Corneal Sub-Basal Nerve Plexus in Non-Diabetic Small Fiber Polyneuropathies and the Diagnostic Role of In Vivo Corneal Confocal Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Small Fibres Neuropathy
2.2. Methods of Evaluation of Small Fiber Neuropathies
- QST is a well-established method that evaluates both pain and loss of sensory function. However, although this method is widely used, on the other hand, there are several limitations. First, the results are quite variable since is a psychophysical test, second is not localizing the lesion (central vs peripheral), third is time-consuming [18,19].
- Nociceptive evoked potentials are methods based on the selective activation of A delta and C fibers. There are several methods available: the radiant heat used for laser-evoked potentials (LEPs) [20,21,22,23,24,25,26], the contact heat for contact-heat-evoked potentials (CHEPs) [27,28,29], and the electrical skin stimulation for pain-related evoked potentials (PREPs) [30,31,32].
- Autonomic nervous system testing may be useful in the syndromic workout as autonomic dysfunction is often present in SFN. It may be detected by several techniques testing of sudomotor function [33]. Among the most used methods to study sudomotor function, are the Thermoregulatory Sweat Test (TST), the quantitative sudomotor axon reflex test (QSART), the quantitative direct and indirect axon reflex testing (QDIRT), the sympathetic skin response test (SSR), electrochemical sweat conductance methods with the SUDOSCAN device (Impeto Medical: Paris, France) [34,35,36,37].
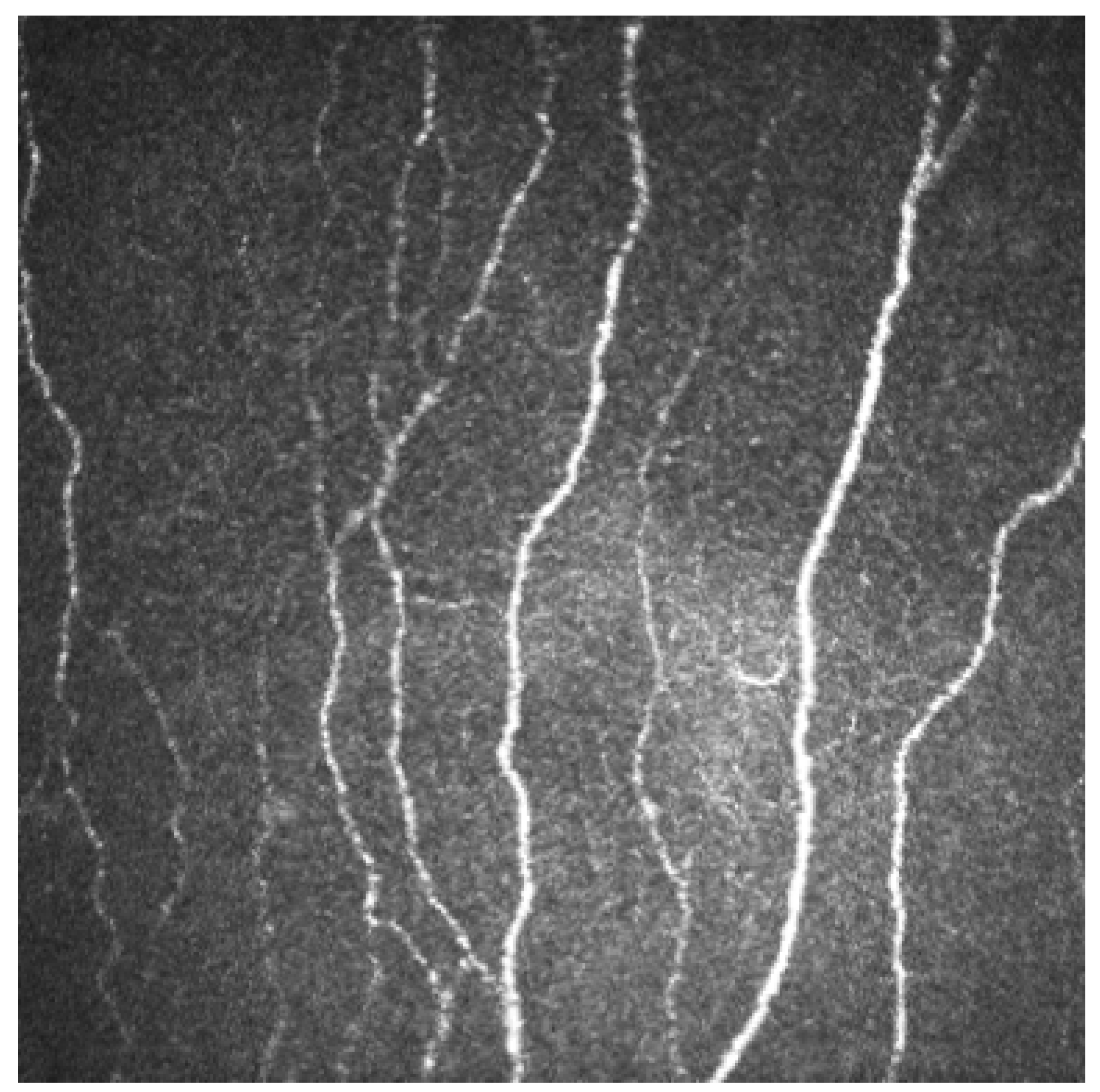
2.3. Corneal Nerve Fibers
2.4. Corneal Confocal Microscopy
2.5. Database and Literature Search
2.6. Inclusion and Exclusion Criteria
3. Results
3.1. Parkinson’s Disease
| Authors | Number of Patients | CNFL | CNFD | CNBD | TC | BF | CCM | Localization | Method Used | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| [Ref.] | (Parkinson/Healthy) | Used | ||||||||
| Andreasson et al. [57]. | 42/13 | x | x | HRT 3 | Central | Automated | No group differences between CNFL (p = 0.81) and CNBD (p = 0.91). | |||
| (21: Parkinson + Restless legs Sindrom; 21: Parkinson) | ||||||||||
| Kass-Iliyya et al. [58]. | 26/26 | x | x | x | HRT 3 | Central | ACCmetrics Automated | Reduction of CNFD in PD (p = 0.003). | ||
| Increase of CNBD (p < 0.001) and CNFL (p = 0.031) in PD. | ||||||||||
| Lim et al. [59]. | 98/26 | X | X | X | HRT 3 | Central | ACCmetrics Automated | Reduction of CNFD (p = 0.001), CNBD (p = 0.003), and CNFL (p = 0.001) in participants with PD compared to controls | ||
| Arrigo et al. [60]. | 3/0 | x | x | Nidek-ConfoScan 4 | Central | NAVIS software | Increase of beading (BF). No significant changes in density, except for a reduction shown by the left eye of PD2. | |||
| Podgorny et al. [61]. | 26/22 | x | x | x | HRT 3 | Central | ACCmetrics Automated | Reduction of CNFL (p = 0.013), (p = 0.013), CNFD, in patients with PD. CNFD | ||
| didn’t have a significantly difference. (p = 0.058) | ||||||||||
| Misra et al. [59]. | 15/15 | x | HRT 2 | Central | Automated | Reduction of CNFD in patients with PD compared with controls. (p < 0.0001) | ||||
| Reddy et al. [62]. | (12: 7 patients with Progressive Sopranuclear Palsy (PSP) and 4 patients with PD) | x | Nidek-ConfoScan 4 | Central | NeuronJ | There were no differences in corneal sub-basal nerve density between the 3 groups. | ||||
| Daggumilli et al. [63]. | 120/30 | x | Nidek-ConfoScan 4 | Central | NeuronJ | Reduction of CNFD in PD amantadine and PD amantadine naive group compared with healthy controls after 1-year follow-up. (p = 0.032 and 0.048) | ||||
| 150 subjects: 90 PD with amantadine, 30 PD naïve amantadine, 30 controls) | ||||||||||
| Anjos R. et al. [64]. | 25/25 | x | x | x | x | HRT 2 | Central | Automated | Corneal nerve fiber morphology differed in both groups, with a lower global fiber density, branch density, and higher tortuosity in Parkinson patients (p < 0.05). These parameters were found to be related to dopaminergic medication exposure. | |
| Avetisov S.E. et al. [65]. | 16/0 | x | x | x | HRT 3 | Central | Increase in a number of branches from the main nerve trunks, an increase in the tortuosity of CNF, and a “beaded” shape. |
3.2. Sjogren Syndrome
| Authors | Number of Patients | CNFL | CNFD | CNBD | TC | BF | CCM | Localization | Method Used | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| [Ref.] | (Sjogren/Healthy) | Used | ||||||||
| Bercelos F. et al. [69]. | −55: SjS; | x | x | x | Heidelberg!Retina Tomograph II, | Central | ImageJ | Reduction of CNFL and CNFD (p < 0.001). | ||
| −63 Sicca; | -Increase TC. | |||||||||
| −18: rheumatoid arthritis (RA). | ||||||||||
| 20: healthy controls | ||||||||||
| Tuominen et al. [70]. | 10/10 | x | model 165A, Tandem Scanning Corp., Reston, VA, USA | Central | No difference was noted in nerve density. | |||||
| McNamara et al. [71]. | 10/10 | x | x | x | Nidek Confoscan 4 | Central | Nerve tracking V1.0 | Reduction of CNFD (p = 0.03) and CNFL (p < 0.05) in SS patients compared to controls. | ||
| Levy et al. [72]. | 30/10 | x | HRT 3 | Central | ImageJ | CNFD was significantly increased (p < 0.0001) associated with a decrease in DC density (p < 0.0001). | ||||
| 30:patients with SS treatment treated with CsA 0.05% twice daily for six months | ||||||||||
| Tepelus et al. [73]. | 44/10 | x | x | HRT 3 | Central | NeuronJ | Reduction of CNFD (p < 0.001) and increase of TC (p < 0.05) in patients with SS syndrome | |||
| Villani et al. [74]. | 35/20 | x | x | Nidek Confoscan 2 | Central | ImageJ | Reduction of CNFD (p < 0.001) and increase of TC (p < 0.0001) |
3.3. Fibromyalgia
| Authors | Number of Patients | CNFL | CNFD | CNBD | TC | BF | CCM | Localization | Method Used | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| [Ref.] | (Fibromyalgia/Healthy) | Used | ||||||||
| Erkan Turan K. et al. [75]. | 34/42 | x | Nidek-ConfoScan 3 | Central | ImageJ | Total nerve density, long nerve fibers, and the number of nerves were all lower in patients with FM compared with controls (p < 0.001). | ||||
| Ramirez et al. [76]. | 7/7 | x | Nidek-ConfoScan 4 | Central | NAVIS software | Reduction of CNFD (p = 0.02) | ||||
| Ramirez et al. [77]. | 28/0 | x | Nidek-ConfoScan 4 | Central | NAVIS software | Reduction of CNFD (nerve density < normality cutoff point found in their previous study). | ||||
| (15: Fibromyalgia + anxiety or depression; 13: Fibromyalgia without depression) | No difference between two groups. | |||||||||
| Oudejans L. et al. [78]. | 39/0 | x | x | x | HRT 3 | Central | ACCmetrics Automated | CNFL was significantly decreased in 44% of patients compared to age- | ||
| and sex-matched reference values; CNFD and CNBD were significantly decreased | ||||||||||
| in 10% and 28% of patients. | ||||||||||
| CNFL values correlated with CNBD (Pearson’s r = 0.81) and CNFD (Pearson’s r = 0.90) (p < 0.01) |
3.4. Multiple Sclerosis
| Authors | Number of Patients | CNFL | CNFD | CNBD | TC | BF | CCM | Localization | Method Used | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| [Ref.] | (SM/Healthy) | Used | ||||||||
| Bitirgen et al. [79]. | 57/30 | X | X | X | HRT3 | Central | ImageJ | Reduction of CNFL (p: 0.001), CNFD (p: 0.002) and CNBD (p: 0.001) | ||
| Mikolajczak et al. [80]. | 26/26 | X | HRT 3 | Central | NeuronJ | Reduction of CNFD in MS patients compared to | ||||
| controls. (p = 0.007) | ||||||||||
| Petropouls et al. [81]. | 25/25 | X | X | X | HRT 3 | Central | ACCmetrics Automated | Reduction of CNFD (p < 0.0001), CNFL (p < 0.0001), and CNBD (p = 0.0003) compared with controls. |
3.5. Other Pathologies
| Authors | SFN | Number of Patients | CNFL | CNFD | CNBD | TC | BF | CCM | Localization | Method Used | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [Ref.] | Type | (SFN/Healthy) | Used | ||||||||
| Rousseau et al. [82]. | TTR-FAP | 15/15 | X | HRT 3 | Central | ImageJ | Reduction of CNFL (p = 0.02). | ||||
| Zangh et al. [83]. | TTR-FAP | 15/15 | X | X | X | HRT 3 | Central/inferior whorl | ImageJ | Reduction of IWL (Inferior whorl length) (p = 0.006), CNFL (p = 0.005), CNBD (p = 0.008), and CNFD (p = 0.014). | ||
| Barnett et al. [84]. | Neurofibromatosis I | 52/0 | x | HRT 2 | ND | Automated | CNFL was below the normative level (10.1 ± 2.7 mm/mm2). | ||||
| Bitirgen et al. [85]. | BEHCET | 49/30 | x | x | x | HRT 3 | Central | ACCmetrics Automated | Reduction of CNFD (p = 0.001) and CNFL (p = 0.031) in patients with Behcet. | ||
| CNBD did not differ significantly (p = 0.067). | |||||||||||
| Bucher et al. [86]. | SNF | 14/14 | x | x | HRT 2 | Central | ImageJ | Reduction CNFD (p = 0.001). | |||
| Increase of TC (p = 0.5). | |||||||||||
| Tavakoli et al. [85]. | Fabry’s Disease | ND | x | x | x | x | HRT 3 | ND | CCM Image Analysis Tools v 0.6 | Nerve damage in idiopathic small fibre neuropathy and Fabry disease. | |
| Ferrari et al. [3]. | SLA | 8.89 | x | x | x | HRT 2 | ND | Automated | Reduction of CNFD (p < 0.011) and CNFL (p = 0.004) in SLA patients. | ||
| Increase TC in SLA patients (p < 0.0005). | |||||||||||
| Fu et al. [87]. | SLA | 66/64 | x | x | x | Nidek-ConfoScan 3 | Inferior whorl | ImageJ | Reduction of CNFL (p < 0.05) and of CNFD (p = 0.011). Increase of CNBD (p = 0.040). | ||
| Gad et al. [88]. | Celiac Disease | 20/20 | x | x | x | x | HRT | Central and inferior whorl | ACCmetrics Automated | CNFL (p = 0.8), CNFD (p = 0.5), CNBD (p = 0.1) did not differ between children with Celiac Disease and controls. | |
| Reduction of TC (p = 0.01). | |||||||||||
| Kemp et al. [89]. | HIV+ | 20/20 | x | x | x | x | HRT | Central | ACCmetrics Automated | Reduction of CNFD (p < 0.001), CNBD (p = 0.01), and CNFL (p = 0.001). | |
| Increase of TC (p = 0.03). | |||||||||||
| Khan et al. [90]. | Diabetes + Charcot | 20/20 | x | x | x | HRT3 | Central | ACCmetrics Automated | Reduction of CNFD (p < 0.001), CNBD (p < 0.01), and CNFL (p < 0.001). | ||
| O’Neill et al. [91]. | Burning mouth Syndrome (BMS) | 17/14 | x | x | x | HRT3 | Central | ACCmetrics Automated | Reduction CNFD (p = 0.007), CNFL (p = 0.007). | ||
| There was no difference in CNBD BMS vs Controls (p = 0.06). | |||||||||||
| Schneider et al. [92]. | Chronic inflammatory demyelinating polyneuropathy (CIDP) | 16/15 | x | x | x | x | HRT3 | Central | ImageJ | Reduction CNFD (p < 0.0001), CNFL (p < 0.001), CNBD (p < 0.0001). | |
| Increase of TC (p < 0.01) | |||||||||||
| Sturniolo et al. [93]. | Wilson Disease | 24/24 | x | x | x | Nidek-ConfoScan 4 | Central | Nerve tracking V1.0 | Reduction of CNFD (p < 0.0001), CNBD (p < 0.0001). | ||
| Increase of TC (p < 0.001). | |||||||||||
| Culver et al. [94]. | Sarcoidosis | 48/16 | X | X | X | X | X | HRT 3 | Central | ACCmetrics Automated | -Double-blind, randomized, placebo-controlled. |
| (16: CIBINETIDE 1MG | -Reduction CNFA: 1 mg and placebo groups (p = 0.748 and p = 0.32). | ||||||||||
| 16: CIBINETIDE 4MG | -Increase CNFA: 4 mg and 8 mg groups (p = 0.084 and p = 0.274). | ||||||||||
| 16: CIBINETIDE 8MG | |||||||||||
| 16: PLACEBO) | |||||||||||
| Pagovich et al. [95]. | Friedreich Ataxia | 23/14 | x | x | x | HRT 3 | Central | ACCmetrics Automated | Reduction of CNFD | ||
| (p < 0.0001) e CNFL (p < 0.002) in FRDA | |||||||||||
| Tavakoli et al. [96]. | Charcot-Marie-Tooth Disease type 1A patients | 12./12 | x | x | x | HRT 3 | Central | ACCmetrics Automated | Reduction of CFND (p = 0.01), CNBD (p = 0.02), and CNFL (p = 0.0001) in CMT1A patients compared with control subjects |
3.6. Correlation of Disease Duration and Nerve Alterations
3.7. Correlation of Disease Severity and Nerve Alterations
3.8. Correlation of Medical Therapy and Nerve Alterations
3.9. Correlation between Skin Biopsy and Nerve Alterations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, A.; Bril, V.; Orsazag, A.; Paulson, J.; Yeung, E.; Ngo, M.; Orlov, S.; Perkins, A. Bruce, Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type1 diabetes: A concurrent validity study. Diabetes Care 2012, 35, 821.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, S.L.; Craig, J.P.; Patel, D.V.; McGhee, C.N.J.; Pradhan, M.; Ellyet, K.; Kilfoyle, D.; Braatvedt, G.D. In vivo confocal microscopy of corneal nerves: An ocular biomarker for peripheral and cardiac autonomic neuropathy in type 1 diabetes mellitus. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5060.e5. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, M.; Malik, R.A. Corneal confocal microscopy: A novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J. Vis. Exp. 2011, 3, e2194. [Google Scholar] [CrossRef] [Green Version]
- Roszkowska, A.M.; Licitra, C.; Tumminello, G.; Postorino, E.I.; Colonna, M.; Aragona, P. Corneal nerves in diabetesdThe role of the in vivo corneal confocal microscopy of the subbasal nerve plexus in the assessment of peripheral small fiber neuropathy. Surv. Ophthalmol. 2020, 10, 1016. [Google Scholar]
- Che, N.N.; Yang, H.Q. Potential use of corneal confocal microscopy in the diagnosis of Parkinson’s disease associated neuropathy. Transl. Neurodegener 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Themistocleous, A.C.; Ramirez, J.D.; Serra, J.; Bennett, D.L. The clinical approach to small fibre neuropathy and painful channelopathy. Pract. Neurol. 2014, 14, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.J.; Bakkers, M.; Merkies, I.S.; Hoeijmakers, J.G.; van Raak, E.P.; Faber, C.G. Incidence and prevalence of small-fiber neuropathy: A survey in the Netherlands. Neurology 2013, 81, 1356–1360. [Google Scholar] [CrossRef]
- Mathias, C.J.; Bannister, R. Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System, 5th ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Khoshnoodi, M.A.; Truelove, S.; Burakgazi, A.; Hoke, A.; Mammen, A.L.; Polydefkis, M. Longitudinal Assessment of Small Fiber Neuropathy: Evidence of a Non-Length-Dependent Distal Axonopathy. JAMA Neurol. 2016, 73, 684–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Zhou, L. Characterization of non-length-dependent small-fiber sensory neuropathy. Muscle Nerve 2012, 45, 86–91. [Google Scholar] [CrossRef]
- Nolano, M.; Crisci, C.; Santoro, L.; Barbieri, F.; Casale, R.; Kennedy, W.R.; Wendelschafer-Crabb, G.; Provitera, V.; Di Lorenzo, N.; Caruso, G. Absent innervation of skin and sweat glands in congenital insensitivity to pain with anhidrosis. Clin. Neurophysiol. 2000, 111, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Themistocleous, A.C.; Ramirez, J.D.; Shillo, P.R.; Lees, J.G.; Selvarajah, D.; Orengo, C.; Tesfaye, S.; Rice, A.S.C.; Bennett, D.L.H. The Pain in Neuropathy Study (PiNS): A cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain 2016, 157, 1132–1145. [Google Scholar] [CrossRef] [Green Version]
- Thaisetthawatkul, P.; Fernandes Filho, J.A.; Herrmann, D.N. Autonomic evaluation is independent of somatic evaluation for small fiber neuropathy. J. Neurol. Sci. 2014, 344, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.A.; Veves, A.; Tesfaye, S.; Smith, G.; Cameron, N.; Zochodne, D.; Lauria, G. Toronto Consensus Panel on Diabetic Neuropathy. Small fibre neuropathy: Role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab. Res. Rev. 2011, 27, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Thaisetthawatkul, P.; Fernandes Filho, J.A.; Herrmann, D.N. Contribution of QSART to the diagnosis of small fiber neuropathy. Muscle Nerve 2013, 48, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Geerts, M.; de Greef, B.T.A.; Sopacua, M.; van Kuijk, S.M.J.; Hoeijmakers, J.G.J.; Faber, C.G.; Merkies, I.S.J. Intravenous Immunoglobulin Therapy in Patients with Painful Idiopathic Small Fiber Neuropathy. Neurology 2021, 96, e2534–e2545. [Google Scholar] [CrossRef]
- Attal, N.; Baron, R.; Bouhassira, D.; Drangholt, M.; Dyck, P.J.; Edwards, R.R.; Freeman, R.; Gracely, R.; Haanpaa, M.H.; Hansson, P. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 2013, 154, 1807–1819. [Google Scholar]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, R.-D.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I.C.; et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef]
- Bargeron, C.B.; McCally, R.L.; Farrell, R.A. Calculated and measured endothelial temperature histories of excised rabbit corneas exposed to infrared radiation. Exp. Eye Res. 1981, 32, 241–250. [Google Scholar] [CrossRef]
- Cummins, L.; Nauenberg, M. Thermal effects of laser radiation in biological tissue. Biophys. J. 1983, 42, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Agostino, R.; Cruccu, G.; Romaniello, A.; Innocenti, P.; Inghilleri, M.; Manfredi, M. Dysfunction of small myelinated afferents in diabetic polyneuropathy, as assessed by laserevoked potentials. Clin. Neurophysiol. 1999, 111, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Lorenz, J.; Baumgartner, U. Clinical usefulness of laserevoked potentials. Neurophysiol. Clin. 2003, 33, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.; Brusa, A.; Creange, A.; Drouot, X.; Jarry, G. Clinical application of laser evoked potentials using the Nd:YAG laser. Neurophysiol. Clin. 2002, 32, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Creange, A. Neurophysiological testing correlates with clinical examination according to fibre type involvement and severity in sensory neuropathy. J. Neurol. Neurosurg. Psyhiatry 2004, 75, 417–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefaucheur, J.-P.; Wahab, A.; Planté-Bordeneuve, V.; Sène, D.; Ménard-Lefaucheur, I.; Rouie, D.; Tebbal, D.; Salhi, H.; Créange, A.; Zouari, H.; et al. Diagnosis of small fiber neuropathy: Comparative study of five neurophysiological tests. Neurphysiol. Clin. 2015, 45, 445–455. [Google Scholar] [CrossRef]
- Chen, A.C.; Niddam, D.M.; Arendt-Nielsen, L. Contact heat evoked potentials as a valid means to study nociceptive pathways in human subjects. Neurosci. Lett. 2001, 316, 79–829. [Google Scholar] [CrossRef]
- Atherton, D.D.; Facer, P.; Roberts, K.M.; Misra, V.P.; Chizh, B.A.; Bountra, C.; Praveen, A. Use of novel contact heat evoked potential stimulator (CHEPS) for the assessment of small fiber neuropathy: Correlations with skin flare responses and intra-epidermal nerve fibre counts. BMC Neurol. 2007, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-W.; Wang, Y.-C.; Hsieh, P.-C.; Tseng, M.-T.; Chiang, M.-C.; Chu, C.-P.; Feng, F.-P.; Lin, Y.-H.; Hsieh, S.-T.; Chao, C.-C. Biomarkers of neuropathic pain in skin nerve degeneration neuropathy: Contact heat-evoked potentials as a physiological signature. Pain 2017, 158, 516–52599. [Google Scholar] [CrossRef]
- Inui, K.; Tran, T.D.; Hoshiyama, M.; Kakigi, R. Preferential stimulation of Adelta fibers by intra-epidermal needle electrode in humans. Pain 2002, 96, 247–252. [Google Scholar] [CrossRef]
- Katsarava, Z.; Yaldizli, Ö.; Voulkoudis, C.; Diener, H.-C.; Kaube, H.; Maschke, M. Pain related potentials by electrical stimulation of skin for detection of small-fiber neuropathy in HIV. J. Neurol. 2006, 253, 1581–1584. [Google Scholar] [CrossRef]
- Obermann, M.; Katsarava, Z.; Esser, S.; Sommer, C.; He, L.; Selter, L.; Yoon, M.-S.; Kaube, H.; Diener, H.-C.; Maschke, M. Correlation of epidermal nerve fiber density with pain-related evoked potentials in HIV neuropathy. Pain 2008, 138, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Terkelsen, A.J.; Karlsson, P.; Lauria, G.; Freeman, R.; Finnerup, N.; Jensen, T.S. The diagnostic challenge of small fibre neuropathy: Clinical presentations, evaluations, and causes. Lancet Neurol. 2017, 16, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Low, V.A.; Sandroni, P.; Fealey, R.D.; Low, P.A. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve 2006, 34, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Siepmann, T. The Investigation of the Cardiovascular and Sudomotor Autonomic Nervous System—A Review. Front. Neurol. 2019, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Lynn, A.; Mamta, J.; Brian, C.; David, R.; Morton, B.; Rodica, P. Sudomotor dysfunction as a measure of small fiber neuropathy in tyoe 1 diabetes. Auton. Neurosci. 2017, 205, 87–92. [Google Scholar]
- Yajnik, C.S.; Kantikar, V.V.; Pande, A.J.; Deslypere, J.P. Quick and simple evaluation of sudomotor function for screening of diabetic neuropathy. ISRN Endocrinol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef]
- Cruzat, A.; Qazi, Y.; Hamrah, P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul. Surf. 2017, 15, 15–47. [Google Scholar] [CrossRef] [Green Version]
- Labetoulle, M.; Baudouin, C.; Calonge, M.; Merayo-Lloves, J.; Boboridis, K.G.; Akova, Y.A.; Aragona, P.; Geerling, G.; Messmer, E.M.; Benítez-Del-Castillo, J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2018, 97, 137–145. [Google Scholar] [CrossRef]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef] [Green Version]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492. [Google Scholar] [CrossRef]
- Eguchi, H.; Hiura, A.; Nakagawa, H.; Kusaka, S.; Shimomura, Y. Corneal nerve fiber structure, its role in corneal function, and its changes in corneal diseases. Biomed. Res. Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sindt, C.W.; Lay, B.; Bouchard, H.; Kern, J.R. Rapid image evaluation system for corneal in vivo confocal microscopy. Cornea 2013, 32, 460–465. [Google Scholar] [CrossRef]
- Patel, D.V.; McGhee, C.N. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4485–4488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.V.; McGhee, C.N. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: A review. Br. J. Ophthalmol. 2009, 93, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Kallinikos, P.; Berhanu, M.; O’Donnell, C. Corneal nerve tortuosity in diabetic patients with neuropathy. Investig. Ophthalmol. Vis. Sci. 2004, 45, 418–422. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Markoulli, M. Automatic analysis of corneal nerves imaged using in vivo confocal microscopy. Clin. Exp. Optom. 2018, 101, 147–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.V.; McGhee, C.N. Contemporary in vivo confocal microscopy of the living human cornea using white light and laser scanning techniques: A major review. Clin. Exp. Ophthalmol. 2007, 35, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.N.; Manzoor, T.; Morgan, P.; Fadavi, H.; Asghar, O.; Alam, U.; Ponirakis, G.; Dabbah, M.A.; Chen, X.; Graham, J.; et al. Repeatability of in vivo corneal confocal microscopy to quantify corneal nerve morphology. Cornea 2013, 32, e83–e89. [Google Scholar] [CrossRef] [Green Version]
- Kokot, J.; Wylęgała, A.; Wowra, B.; Wójcik, Ł.; Dobrowolski, D.; Wylęgała, E. Corneal confocal sub-basal nerve plexus evaluation: A review. Acta Ophthalmol. 2018, 96, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Batawi, H.; Shalabi, N.; Joag, M.; Koru-Sengul, T.; Rodriguez, J.; Green, P.T.; Campigotto, M.; Karp, C.L.; Galor, A. Sub-basal Corneal Nerve Plexus Analysis Using a New Software Technology. Eye Contact Lens. 2018, 44, S199–S205. [Google Scholar] [CrossRef]
- Roszkowska, A.; Wylegala, A.; Gargano, R.; Spinella, R.; Inferrera, L.; Orzechowska-Wylegala, B.; Aragona, P. Impact of corneal parameters, refractive error and age on density and morphology of the subbasal nerve plexus fibers in healthly adults. Sci. Rep. 2021, 16, 6076. [Google Scholar] [CrossRef] [PubMed]
- Erie, J.C.; McLaren, J.W.; Patel, S.V. Confocal microscopy in ophthalmology. Am. J. Ophthalmol. 2009, 148, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Parissi, M.; Karanis, G.; Randjelovic, S.; Germundsson, J.; Poletti, E.; Ruggeri, A.; Utheim, T.P.; Lagali, N. Standardized baseline human corneal subbasal nerve density for clinical investigations with laser-scanning in vivo confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7091–7102. [Google Scholar] [CrossRef] [Green Version]
- Efron, N.; Edwards, K.; Roper, N.; Pritchard, N.; Sampson, G.P.; Shahidi, A.M.; Vagenas, D.; Russell, A.; Graham, J.; Dabbah, M.A.; et al. Repeatability of measuring corneal subbasal nerve fiber length in individuals with type 2 diabetes. Eye Contact Lens. 2010, 36, 245–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertz, P.; Bril, V.; Orszag, A. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet. Med. 2011, 28, 1253–1260. [Google Scholar] [CrossRef]
- Misra, S.L.; Kersten, H.M.; Roxburgh, R.H.; Danesh-Meyer, H.V.; McGhee, C.N. Corneal nerve microstructure in Parkinson’s disease. J. Clin. Neurosci. 2017, 39, 53–58. [Google Scholar] [CrossRef]
- Arrigo, A.; Rania, L.; Calamuneri, A.; Postorino, E.I.; Mormina, E.; Gaeta, M.; Marino, S.; Di Lorenzo, G.; Quartarone, A.; Anastasi, G.; et al. Early corneal innervation and trigeminal alterations in Parkinson disease: A pilot study. Cornea 2018, 37, 448–454. [Google Scholar] [CrossRef]
- Podgorny, P.J.; Suchowersky, O.; Romanchuk, K.G.; Feasby, T.E. Evidence for small fiber neuropathy in early Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 28, 94–99. [Google Scholar] [CrossRef]
- Reddy, V.C.; Patel, S.V.; Hodge, D.O.; Leavitt, J.A. Corneal sensitivity, blink rate, and corneal nerve density in progressive supranuclear palsy and Parkinson disease. Cornea 2013, 32, 631–635. [Google Scholar] [CrossRef]
- Daggumilli, S.; Vanathi, M.; Ganger, A.; Goyal, V.; Tandon, R. Corneal evaluation in patients with parkinsonism on long-term amantadine therapy. Cornea 2019, 38, 1131–1136. [Google Scholar] [CrossRef]
- Anjos, R.; Vieira, L.; Sousa, A.; Maduro, V.; Alves, N.; Candelaria, P. Peripheral neuropathy in Parkinson disease: An in vivo confocal microscopy study. Acta Ophtal. 2014, 92. [Google Scholar] [CrossRef]
- Avetisov, S.E.; Karabanov, A.V.; Surnina, Z.V.; Gamidov, A.A. Changes in corneal nerves fibers in the early stages of Parkinson’s disease according to in vivo confocal microscopy (preliminary report). Vestn. Oftalmol. 2020, 136, 191–196. [Google Scholar] [CrossRef]
- Andréasson, M.; Lagali, N.; Badian, R.A.; Utheim, T.P.; Scarpa, F.; Colonna, A.; Allgeier, S.; Bartschat, A.; Köhler, B.; Mikut, R.; et al. Parkinson’s disease with restless legs syndrome-an in vivo corneal confocal microscopy study. NPJ Parkinsons. Dis. 2021, 7, 4. [Google Scholar] [CrossRef]
- Kass-Iliyya, L.; Javed, S.; Gosal, D.; Kobylecki, C.; Marshall, A.; Petropoulos, I.N.; Ponirakis, G.; Tavakoli, M.; Ferdousi, M.; Chaudhuri, K.R. Small fiber neuropathy in Parkinson’s disease: A clinical, pathological and corneal confocal microscopy study. Parkinsonism Relat. Disord. 2015, 21, 1454–1460. [Google Scholar] [CrossRef]
- Lim, S.H.; Ferdousi, M.; Kalteniece, A.; Mahfoud, Z.R.; Petropoulos, I.N.; Malik, R.A.; Kobylecki, C.; Silverdale, M. Corneal Confocal Microscopy Identifies Parkinson’s Disease with More Rapid Motor Progression. Mov. Disord. 2021, 36, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, F.; Hipólito-Fernandes, D.; Martins, C.; Ângelo-Dias, M.; Cardigos, J.; Monteiro, R.; Alves, N.; Vaz-Patto, J.; da Cunha-Branco, J.; Borrego, L.M. Corneal sub-basal nerve plexus assessment and its association with phenotypic features and lymphocyte subsets in Sjögren’s Syndrome. Acta Ophthalmol. 2021, 99, e1315–e1325. [Google Scholar] [CrossRef]
- Tuominen, I.S.; Konttinen, Y.T.; Vesaluoma, M.H.; Moilanen, J.A.; Helintö, M.; Tervo, T.M. Corneal innervation and morphology in primary Sjögren’s syndrome. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2545–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, N.A.; Ge, S.; Lee, S.M.; Enghauser, A.M.; Kuehl, L.; Chen, F.Y.; Gallup, M.; McKown, R.L. Reduced Levels of Tear Lacritin Are Associated with Corneal Neuropathy in Patients with the Ocular Component of Sjögren’s Syndrome. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5237–5243. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Labbé, A.; Borderie, V.; Hamiche, T.; Dupas, B.; Laroche, L.; Baudouin, C.; Bouheraoua, N. Increased corneal sub-basal nerve density in patients with Sjögren syndrome treated with topical cyclosporine A. Clin. Exp. Ophthalmol. 2017, 45, 455–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepelus, T.C.; Chiu, G.B.; Huang, J.; Huang, P.; Sadda, S.R.; Irvine, J.; Lee, O.L. Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: A preliminary study. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1771–1778. [Google Scholar] [CrossRef]
- Villani, E.; Galimberti, D.; Viola, F.; Mapelli, C.; Ratiglia, R. The cornea in Sjogren’s syndrome: An in vivo confocal study. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Erkan Turan, K.; Kocabeyoglu, S.; Unal-Cevik, I.; Bezci, F.; Akinci, A.; Irkec, M. Ocular Surface Alterations in the Context of Corneal In Vivo Confocal Microscopic Characteristics in Patients with Fibromyalgia. Cornea 2018, 37, 205–210. [Google Scholar] [CrossRef]
- Ramírez, M.; Martínez-Martínez, L.A.; Hernández-Quintela, E.; Velazco-Casapía, J.; Vargas, A.; Martínez-Lavín, M. Small fiber neuropathy in women with fibromyalgia. An in vivo assessment using corneal confocal bio-microscopy. Semin. Arthritis. Rheum. 2015, 45, 214–219. [Google Scholar] [CrossRef]
- Ramírez, M.; Guerra-Juárez, A.; Miyake, D.-Y.; Sebastian-Arellano, C.; Estrada-Mata, A.-G.; González-Moyotl, N.-J.; Rodríguez-Aguayo, A.-M.; Martínez-Lavin, M.; Martínez-Martínez, L.-A. Correlation between Corneal Nerve Density and Symptoms of Small Fiber Neuropathy in Patients with Fibromyalgia: The Confounding Role of Severe Anxiety or Depression. J. Clin. Rheumatol. 2020, 10, 1536–7355. [Google Scholar] [CrossRef] [PubMed]
- Oudejans, L.; He, X.; Niesters, M.; Dahan, A.; Brines, M.; van Velzen, M. Cornea nerve fiber quantification and construction of phenotypes in patients with fibromyalgia. Sci. Rep. 2016, 6, 23573. [Google Scholar] [CrossRef] [Green Version]
- Bitirgen, G.; Akpinar, Z.; Malik, R.A.; Ozkagnici, A. Use of corneal confocal microscopy to detect corneal nervelossand increased dendritic cells in patients with multiple sclerosis. JAMA Ophthalmol. 2017, 135, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczak, J.; Zimmermann, H.; Kheirkhah, A.; Kadas, E.M.; Oberwahrenbrock, T.; Muller, R.; Ren, A.; Kuchling, J.; Dietze, H.; Prüss, H.; et al. Patients with multiple sclerosis demonstrate reduced subbasal corneal nerve fibre density. Mult. Scler. 2017, 23, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.N.; Kamran, S.; Li, Y.; Khan, A.; Ponirakis, G.; Akhtar, N.; Deleu, D.; Shuaib, A.; Malik, R.A. Corneal Confocal Microscopy: An imaging endpoint for axonal degeneration in multiple sclerosis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3677–3681. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, A.; Cauquil, C.; Dupas, B.; Labbé, A.; Baudouin, C.; Barreau, E.; Théaudin, M.; Lacroix, C.; Guiochon-Mantel, A.; Benmalek, A.; et al. Potential Role of In Vivo Confocal Microscopy for Imaging Corneal Nerves in Transthyretin Familial Amyloid Polyneuropathy. JAMA Ophthalmol. 2016, 134, 983–989. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wang, H.; Liu, X.; Zhang, S.; Fan, D. Corneal sub-basal whorl-like nerve plexus: A landmark for early and follow-up evaluation in transthyretin familial amyloid polyneuropathy. Eur. J. Neurol. 2021, 28, 630–638. [Google Scholar] [CrossRef]
- Barnett, C.; Alon, T.; Abraham, A.; Kim, R.; McCuaig, J.M.; Kongkham, P.; Maurice, C.; Suppiah, S.; Zadeh, G.; Bril, V. Evidence of small-fiber neuropathy in neurofibromatosis type 1. Muscle Nerve 2019, 60, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Bitirgen, G.; Tinkir Kayitmazbatir, E.; Satirtav, G.; Malik, R.A.; Ozkagnici, A. In Vivo Confocal Microscopic Evaluation of Corneal Nerve Fibers and Dendritic Cells in Patients with Behçet’s Disease. Front. Neurol. 2018, 28, 204. [Google Scholar] [CrossRef] [Green Version]
- Bucher, F.; Schneider, C.; Blau, T.; Claus, C.; Gereon, F.R.; Lehmann, H.; Ludwig, H.M. Small-Fiber Neuropathy Is Associated with Corneal Nerve and Dendritic Cell Alterations: An In Vivo Confocal Microscopy Study. Cornea 2015, 34, 1114–1119. [Google Scholar] [CrossRef]
- Fu, J.; He, J.; Zhang, Y.; Liu, Z.; Wang, H.; Li, J.; Chen, L.; Fan, D. Small fiber neuropathy for assessment of disease severity in amyotrophic lateral sclerosis: Corneal confocal microscopy findings. Orphanet 2022, 17, 7. [Google Scholar] [CrossRef]
- Gad, H.; Saraswathi, S.; Al-Jarrah, B.; Petropoulos, I.N.; Ponirakis, G.; Khan, A.; Singh, P.; Al Khodor, S.; Elawad, M.; Almasri, W.; et al. Corneal confocal microscopy demonstrates minimal evidence of distal neuropathy in children with celiac disease. PLoS ONE 2020, 15, e0238859. [Google Scholar] [CrossRef]
- Kemp, H.I.; Petropoulos, I.N.; Rice, A.S.C.; Vollert, J.; Maier, C.; Sturm, D.; Schargus, M.; Peto, T.; Hau, S.; Chopra, R.; et al. Use of Corneal Confocal Microscopy to Evaluate Small Nerve Fibers in Patients with Human Immunodeficiency Virus. JAMA Ophthalmol. 2017, 135, 95–800. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Petropoulos, I.N.; Ponirakis, G.; Menzies, R.; Chidiac, O.; Pasquier, J.; Khalil, A.K.; Talal, T.K.; Malik, A.M. Corneal confocal microscopy detects severe small fiber neuropathy in diabetic patients with Charcot neuroarthropathy. J. Diabetes Investig. 2018, 9, 1167–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, F.; Marshall, A.; Ferdousi, M.; Malik, R.A. Corneal Confocal Microscopy Detects Small-Fiber Neuropathy in Burning Mouth Syndrome: A Cross-Sectional Study. J. Oral. Facial Pain Headache 2019, 33, 337–341. [Google Scholar] [CrossRef]
- Schneider, C.; Bucher, F.; Cursiefen, C.; Fink, G.R.; Heindl, L.M.; Lehmann, H.C. Corneal confocal microscopy detects small fiber damage in chronic inflammatory demyelinating polyneuropathy (CIDP). Peripher. Nerv. Syst. 2014, 19, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Sturniolo, G.C.; Lazzarini, D.; Bartolo, O.; Berton, M.; Leonardi, A.; Iva, A.F.; Parrozzani, R.; Midena, E. Small fiber peripheral neuropathy in Wilson disease: An in vivo documentation by corneal confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2015, 22, 1390–1395. [Google Scholar] [CrossRef] [Green Version]
- Culver, D.A.; Dahan, A.; Bajorunas, D.; Jeziorska, M.; van Velzen, M.; Aarts, L.P.H.J.; Tavee, J.; Tannemaat, R.T.; Dunne, A.N.; Kirk, I.R.; et al. Cibinetide Improves Corneal Nerve Fiber Abundance in Patients with Sarcoidosis-Associated Small Nerve Fiber Loss and Neuropathic Pain. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO52–BIO60. [Google Scholar] [CrossRef] [PubMed]
- Pagovich, O.E.; Vo, M.L.; Zhao, Z.Z.; Petropoulos, I.N.; Yuan, M.; Lertsuwanroj, B.; Ciralsky, J.; Lai, E.; Kiss, S.; D'Amico, D.J.; et al. Corneal confocal microscopy: Neurologic disease biomarker in Friedreich ataxia. Ann. Neurol. 2018, 84, 893–904. [Google Scholar] [CrossRef]
- Tavakoli, M.; Marshall, A.; Banka, S.; Petropoulos, I.N.; Fadavi, H.; Kingston, H.; Malik, R.A. Corneal confocal microscopy detects small-fiber neuropathy in Charcot-Marie-Tooth disease type 1A patients. Muscle Nerve 2012, 46, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, G.; Grisan, E.; Scarpa, F.; Efazio, R.; Ecomola, M.; Quattrini, A.; Ecomi, G.; Rama, P.; Eriva, N. Corneal confocal microscopy reveals trigeminal small sensory fiber neuropathy in amyotrophic lateral sclerosis. Front. Aging Neurosci. 2014, 16, 278. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Treister, R.; Lang, M.; Oaklander, A. IVIg for apparently autoimmune small-fiber polyneuropathy: First analysis of efficacy and safety. Ther. Adv. Neurol. Disord. 2018, 11, 1756285617744484. [Google Scholar] [CrossRef] [Green Version]
- Nolano, M.; Provitera, V.; Lanzillo, B.; Santoro, L. Neuropathy in idiopathic Parkinson disease: An iatrogenic problem? Ann. Neurol. 2011, 69, 427–428. [Google Scholar] [CrossRef] [Green Version]
- Ceravolo, R.; Cossu, G.; Bandettini di Poggio, M.; Santoro, L.; Barone, P.; Zibetti, M. Neuropathy and levodopa in Parkinson’s disease: Evidence from a multicenter study. Mov. Disord. 2013, 28, 1391–1397. [Google Scholar] [CrossRef]
- Serra, J.; Collado, A.; Roma, S.; Francesca, A.; Xavier, T.; Salguiero, M.; Quiles, C.; Bostock, H. Hyperexcitable C nociceptors in fibromyalgia. Ann. Neurol. 2014, 75, 196–208. [Google Scholar] [CrossRef]
- Lukashenko, M.V.; Gavrilova, N.Y.; Bregovskaya, A.V.; Soprun, L.A.; Churilov, L.P.; Petropoulos, I.N.; A Malik, R.; Shoenfeld, Y. Corneal confocal microscopy in the diagnosis of small fiber neuropathy: Faster, easier and more efficient than skin biopsy? Pathophysiology 2022, 29, 1–8. [Google Scholar] [CrossRef]
- Li, F.; Pan, J.; Yang, D.; Wu, J.; Ou, Y.; Li, H.; Huang, J.; Xie, H.; Ou, D.; Wu, X.; et al. A multicenter clinical study of the automated fundus screening algorithm. Transl. Vis. Sci. Technol. 2022, 11, 22. [Google Scholar] [CrossRef]
- Han, R.; Cheng, G.; Zhang, B.; Yang, J.; Yuan, M.; Yang, D.; Wu, J.; Liu, J.; Zhao, C.; Chen, Y.; et al. Validating automated eye disease screening AI algorithm in community and in-hospital scenarios. Front. Public Health 2022, 10, 944967. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roszkowska, A.M.; Wylęgała, A.; Gargiulo, L.; Inferrera, L.; Russo, M.; Mencucci, R.; Orzechowska-Wylęgała, B.; Aragona, E.; Mancini, M.; Quartarone, A. Corneal Sub-Basal Nerve Plexus in Non-Diabetic Small Fiber Polyneuropathies and the Diagnostic Role of In Vivo Corneal Confocal Microscopy. J. Clin. Med. 2023, 12, 664. https://doi.org/10.3390/jcm12020664
Roszkowska AM, Wylęgała A, Gargiulo L, Inferrera L, Russo M, Mencucci R, Orzechowska-Wylęgała B, Aragona E, Mancini M, Quartarone A. Corneal Sub-Basal Nerve Plexus in Non-Diabetic Small Fiber Polyneuropathies and the Diagnostic Role of In Vivo Corneal Confocal Microscopy. Journal of Clinical Medicine. 2023; 12(2):664. https://doi.org/10.3390/jcm12020664
Chicago/Turabian StyleRoszkowska, Anna M., Adam Wylęgała, Ludovica Gargiulo, Leandro Inferrera, Massimo Russo, Rita Mencucci, Bogusława Orzechowska-Wylęgała, Emanuela Aragona, Maura Mancini, and Angelo Quartarone. 2023. "Corneal Sub-Basal Nerve Plexus in Non-Diabetic Small Fiber Polyneuropathies and the Diagnostic Role of In Vivo Corneal Confocal Microscopy" Journal of Clinical Medicine 12, no. 2: 664. https://doi.org/10.3390/jcm12020664
APA StyleRoszkowska, A. M., Wylęgała, A., Gargiulo, L., Inferrera, L., Russo, M., Mencucci, R., Orzechowska-Wylęgała, B., Aragona, E., Mancini, M., & Quartarone, A. (2023). Corneal Sub-Basal Nerve Plexus in Non-Diabetic Small Fiber Polyneuropathies and the Diagnostic Role of In Vivo Corneal Confocal Microscopy. Journal of Clinical Medicine, 12(2), 664. https://doi.org/10.3390/jcm12020664







