Validation of the Prognostic Role for Surgical Treatment in Stage II Intrahepatic Cholangiocarcinoma: A SEER Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. OS Analysis between T2sN0M0 and T2mN0M0
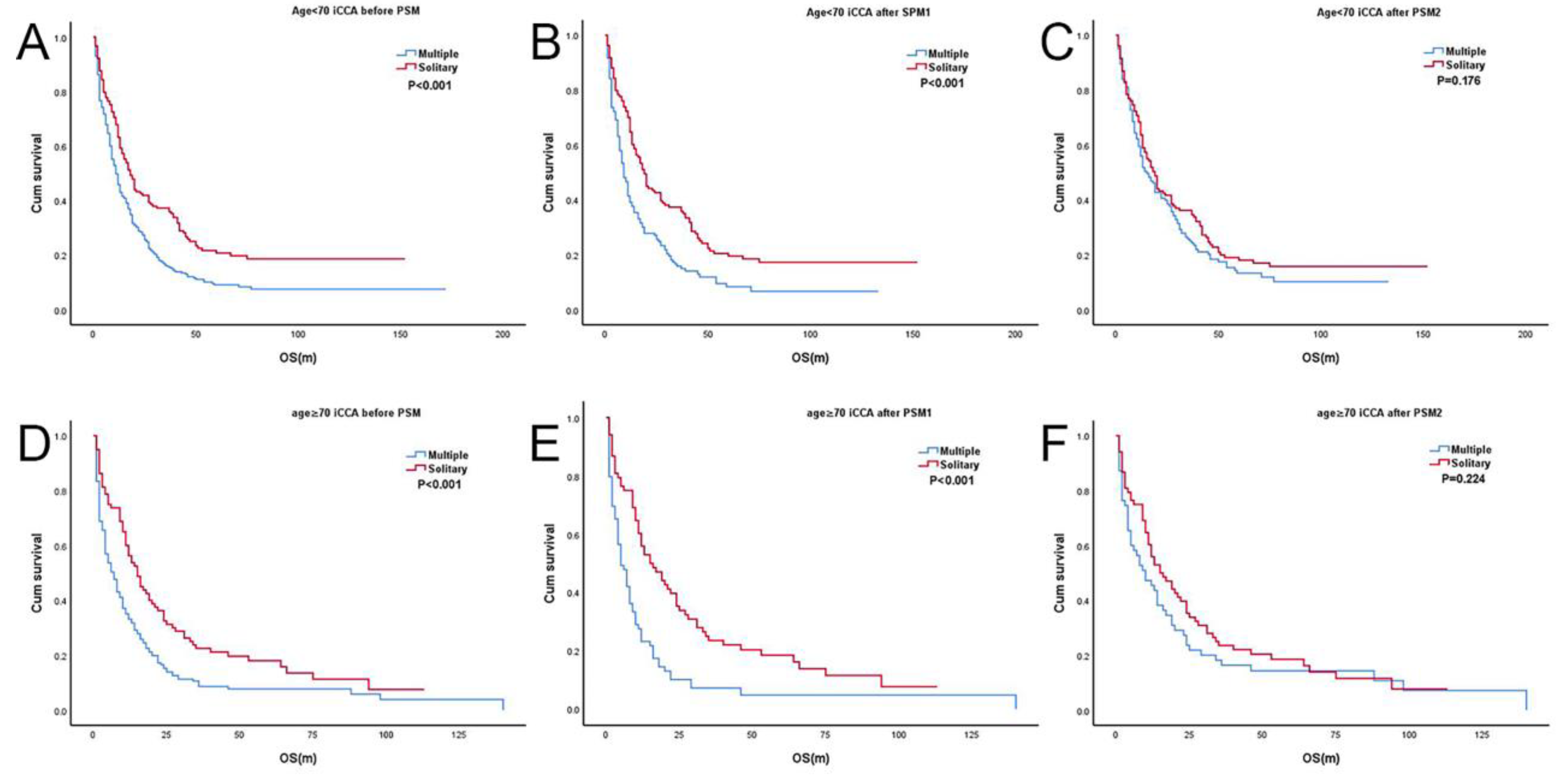
3.3. Subgroup Analysis According to Age (T2sN0M0 and T2mN0M0)
3.4. Subgroup Analysis According to Sex (T2sN0M0 and T2mN0M0)
3.5. Subgroup Analysis According to Types of Surgery (T2sN0M0 and T2mN0M0)
3.6. OS Analysis between T2mN0M0 and Stage IIIB
3.7. Cox Regression Analysis for T2sN0M0 and T2mN0M0
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Shaib, Y.H.; Davila, J.A.; McGlynn, K.; El-Serag, H.B. Rising incidence of intrahepatic cholangiocarcinoma in the United States: A true increase? J. Hepatol. 2004, 40, 472–477. [Google Scholar] [CrossRef]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Kim, R. Adjuvant therapy for resected extrahepatic cholangiocarcinoma: A review of the literature and future directions. Cancer Treat. Rev. 2009, 35, 322–327. [Google Scholar] [CrossRef]
- Raoof, M.; Dumitra, S.; Ituarte, P.H.G.; Melstrom, L.; Warner, S.G.; Fong, Y.; Singh, G. Development and Validation of a Prognostic Score for Intrahepatic Cholangiocarcinoma. JAMA Surg. 2017, 152, e170117. [Google Scholar] [CrossRef] [Green Version]
- Ahn, D.H.; Bekaii-Saab, T. AJCC 8th edition staging system for pathologically versus clinically staged intrahepatic cholangiocarcinoma (iCCA): Ready for prime time? Chin. Clin. Oncol. 2019, 8, S19. [Google Scholar] [CrossRef] [PubMed]
- Hyder, O.; Marques, H.; Pulitano, C.; Marsh, J.W.; Alexandrescu, S.; Bauer, T.W.; Pawlik, T.M. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: An Eastern and Western experience. JAMA Surg. 2014, 149, 432–438. [Google Scholar] [CrossRef]
- De Jong, M.C.; Nathan, H.; Sotiropoulos, G.C.; Paul, A.; Alexandrescu, S.; Marques, H.; Pawlik, T.M. Intrahepatic cholangiocarcinoma: An international multi-institutional analysis of prognostic factors and lymph node assessment. J. Clin. Oncol. 2011, 29, 3140–3145. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, J.; Xia, Y.; Gong, R.; Wang, K.; Yan, Z.; Wan, X.; Liu, G.; Wu, D.; Shi, L.; et al. Prognostic Nomogram for Intrahepatic Cholangiocarcinoma After Partial Hepatectomy. J. Clin. Oncol. 2013, 31, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.-W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef] [Green Version]
- Kamarajah, S.K. Evaluation of the AJCC 8th Edition Staging System for Pathologically Versus Clinically Staged Intrahepatic Cholangiocarcinoma (iCCA): A Time to Revisit a Dogma? A Surveillance, Epidemiology, and End Results (SEER) Analysis. J. Gastrointest. Cancer 2018, 50, 392–399. [Google Scholar] [CrossRef]
- Ronnekleiv-Kelly, S.M.; Pawlik, T.M. Staging of intrahepatic cholangiocarcinoma. HepatoBiliary Surg. Nutr. 2017, 6, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarca, A.; Santos-Laso, A.; Utpatel, K.; La Casta, A.; Stock, S.; Forner, A.; Adeva, J.; Folseraas, T.; Fabris, L.; Macias, R.I.; et al. Liver Metastases of Intrahepatic Cholangiocarcinoma: Implications for an Updated Staging System. Hepatology 2020, 73, 2311–2325. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, T.; Wu, M.; Shen, F. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016, 379, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Darlow, S.D. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef]
- Alvaro, D.; Cannizzaro, R.; Labianca, R.; Valvo, F.; Farinati, F. Cholangiocarcinoma: A position paper by the Italian Society of Gastroenterology (SIGE), the Italian Association of Hospital Gastroenterology (AIGO), the Italian Association of Medical Oncology (AIOM) and the Italian Association of Oncological Radiotherapy (AIRO). Dig. Liver Dis. 2010, 42, 831–838. [Google Scholar] [PubMed]
- Khan, S.A.; Davidson, B.R.; Goldin, R.; Heaton, N.; Karani, J.; Pereira, S.; Rosenberg, W.; Tait, P.; Taylor-Robinson, S.D.; Thillainayagam, A.V.; et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut 2012, 61, 1657–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodson, R.M.; Weiss, M.J.; Cosgrove, D.; Herman, J.M.; Kamel, I.; Anders, R.; Geschwind, J.-F.H.; Pawlik, T.M. Intrahepatic Cholangiocarcinoma: Management Options and Emerging Therapies. J. Am. Coll. Surg. 2013, 217, 736–750.e4. [Google Scholar] [CrossRef]
- Cai, J.-Q.; Cai, S.-W.; Cong, W.-M.; Chen, M.-S.; Chen, P.; Chen, X.-P.; Chen, Y.-L.; Chen, Y.-F.; Dai, C.-L.; Huang, Q.; et al. Diagnosis and treatment of cholangiocarcinoma: A consensus from surgical specialists of China. J. Huazhong Univ. Sci. Technol. 2014, 34, 469–475. [Google Scholar] [CrossRef]
- Gómez-España, M.A.; Montes, A.F.; Garcia-Carbonero, R.; Mercadé, T.M.; Maurel, J.; Martín, A.M.; Pazo-Cid, R.; Vera, R.; Carrato, A.; Feliu, J. SEOM clinical guidelines for pancreatic and biliary tract cancer (2020). Clin. Transl. Oncol. 2021, 23, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Kim, Y.; Alexandrescu, S.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Gamblin, T.C.; Miura, J.; Maithel, S.K.; Squires, M.H.; et al. Is Hepatic Resection for Large or Multifocal Intrahepatic Cholangiocarcinoma Justified? Results from a Multi-Institutional Collaboration. Ann. Surg. Oncol. 2014, 22, 2218–2225. [Google Scholar] [CrossRef] [Green Version]
- Sotiropoulos, G.C.; Miyazaki, M.; Konstadoulakis, M.M.; Paul, A.; Molmenti, E.P.; Gomatos, I.P.; Radtke, A.; Baba, H.A.; Beckebaum, S.; Brokalaki, E.I.; et al. Multicentric evaluation of a clinical and prognostic scoring system predictive of survival after resection of intrahepatic cholangiocarcinomas. Liver Int. 2010, 30, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, H.; Li, A.-J.; Lau, W.Y.; Pan, Z.-Y.; Lai, E.C.; Wu, M.-C.; Zhou, W.-P. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: A RCT. J. Hepatol. 2014, 61, 82–88. [Google Scholar] [CrossRef]
- Konstadoulakis, M.M.; Roayaie, S.; Gomatos, I.P.; Labow, D.; Fiel, M.-I.; Miller, C.M.; Schwartz, M.E. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: Operative results and long-term outcome. Surgery 2008, 143, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-B.; Kim, K.-S.; Choi, J.-Y.; Park, S.-W.; Lee, W.-J.; Chung, J.-B. The Prognosis and Survival Outcome of Intrahepatic Cholangiocarcinoma Following Surgical Resection: Association of Lymph Node Metastasis and Lymph Node Dissection with Survival. Ann. Surg. Oncol. 2009, 16, 3048–3056. [Google Scholar] [CrossRef]
- Cillo, U.; Fondevila, C.; Donadon, M.; Gringeri, E.; Mocchegiani, F.; Schlitt, H.J.; Groot Koerkamp, B. Surgery for cholangiocarcinoma. Liver Int. 2019, 39, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Zhao, S.; Zhu, H.; Ji, G.; Zhang, X. Primary tumor resection improves survival in patients with multifocal intrahepatic cholangiocarcinoma based on a population study. Sci. Rep. 2021, 11, 12166. [Google Scholar] [CrossRef]


| Estimate | Before PSM | After PSM1 | After PSM2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Single
(n = 233) | Multiple (n = 434) | p |
Single
(n = 202) | Multiple (n = 202) | p |
Single
(n = 198) | Multiple (n = 198) | p | ||
| Age (Median) | 65 | 65 | 65 | 65 | 65 | 65 | 63 | |||
| <70 y | 436 (65.4) | 153 (65.7) | 283 (65.2) | 0.906 | 134 (66.3) | 133 (65.8) | 0.916 | 130 (65.7) | 143 (72.2) | 0.158 |
| ≥70 y | 231 (34.6) | 80 (34.3) | 151 (34.8) | 68 (33.6) | 69 (34.2) | 68 (34.3) | 55 (27.8) | |||
| Gender | 0.671 | 0.232 | 0.314 | |||||||
| Female | 351 (52.6) | 120 (51.5) | 231 (53.2) | 109 (54.0) | 97 (48.0) | 109 (55.1) | 99 (50.0) | |||
| Male | 316 (47.4) | 113 (48.5) | 203 (46.7) | 93 (46.0) | 105 (52.0) | 89 (44.9) | 99 (50.0) | |||
| Race | 0.192 | 0.796 | 0.495 | |||||||
| White | 502 (75.3) | 169 (72.5) | 333 (76.7) | 148 (73.3) | 153 (75.7) | 148 (74.7) | 154 (77.8) | |||
| Black | 62 (9.3) | 20 (8.6) | 42 (9.7) | 18 (8.9) | 18 (8.9) | 17 (8.6) | 11 (5.6) | |||
| Other * | 103 (15.4) | 44 (18.9) | 59 (13.6) | 36 (17.8) | 31 (15.3) | 33 (16.7) | 33 (16.7) | |||
| Size | 0.026 | 0.837 | 0.534 | |||||||
| ≤5 cm | 189 (28.3) | 80 (34.3) | 109 (25.1) | 75 (37.1) | 77 (38.1) | 72 (36.4) | 78 (39.4) | |||
| >5 cm | 384 (57.6) | 127 (54.5) | 257 (59.2) | 127 (62.9) | 125 (61.9) | 126 (63.6) | 120 (60.6) | |||
| unknown | 94 (14.1) | 26 (11.2) | 68 (15.7) | 0 | 0 | 0 | 0 | |||
| Treatment | <0.001 | <0.001 | 1.000 | |||||||
| No surgical procedure | 424 (63.6) | 114 (48.9) | 310 (71.4) | 89 (44.1) | 163 (80.7) | 90 (45.5) | 90 (45.5) | |||
| resection | 243 (36.4) | 119 (51.1) | 124 (28.6) | 113 (55.9) | 39 (19.3) | 108 (54.5) | 108 (54.5) | |||
| Survival Rate | <0.001 | <0.001 | 0.079 | |||||||
| 1 year (%) | 60.8 | 41.4 | 61.7 | 33.7 | 60.9 | 53.0 | ||||
| 3 years (%) | 32.1 | 13.4 | 32.6 | 12.9 | 31.8 | 22.6 | ||||
| 5 years (%) | 22.0 | 10.0 | 21.6 | 9.5 | 20.6 | 16.6 | ||||
| Before PSM | After PSM1 | After PSM2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage ⅢB (n = 788) | Multiple (n = 434) | p | Stage ⅢB (n = 366) | Multiple (n = 366) | p | Stage ⅢB (n = 361) | Multiple (n = 361) | p | |
| Age (Median) | 63 | 65 | 64 | 65 | 64 | 65 | |||
| <70 y | 562 (71.3) | 283 (65.2) | 0.027 | 236 (64.5) | 236 (64.5) | 1.000 | 233 (64.5) | 236 (65.4) | 0.815 |
| ≥70 y | 226 (28.7) | 151 (34.8) | 130 (35.5) | 130 (35.5) | 128 (35.5) | 125 (34.6) | |||
| Gender | 0.409 | 0.375 | 0.551 | ||||||
| Female | 400 (50.8) | 231 (53.2) | 180 (49.2) | 192 (52.5) | 180 (49.9) | 188 (52.1) | |||
| Male | 388 (49.2) | 203 (46.8) | 186 (50.8) | 174 (47.5) | 181 (50.1) | 173 (47.9) | |||
| Race | 0.368 | 1.000 | 0.926 | ||||||
| White | 631 (80.1) | 333 (76.7) | 285 (77.9) | 285 (77.9) | 282 (78.1) | 285 (78.9) | |||
| Black | 62 (7.9) | 42 (9.7) | 33 (9.0) | 33 (9.0) | 34 (9.4) | 31 (8.6) | |||
| Other * | 95 (12.1) | 59 (13.6) | 48 (13.1) | 48 (13.1) | 45 (12.5) | 45 (12.5) | |||
| Size | 0.034 | 1.000 | 0.470 | ||||||
| ≤5 cm | 231 (29.3) | 109 (25.1) | 109 (29.8) | 109 (29.8) | 117 (32.4) | 108 (29.9) | |||
| >5 cm | 406 (51.5) | 257 (59.2) | 257 (70.2) | 257 (70.2) | 244 (67.6) | 253 (70.1) | |||
| unknown | 151 (19.2) | 68 (15.7) | 0 | 0 | 0 | 0 | |||
| Treatment | 0.114 | 0.473 | 0.460 | ||||||
| No surgical procedure | 551 (69.9) | 322 (74.2) | 247 (67.5) | 256 (69.9) | 261 (72.3) | 252 (69.8) | |||
| Surgery | 237 (30.1) | 112 (25.8) | 119 (32.5) | 110 (30.1) | 100 (27.7) | 109 (30.2) | |||
| Survival Rate | 0.857 | 0.907 | 0.699 | ||||||
| 1 year (%) | 48.9 | 41.4 | 54.4 | 43.9 | 53.5 | 44.5 | |||
| 3 years (%) | 12.8 | 13.4 | 13.8 | 15.3 | 13.1 | 15.5 | |||
| 5 years (%) | 6.7 | 8.6 | 5.0 | 9.6 | 5.3 | 9.7 | |||
| Female iCCA before PSM | Male iCCA before PSM | Female iCCA after PSM1 | Male iCCA after PSM1 | Female iCCA after PSM2 | Male iCCA after PSM2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single (n = 120) | Multiple (n = 231) | p | Single (n = 113) | Multiple (n = 203) | p | Single (n = 109) | Multiple (n = 97) | p | Single (n = 93) | Multiple (n = 105) | p | Single (n = 109) | Multiple (n = 99) | p | Single (n = 89) | Multiple (n = 99) | p | |
| Age (Median) | 65 | 64 | 65 | 65 | 65 | 65 | 66 | 65 | 61 | 60 | 75 | 75 | ||||||
| 0.693 | 0.555 | 0.868 | 1.000 | 0.125 | 0.608 | |||||||||||||
| <70 y | 78 (65.0) | 155 (67.1) | 75 (66.4) | 128 (63.1) | 72 (66.1) | 63 (64.9) | 62 (66.7) | 70 (66.7) | 72 (66.1) | 75 (75.8) | 58 (65.2) | 68 (68.7) | ||||||
| ≥70 y | 42 (35.0) | 76 (32.9) | 38 (33.6) | 75 (36.9) | 37 (33.9) | 34 (35.1) | 31 (33.3) | 35 (33.3) | 37 (33.9) | 24 (24.2) | 31 (34.8) | 31 (31.3) | ||||||
| Race | 0.562 | 0.094 | 0.602 | 0.930 | 0.483 | 1.000 | ||||||||||||
| White | 84 (70.0) | 174 (75.3) | 85 (75.2) | 159 (78.3) | 76 (69.7) | 73 (75.3) | 72 (77.4) | 80 (76.2) | 76 (69.7) | 74 (74.7) | 72 (80.9) | 80 (80.8) | ||||||
| Black | 15 (12.5) | 24 (10.4) | 5 (4.4) | 18 (8.9) | 13 (11.9) | 11 (11.3) | 5 (5.4) | 7 (6.7) | 13 (11.9) | 7 (7.1) | 4 (4.5) | 4 (4.0) | ||||||
| Other * | 21 (17.5) | 33 (14.3) | 23 (20.4) | 26 (12.8) | 20 (18.3) | 13 (13.4) | 16 (17.2) | 18 (17.1) | 20 (18.3) | 18 (18.2) | 13 (14.6) | 15 (15.2) | ||||||
| Size | 0.007 | 0.781 | 0.688 | 0.540 | 0.413 | 0.110 | ||||||||||||
| ≤5 cm | 40 (33.3) | 45 (19.5) | 40 (35.4) | 64 (31.5) | 40 (36.7) | 33 (34.0) | 35 (37.6) | 44 (41.9) | 40 (36.7) | 31 (31.3) | 32 (36.0) | 47 (47.5) | ||||||
| >5 cm | 69 (57.5) | 147 (63.6) | 58 (51.3) | 110 (54.2) | 69 (63.3) | 64 (66.0) | 58 (62.4) | 61 (58.1) | 69 (63.3) | 68 (68.7) | 57 (64.0) | 52 (52.5) | ||||||
| unknown | 11 (9.2) | 39 (16.9) | 15 (13.3) | 29 (14.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Treatment | <0.001 | <0.001 | <0.001 | <0.001 | 0.724 | 0.728 | ||||||||||||
| No surgery procedure | 61 (50.8) | 162 (70.1) | 53 (46.9) | 148 (72.9) | 50 (45.9) | 79 (81.4) | 39 (41.9) | 84 (80.0) | 50 (45.9) | 43 (43.4) | 40 (44.9) | 47 (47.5) | ||||||
| surgery | 59 (49.2) | 69 (29.9) | 60 (53.1) | 55 (27.1) | 59 (54.1) | 18 (18.6) | 54 (58.1) | 21 (20.0) | 59 (54.1) | 56 (56.6) | 49 (55.1) | 52 (52.5) | ||||||
| Survival Rate | 0.007 | <0.001 | 0.010 | <0.001 | 0.943 | 0.015 | ||||||||||||
| 1 years (%) | 60.7 | 46.8 | 60.9 | 35.3 | 59.4 | 40.2 | 64.4 | 27.6 | 59.4 | 60.6 | 62.8 | 45.5 | ||||||
| 3 years (%) | 28.7 | 15.9 | 35.8 | 10.5 | 28.8 | 15.5 | 37.1 | 10.5 | 28.8 | 28.0 | 35.4 | 17.2 | ||||||
| 5 years (%) | 16.5 | 10.7 | 23.0 | 6.2 | 15.4 | 10.4 | 23.3 | 4.1 | 15.4 | 18.4 | 21.2 | 9.4 | ||||||
| Variable | Cox Proportional Hazards Analysis | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | ||||
| <70 y | Referent | NA | NA | NA |
| ≥70 y | 1.374 (1.144–1.650) | 0.001 | 1.208 (1.005–1.453) | 0.044 |
| Sex | ||||
| Female | Referent | NA | NA | NA |
| male | 1.124 (0.943–1.340) | 0.193 | NA | NA |
| Race | ||||
| White | Referent | NA | NA | NA |
| Black | 0.991 (0.725–1.355) | 0.955 | NA | NA |
| Other * | 0.918 (0.716–1.177) | 0.500 | NA | NA |
| Size | ||||
| ≤5 cm | Referent | NA | NA | NA |
| >5 cm | 1.216 (1.006–1.470) | 0.044 | NA | NA |
| Treatment | ||||
| No surgery procedure | Referent | NA | NA | NA |
| Surgery treatment | 0.284 (0.233–0.346) | <0.001 | 0.304 (0.248–0.372) | <0.001 |
| Number of lesions | ||||
| Solitary | Referent | NA | NA | NA |
| Multiple | 1.539 (1.276–1.855) | <0.001 | 1.236 (1.021–1.496) | 0.030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Wu, L.; Li, M.; Wang, J.; Wang, C.; Yang, J.; Zhang, L.; Ge, J.; Sun, C.; Li, E.; et al. Validation of the Prognostic Role for Surgical Treatment in Stage II Intrahepatic Cholangiocarcinoma: A SEER Population-Based Study. J. Clin. Med. 2023, 12, 675. https://doi.org/10.3390/jcm12020675
Luo S, Wu L, Li M, Wang J, Wang C, Yang J, Zhang L, Ge J, Sun C, Li E, et al. Validation of the Prognostic Role for Surgical Treatment in Stage II Intrahepatic Cholangiocarcinoma: A SEER Population-Based Study. Journal of Clinical Medicine. 2023; 12(2):675. https://doi.org/10.3390/jcm12020675
Chicago/Turabian StyleLuo, Shuaiwu, Linquan Wu, Min Li, Jiakun Wang, Cong Wang, Jun Yang, Ligan Zhang, Jin Ge, Chi Sun, Enliang Li, and et al. 2023. "Validation of the Prognostic Role for Surgical Treatment in Stage II Intrahepatic Cholangiocarcinoma: A SEER Population-Based Study" Journal of Clinical Medicine 12, no. 2: 675. https://doi.org/10.3390/jcm12020675




