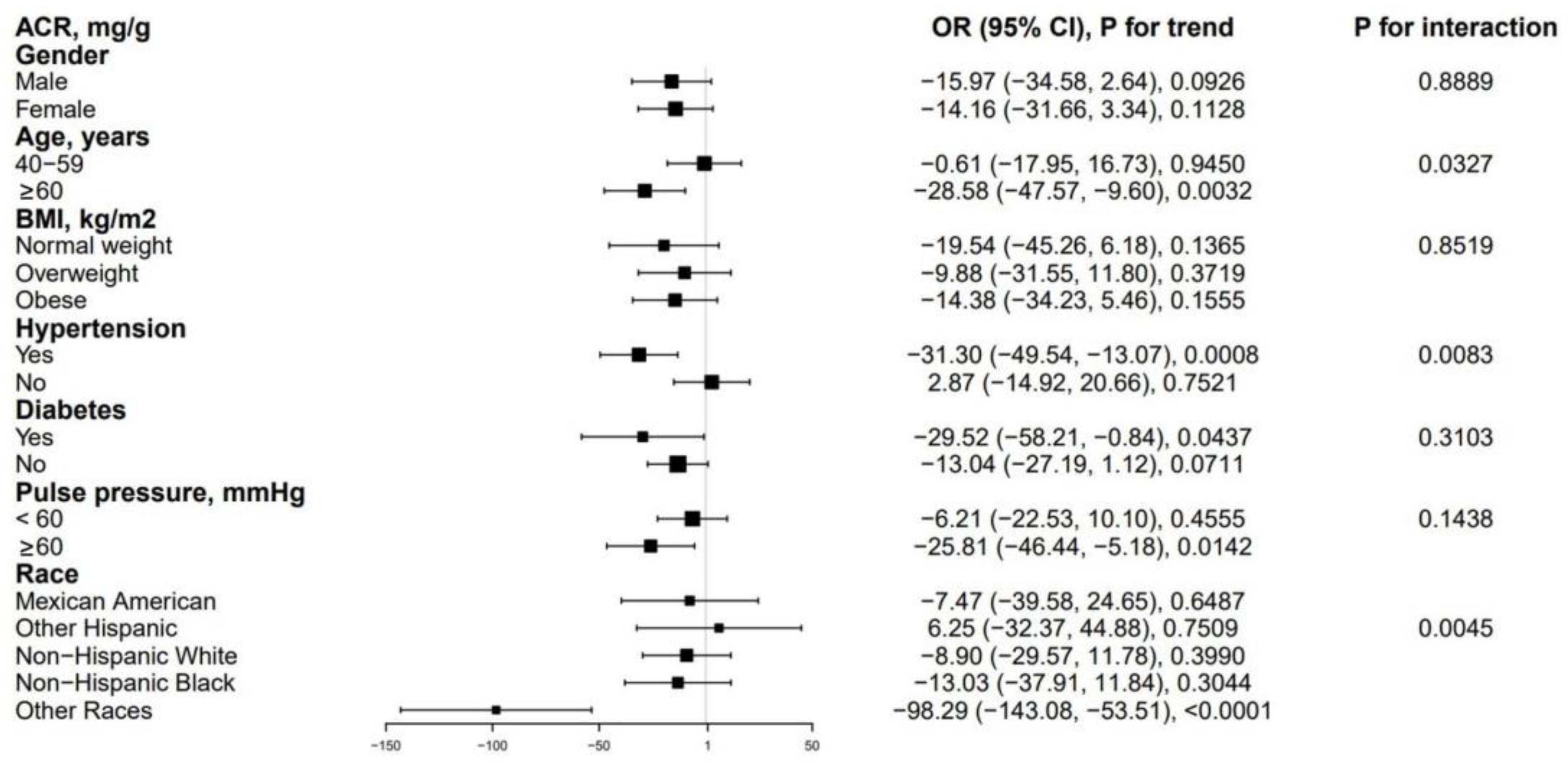
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olauson, H.; Mencke, R.; Hillebrands, J.L.; Larsson, T.E. Tissue expression and source of circulating αKlotho. Bone 2017, 100, 19–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Liu, Y.; Goetz, R.; Fu, L.; Jayaraman, S.; Hu, M.C.; Moe, O.W.; Liang, G.; Li, X.; Mohammadi, M. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 2018, 553, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 2004, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kinoshita, S.; Shiraishi, N.; Nakagawa, S.; Sekine, S.; Fujimori, T.; Nabeshima, Y.I. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech. Dev. 2000, 98, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Onishi, K.; Miyake, M.; Hori, S.; Onishi, S.; Iida, K.; Morizawa, Y.; Tatsumi, Y.; Nakai, Y.; Tanaka, N.; Fujimoto, K. γ-Klotho is correlated with resistance to docetaxel in castration-resistant prostate cancer. Oncol. Lett. 2020, 19, 2306–2316. [Google Scholar] [CrossRef] [Green Version]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Yanucil, C.; Kentrup, D.; Campos, I.; Czaya, B.; Heitman, K.; Westbrook, D.; Osis, G.; Grabner, A.; Wende, A.R.; Vallejo, J.; et al. Soluble α-klotho and heparin modulate the pathologic cardiac actions of fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2022, 102, 261–279. [Google Scholar] [CrossRef]
- Ciardullo, S.; Perseghin, G. Soluble α-Klotho levels, glycemic control and renal function in US adults with type 2 diabetes. Acta Diabetol. 2022, 59, 803–809. [Google Scholar] [CrossRef]
- Yu, L.; Kang, L.; Ren, X.Z.; Diao, Z.L.; Liu, W.H. Circulating α-Klotho Levels in Hemodialysis Patients and Their Relationship to Atherosclerosis. Kidney Blood Press. Res. 2018, 43, 1174–1182. [Google Scholar] [CrossRef]
- Ligumsky, H.; Merenbakh-Lamin, K.; Keren-Khadmy, N.; Wolf, I.; Rubinek, T. The role of α-klotho in human cancer: Molecular and clinical aspects. Oncogene 2022, 41, 4487–4497. [Google Scholar] [CrossRef]
- Kresovich, J.K.; Bulka, C.M. Low Serum Klotho Associated With All-cause Mortality Among a Nationally Representative Sample of American Adults. J. Gerontol A Biol. Sci. Med. Sci. 2022, 77, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Koyama, D.; Sato, Y.; Aizawa, M.; Maki, T.; Kurosawa, M.; Kuro-o, M.; Furukawa, Y. Soluble αKlotho as a candidate for the biomarker of aging. Biochem. Biophys. Res. Commun. 2015, 467, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Kuro, O.M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Davidsohn, N.; Pezone, M.; Vernet, A.; Graveline, A.; Oliver, D.; Slomovic, S.; Punthambaker, S.; Sun, X.; Liao, R.; Bonventre, J.V.; et al. A single combination gene therapy treats multiple age-related diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 23505–23511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.H.; Kuro, O.M.; Chen, C.H.; Sue, Y.M.; Chen, Y.C.; Wu, H.H.; Cheng, C.Y. The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur. J. Pharm. 2013, 698, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yin, X.; Zhao, Y.; Chen, H.; Tan, T.; Yao, P.; Tang, Y. Biological ageing and the risks of all-cause and cause-specific mortality among people with diabetes: A prospective cohort study. J. Epidemiol. Community Health 2022, 76, 771–778. [Google Scholar] [CrossRef]
- Ortiz, A.; Mattace-Raso, F.; Soler, M.J.; Fouque, D. Ageing meets kidney disease. Age Ageing 2022, 51, afac157. [Google Scholar] [CrossRef]
- Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [CrossRef] [Green Version]
- Kim, H.R.; Nam, B.Y.; Kim, D.W.; Kang, M.W.; Han, J.H.; Lee, M.J.; Shin, D.H.; Doh, F.M.; Koo, H.M.; Ko, K.I.; et al. Circulating α-klotho levels in CKD and relationship to progression. Am. J. Kidney Dis. 2013, 61, 899–909. [Google Scholar] [CrossRef]
- Buchanan, S.; Combet, E.; Stenvinkel, P.; Shiels, P.G. Klotho, Aging, and the Failing Kidney. Front. Endocrinol. 2020, 11, 560. [Google Scholar] [CrossRef]
- Neyra, J.A.; Hu, M.C. Potential application of klotho in human chronic kidney disease. Bone 2017, 100, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. The emerging role of Klotho in clinical nephrology. Nephrol. Dial. Transplant. 2012, 27, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat. Rev. Nephrol 2013, 9, 650–660. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol 2011, 22, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonelli, M.; Muntner, P.; Lloyd, A.; Manns, B.J.; James, M.T.; Klarenbach, S.; Quinn, R.R.; Wiebe, N.; Hemmelgarn, B.R. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: A cohort study. Ann. Intern. Med. 2011, 154, 12–21. [Google Scholar] [CrossRef]
- Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; van der Velde, M.; Woodward, M.; Levey, A.S.; Jong, P.E.; Coresh, J.; Astor, B.C.; Matsushita, K.; et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011, 79, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Fernández, B.; Valiño-Rivas, L.; Sánchez-Niño, M.D.; Ortiz, A. Albuminuria Downregulation of the Anti-Aging Factor Klotho: The Missing Link Potentially Explaining the Association of Pathological Albuminuria with Premature Death. Adv. Ther. 2020, 37, 62–72. [Google Scholar] [CrossRef]
- Rabelink, T.J.; de Zeeuw, D. The glycocalyx--linking albuminuria with renal and cardiovascular disease. Nat. Rev. Nephrol. 2015, 11, 667–676. [Google Scholar] [CrossRef]
- Ortiz, A.; Fernandez-Fernandez, B. Humble kidneys predict mighty heart troubles. Lancet Diabetes Endocrinol. 2015, 3, 489–491. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008, 31 (Suppl. S1), S12–S54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Katz, R.; Kritchevsky, S.; Ix, J.; Shlipak, M.; Gutiérrez, O.M.; Newman, A.; Hoofnagle, A.; Fried, L.; Semba, R.D.; et al. Association between Soluble Klotho and Change in Kidney Function: The Health Aging and Body Composition Study. J. Am. Soc. Nephrol. 2017, 28, 1859–1866. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhou, X.; Deng, L.; Jin, K.; Xiong, X.; Su, X.; Qiu, S.; Yang, L. The association between serum soluble Klotho and chronic kidney disease among us adults ages 40 to 79 years: Cross-sectional study. Front. Public Health 2022, 10, 995314. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- WHO. Obesity: Preventing and Managing the Global Epidemic. Technical Report Series 894. WHO. 2000. Available online: http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ (accessed on 5 September 2016).
- Landry, T.; Shookster, D.; Huang, H. Circulating α-klotho regulates metabolism via distinct central and peripheral mechanisms. Metabolism 2021, 121, 154819. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020, 72, 785–801. [Google Scholar] [CrossRef] [Green Version]
- Menke, A.; Casagrande, S.; Geiss, L.; Cowie, C.C. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA 2015, 314, 1021–1029. [Google Scholar] [CrossRef] [Green Version]
- Ushigome, E.; Fukui, M.; Hamaguchi, M.; Matsumoto, S.; Mineoka, Y.; Nakanishi, N.; Senmaru, T.; Yamazaki, M.; Hasegawa, G.; Nakamura, N. Morning pulse pressure is associated more strongly with elevated albuminuria than systolic blood pressure in patients with type 2 diabetes mellitus: Post hoc analysis of a cross-sectional multicenter study. Diabetes Res. Clin. Pract. 2013, 101, 270–277. [Google Scholar] [CrossRef]
- Fryar, C.D.; Ostchega, Y.; Hales, C.M.; Zhang, G.; Kruszon-Moran, D. Hypertension Prevalence and Control Among Adults: United States, 2015–2016. NCHS Data Brief. 2017, 289, 1–8. [Google Scholar]
- US Department of Health and Human Services, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes.htm (accessed on 6 January 2023).
- Abraham, C.R.; Li, A. Aging-suppressor Klotho: Prospects in diagnostics and therapeutics. Ageing Res. Rev. 2022, 82, 101766. [Google Scholar] [CrossRef] [PubMed]
- Alkalbani, M.; Prabhu, G.; Lagbo, J.; Qayyum, R. Serum Klotho and pulse pressure; insight from NHANES. Int J. Cardiol. 2022, 355, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Liu, F.; Peng, Y.; Wang, P.; Ma, B.; Li, L.; Si, C.; Wang, X.; Zhang, M.; Song, F. Association of serum Klotho levels with cancer and cancer mortality: Evidence from National Health and Nutrition Examination Survey. Cancer Med. 2022. [CrossRef] [PubMed]
- Maltese, G.; Fountoulakis, N.; Siow, R.C.; Gnudi, L.; Karalliedde, J. Perturbations of the anti-ageing hormone Klotho in patients with type 1 diabetes and microalbuminuria. Diabetologia 2017, 60, 911–914. [Google Scholar] [CrossRef] [Green Version]
- Navarro-González, J.F.; Sánchez-Niño, M.D.; Donate-Correa, J.; Martín-Núñez, E.; Ferri, C.; Pérez-Delgado, N.; Górriz, J.L.; Martínez-Castelao, A.; Ortiz, A.; Mora-Fernández, C. Effects of Pentoxifylline on Soluble Klotho Concentrations and Renal Tubular Cell Expression in Diabetic Kidney Disease. Diabetes Care 2018, 41, 1817–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.C.; Shi, M.; Zhang, J.; Addo, T.; Cho, H.J.; Barker, S.L.; Ravikumar, P.; Gillings, N.; Bian, A.; Sidhu, S.S.; et al. Renal Production, Uptake, and Handling of Circulating αKlotho. J. Am. Soc. Nephrol. 2016, 27, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, M.; Acikgoz, S.B.; Genc, A.B.; Yaylaci, S.; Dheir, H.; Sipahi, S.S. The levels of inflammatory biomarkers in hemodialysis and peritoneal dialysis patients. Rev. Assoc. Med. Bras. (1992) 2021, 67, 718–723. [Google Scholar] [CrossRef]
- Lisowska, K.A.; Storoniak, H.; Soroczyńska-Cybula, M.; Maziewski, M.; Dębska-Ślizień, A. Serum Levels of α-Klotho, Inflammation-Related Cytokines, and Mortality in Hemodialysis Patients. J. Clin. Med. 2022, 11, 6518. [Google Scholar] [CrossRef]
- Kadoya, H.; Satoh, M.; Haruna, Y.; Sasaki, T.; Kashihara, N. Klotho attenuates renal hypertrophy and glomerular injury in Ins2Akita diabetic mice. Clin. Exp. Nephrol. 2016, 20, 671–678. [Google Scholar] [CrossRef]
- Kim, J.H.; Xie, J.; Hwang, K.H.; Wu, Y.L.; Oliver, N.; Eom, M.; Park, K.S.; Barrezueta, N.; Kong, I.D.; Fracasso, R.P.; et al. Klotho May Ameliorate Proteinuria by Targeting TRPC6 Channels in Podocytes. J. Am. Soc. Nephrol. 2017, 28, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Li, S.; Lin, Q.; Shao, X.; Wu, J.; Zhang, W.; Cai, H.; Zhou, W.; Jiang, N.; Zhang, Z.; et al. αKlotho protein has therapeutic activity in contrast-induced acute kidney injury by limiting NLRP3 inflammasome-mediated pyroptosis and promoting autophagy. Pharmacol. Res. 2021, 167, 105531. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Kim, E.N.; Kim, M.Y.; Chung, S.; Shin, S.J.; Kim, H.W.; Yang, C.W.; Kim, Y.S.; Chang, Y.S.; Park, C.W.; et al. Age-associated molecular changes in the kidney in aged mice. Oxid. Med. Cell Longev. 2012, 2012, 171383. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, T.; Kobori, H.; Miyazaki, T.; Suzuki, H.; Nishiyama, A.; Ishii, N.; Yamashita, M.; Hayashi, M. Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes. Acta Physiol. 2019, 225, e13190. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.J.; Oh, K.W.; Lee, W.Y.; Kim, S.Y.; Jung, C.H.; Kim, B.J.; Sung, K.C.; Kim, B.S.; Kang, J.H.; Lee, M.H.; et al. The differential effects of age on the association of KLOTHO gene polymorphisms with coronary artery disease. Metabolism 2006, 55, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.J.; Jurado-Fasoli, L.; Sanchez-Delgado, G.; García-Lario, J.V.; Castillo, M.J.; Ruiz, J.R. Relationship between plasma S-Klotho and cardiometabolic risk in sedentary adults. Aging 2020, 12, 2698–2710. [Google Scholar] [CrossRef] [PubMed]
- Żelaźniewicz, A.; Nowak-Kornicka, J.; Pawłowski, B. S-Klotho level and physiological markers of cardiometabolic risk in healthy adult men. Aging 2022, 14, 708–727. [Google Scholar] [CrossRef]
- Marino, F.; Scalise, M.; Salerno, N.; Salerno, L.; Molinaro, C.; Cappetta, D.; Torella, M.; Greco, M.; Foti, D.; Sasso, F.C.; et al. Diabetes-Induced Cellular Senescence and Senescence-Associated Secretory Phenotype Impair Cardiac Regeneration and Function Independently of Age. Diabetes 2022, 71, 1081–1098. [Google Scholar] [CrossRef]
- Xiao, L.; Zan, G.; Liu, C.; Xu, X.; Li, L.; Chen, X.; Zhang, Z.; Yang, X. Associations Between Blood Pressure and Accelerated DNA Methylation Aging. J. Am. Heart Assoc. 2022, 11, e022257. [Google Scholar] [CrossRef]
- Shimada, T.; Takeshita, Y.; Murohara, T.; Sasaki, K.; Egami, K.; Shintani, S.; Katsuda, Y.; Ikeda, H.; Nabeshima, Y.; Imaizumi, T. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation 2004, 110, 1148–1155. [Google Scholar] [CrossRef] [Green Version]
- Citterio, L.; Delli Carpini, S.; Lupoli, S.; Brioni, E.; Simonini, M.; Fontana, S.; Zagato, L.; Messaggio, E.; Barlassina, C.; Cusi, D.; et al. Klotho Gene in Human Salt-Sensitive Hypertension. Clin. J. Am. Soc. Nephrol. 2020, 15, 375–383. [Google Scholar] [CrossRef]
- Luo, L.; Hao, Q.; Dong, B.; Yang, M. The Klotho gene G-395A polymorphism and metabolic syndrome in very elderly people. BMC Geriatr. 2016, 16, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, I.; Stein, D.; Shahmoon, S.; Ziv, S.I.; Hemi, R.; Kanety, H.; Rubinek, T.; Modan-Moses, D. Alteration in serum klotho levels in anorexia nervosa patients. Clin. Nutr. 2016, 35, 958–962. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takashi, Y.; Maeda, Y.; Toyokawa, K.; Oda, N.; Yoshioka, R.; Sekiguchi, D.; Minami, M.; Kawanami, D. Fibroblast growth factor 23 and kidney function in patients with type 1 diabetes. PLoS ONE 2022, 17, e0274182. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, J.; Wolf, M. Regulation and Effects of FGF23 in Chronic Kidney Disease. Annu. Rev. Physiol. 2020, 82, 365–390. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.P.; Mendes, F.; Pereira, L.; Fragoso, A.; Gonçalves, R.B.; Santos, N.; Rato, F.; Neves, P.L. Klotho levels: Association with insulin resistance and albumin-to-creatinine ratio in type 2 diabetic patients. Int. Urol. Nephrol. 2017, 49, 1809–1814. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.; Kang, M.; Kim, S.; Park, S.K.; Sung, S.; Hyun, Y.Y.; Jung, J.Y.; Ahn, C.; Oh, K.H. Low Klotho/Fibroblast Growth Factor 23 Ratio Is an Independent Risk Factor for Renal Progression in Chronic Kidney Disease: Finding From KNOW-CKD. Front. Med. 2022, 9, 904963. [Google Scholar] [CrossRef]

| Characteristic | α-Klotho Levels Tertiles, pg/mL | p-Value for Trend | |||
|---|---|---|---|---|---|
| Overall | <704.00 | 704.00–918.90 | ≥918.90 | ||
| No. of participants | 13,584 | 4528 | 4526 | 4530 | |
| α-Klotho (pg/mL), median (IQR) | 803.10 (655.50–994.23) | 594.10 (520.58–655.50) | 803.05 (752.40–856.30) | 1100.20 (994.20–1269.85) | <0.001 |
| Age (year), mean (SD) | 57.65 ± 10.83 | 58.86 ± 11.01 | 57.49 ± 10.77 | 56.59 ± 10.59 | <0.001 |
| 40–59 years, % | 7415, 54.59% | 49.71 | 55.52 | 58.52 | |
| 60–79 years, % | 6169, 45.41% | 50.29 | 44.48 | 41.48 | |
| Gender, % | <0.001 | ||||
| Male | 48.43 | 51.46 | 49.60 | 44.24 | |
| Female | 51.57 | 48.54 | 50.40 | 55.76 | |
| Race, % | <0.001 | ||||
| Mexican American | 15.92 | 15.90 | 16.59 | 15.25 | |
| Other Hispanic | 11.52 | 10.29 | 11.73 | 12.54 | |
| Non-Hispanic White | 42.99 | 45.85 | 44.56 | 38.57 | |
| Non-Hispanic Black | 19.71 | 19.21 | 16.28 | 23.62 | |
| Other Races | 9.86 | 8.75 | 10.83 | 10.02 | |
| Education level, % | <0.001 | ||||
| below high school | 28.14 | 29.09 | 27.57 | 27.75 | |
| High school or GED | 22.11 | 23.37 | 22.29 | 20.68 | |
| Above high school | 49.75 | 47.55 | 50.13 | 51.57 | |
| BMI (kg/m2), mean (SD) | 29.71 ± 6.66 | 29.81 ± 6.38 | 29.75 ± 6.64 | 29.58 ± 6.94 | 0.006 |
| Normal weight <25, % | 23.88 | 22.11 | 23.49 | 26.02 | |
| Overweight 25–29.9, % | 34.66 | 35.83 | 34.80 | 33.36 | |
| Obese ≥ 30, % | 41.46 | 42.06 | 41.71 | 40.62 | |
| HDL-C (mmol/L) | 1.37 ± 0.43 | 1.37 ± 0.44 | 1.36 ± 0.42 | 1.39 ± 0.43 | <0.001 |
| Triglycerides (mmol/L) | 1.90 ± 1.60 | 1.99 ± 1.92 | 1.89 ± 1.38 | 1.81 ± 1.46 | <0.001 |
| ALT (IU/L) | 25.56 ± 18.99 | 24.68 ± 15.60 | 25.06 ± 19.58 | 26.94 ± 21.28 | <0.001 |
| AST (IU/L) | 26.42 ± 16.68 | 25.62 ± 13.71 | 25.66 ± 13.74 | 27.98 ± 21.30 | <0.001 |
| Diabetes, % | 0.018 | ||||
| Yes | 17.71 | 18.73 | 16.48 | 17.92 | |
| no | 82.29 | 81.27 | 83.52 | 82.08 | |
| Hypertension, % | <0.001 | ||||
| Yes | 46.25 | 49.76 | 43.70 | 45.30 | |
| no | 53.75 | 50.24 | 56.30 | 54.70 | |
| Pulse pressure, % | <0.001 | ||||
| <60 | 63.86 | 60.04 | 65.21 | 66.32 | |
| ≥60 | 36.14 | 39.96 | 34.79 | 33.68 | |
| Urine albumin, % | <0.001 | ||||
| Normal (<30) | 86.01 | 83.92 | 87.27 | 86.82 | |
| Microalbuminuria (30–299) | 11.35 | 12.52 | 10.41 | 11.13 | |
| Macroalbuminuria (≥300) | 2.64 | 3.56 | 2.32 | 2.05 | |
| Outcome | β (95%CI) 1, p-Value | ||
|---|---|---|---|
| Crude Model 2 | Model 1 3 | Model 2 4 | |
| α-Klotho (continuous) | −18.07 (−32.95, −3.19), 0.0197 | −3.24 (−6.39, −0.09), 0.0476 | −12.22 (−23.91, −0.53), 0.0448 |
| α-Klotho (tertiles) | |||
| T1 | Reference | Reference | Reference |
| T2 | −20.26 (−32.85, −7.67), 0.0023 | −3.04 (−5.93, −0.16), 0.0422 | −13.92 (−24.68, −3.16), 0.0139 |
| T3 | −19.31 (−32.19, −6.44), 0.0043 | −3.34 (−6.30, −0.38), 0.0300 | −12.67 (−22.86, −2.47), 0.0179 |
| p for trend | 0.0041 | 0.0294 | 0.0176 |
| Variables | β (95%CI) | p-Value |
|---|---|---|
| Log 2 α-Klotho | ||
| per one-unit increase | −12.22 (−23.91, −0.53) | 0.0448 |
| Age (year) | −0.53 (−1.04, −0.01) | 0.0503 |
| Female (versus male) | −13.37 (−25.51, −1.23) | 0.0348 |
| Race (versus Mexican American) | ||
| Other Hispanic | 3.40 (−32.63, 39.44) | 0.8538 |
| Non-Hispanic white | −26.45 (−47.52, −5.39) | 0.0168 |
| Non-Hispanic black | −17.47 (−38.89, 3.95) | 0.1151 |
| Other race/ethnicity | 4.93 (−36.32, 46.19) | 0.8155 |
| Education level (versus less than high school) | ||
| High school or GED | −0.68 (−17.15, 15.80) | 0.9363 |
| Above high school | −7.03 (−20.69, 6.62) | 0.3170 |
| BMI (kg/m2) (versus normal weight < 25) | ||
| Overweight 25–29.9 | −10.68 (−17.04, −4.31) | 0.0018 |
| Obese ≥ 30 | −3.85 (−0.68, −14.99) | 0.5014 |
| HDL-C (mmol/L) | 18.58 (−0.26, 37.41) | 0.0579 |
| Triglycerides (mmol/L) | 7.86 (1.04, 14.67) | 0.0277 |
| ALT (IU/L) | −0.58 (−0.99, −0.17) | 0.0069 |
| AST (IU/L) | 0.34 (−0.10, 0.77) | 0.1360 |
| Diabetes (no versus yes) | −85.73 (−114.11, −57.35) | <0.0001 |
| Hypertension (no versus yes) | −21.24 (−29.30, −13.18) | <0.0001 |
| Pulse pressure (elevated versus normal) | 34.65 (21.78, 47.53) | <0.0001 |
| Models | ACR | |
|---|---|---|
| β (95%CI) | p-Value | |
| Model I | ||
| One line slope | −14.31 (−27.13, −1.50) | 0.0286 |
| Model II | ||
| Turning point (K) | 9.91 | |
| <9.91 slope 1 | −39.95 (−58.82, −21.07) | <0.0001 |
| >9.91 slope 2 | 41.02 (8.47, 73.57) | 0.0135 |
| slope 2-slope 1 | 80.97 (37.18, 124.76) | 0.0003 |
| Predicted at 9.91 | 28.15 (17.98, 38.33) | |
| Log likelihood ratio test | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.; Li, Y.; Qin, Z.; Zhang, Z.; Wang, L.; Yang, Q.; Su, B. Association between Serum Soluble α-Klotho and Urinary Albumin Excretion in Middle-Aged and Older US Adults: NHANES 2007–2016. J. Clin. Med. 2023, 12, 637. https://doi.org/10.3390/jcm12020637
Chang K, Li Y, Qin Z, Zhang Z, Wang L, Yang Q, Su B. Association between Serum Soluble α-Klotho and Urinary Albumin Excretion in Middle-Aged and Older US Adults: NHANES 2007–2016. Journal of Clinical Medicine. 2023; 12(2):637. https://doi.org/10.3390/jcm12020637
Chicago/Turabian StyleChang, Kaixi, Yupei Li, Zheng Qin, Zhuyun Zhang, Liya Wang, Qinbo Yang, and Baihai Su. 2023. "Association between Serum Soluble α-Klotho and Urinary Albumin Excretion in Middle-Aged and Older US Adults: NHANES 2007–2016" Journal of Clinical Medicine 12, no. 2: 637. https://doi.org/10.3390/jcm12020637





