Which Biomarker(s) Augment the Diagnostic Value of the Positive Exercise Electrocardiography Test: Systemic Inflammatory Index, Plasma Atherogenic Index, or Monocyte/HDL-C Ratio?
Abstract
:1. Introduction
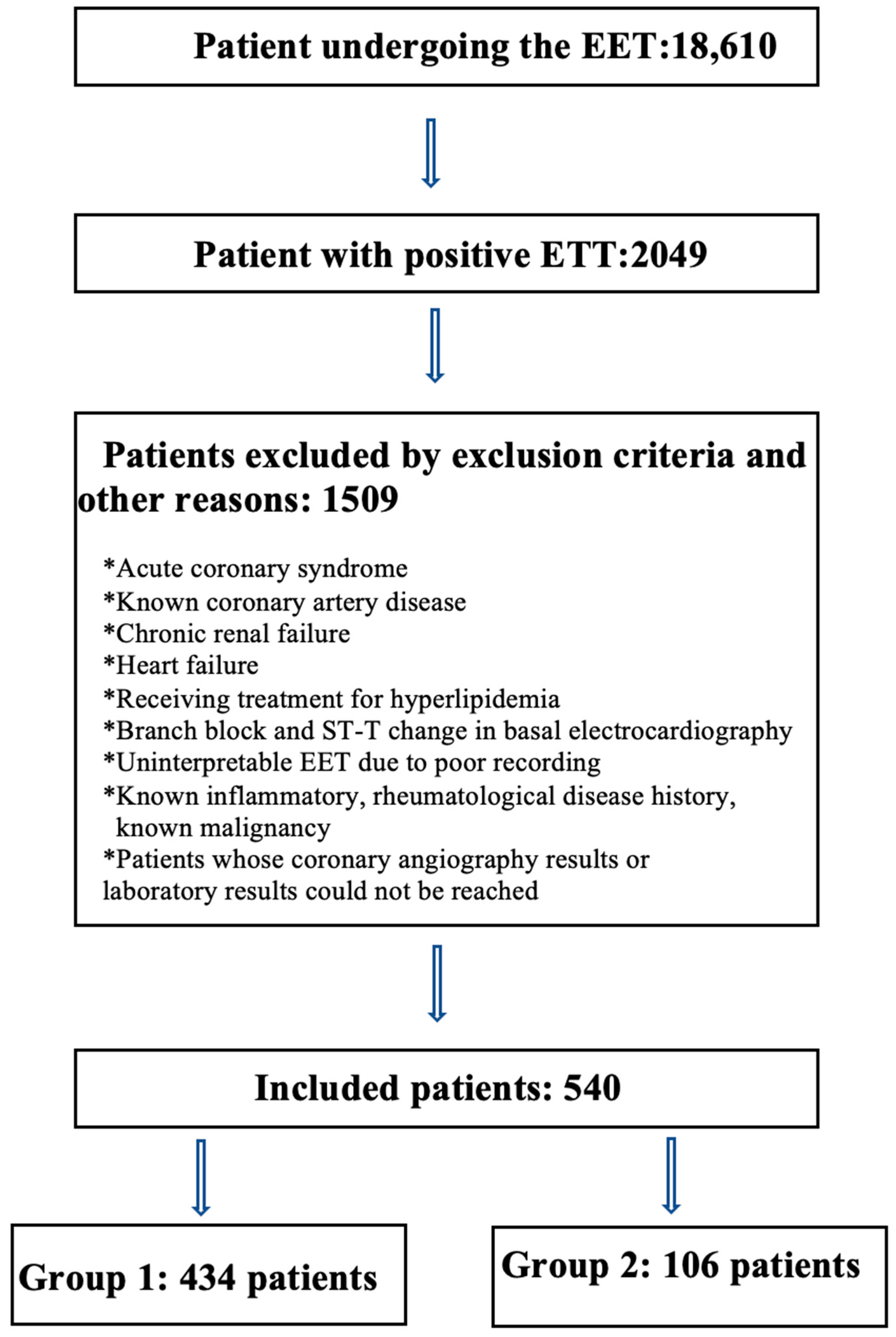
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Gianrossi, R.; Detrano, R.; Mulvihill, D.; Lehmann, K.; Dubach, P.; Colombo, A.; McArthur, D.; Froelicher, V. Exercise-induced ST depression in the diagnosis of coronary artery disease. A meta-analysis. Circulation 1989, 80, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Roifman, I.; Wijeysundera, H.C.; Austin, P.C.; Rezai, M.R.; Wright, G.A.; Tu, J.V. Comparison of Anatomic and Clinical Outcomes in Patients Undergoing Alternative Initial Noninvasive Testing Strategies for the Diagnosis of Stable Coronary Artery Disease. J. Am. Heart Assoc. 2017, 6, e005462. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Yang, Y.L.; Wu, C.H.; Hsu, P.F.; Chen, S.C.; Huang, S.S.; Chan, W.L.; Lin, S.J.; Chou, C.Y.; Chen, J.W.; Pan, J.P.; et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur. J. Clin. Investig. 2020, 50, e13230. [Google Scholar] [CrossRef]
- Gur, D.O.; Efe, M.M.; Alpsoy, S.; Akyüz, A.; Uslu, N.; Çelikkol, A.; Gur, O. Systemic Immune-Inflammatory Index as a Determinant of Atherosclerotic Burden and High-Risk Patients with Acute Coronary Syndromes. Arq. Bras. Cardiol. 2022, 119, 382–390. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, T.; Chen, L.; Jin, T.; Sheng, Y.; Wu, G.; Zong, G. Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron. Artery Dis. 2021, 32, 715–720. [Google Scholar] [CrossRef]
- Dziedzic, E.A.; Gąsior, J.S.; Tuzimek, A.; Paleczny, J.; Junka, A.; Dąbrowski, M.; Jankowski, P. Investigation of the Associations of Novel Inflammatory Biomarkers-Systemic Inflammatory Index (SII) and Systemic Inflammatory Response Index (SIRI)-With the Severity of Coronary Artery Disease and Acute Coronary Syndrome Occurrence. Int. J. Mol. Sci. 2022, 23, 9553. [Google Scholar] [CrossRef]
- Han, S.H.; Nicholls, S.J.; Sakuma, I.; Zhao, D.; Koh, K.K. Hypertriglyceridemia and Cardiovascular Diseases: Revisited. Korean Circ. J. 2016, 46, 135–144. [Google Scholar] [CrossRef]
- Goldbourt, U.; Yaari, S.; Medalie, J.H. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21-year follow-up of 8000 men. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Lu, Y.; Qi, H.; Li, F.; Shen, Z.; Wu, L.; Yang, C.; Wang, L.; Shui, K.; Wang, Y.; et al. Association between ideal cardiovascular health and the atherogenic index of plasma. Medicine 2016, 95, e3866. [Google Scholar] [CrossRef] [PubMed]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med. J. Islam Repub. Iran. 2015, 29, 240. [Google Scholar] [PubMed]
- Fernández-Macías, J.C.; Ochoa-Martínez, A.C.; Varela-Silva, J.A.; Pérez-Maldonado, I.N. Atherogenic Index of Plasma: Novel Predictive Biomarker for Cardiovascular Illnesses. Arch. Med. Res. 2019, 50, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, Q.; Wei, Z.; Wei, J.; Cui, M. Atherogenic Index of Plasma and Coronary Artery Disease in the Adult Population: A Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 817441, Erratum in: Front. Cardiovasc. Med. 2023, 10, 1153914. https://doi.org/10.3389/fcvm.2023.1153914. [Google Scholar] [CrossRef]
- Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Alarcon-Braga, E.A.; Mosquera-Rojas, M.D.; Campos-Aspajo, A.; Salazar-Valdivia, F.E.; Valdez-Cornejo, V.A.; Benites-Zapata, V.A.; Herrera-Añazco, P.; Valenzuela-Rodríguez, G.; et al. Atherogenic index of plasma and coronary artery disease: A systematic review. Open Med. 2022, 17, 1915–1926. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, J.; Zou, H.; Li, M.; Sun, W.; Kong, X. Monocyte-to-high-density lipoprotein-cholesterol ratio (MHR) and the risk of all-cause and cardiovascular mortality: A nationwide cohort study in the United States. Lipids Health Dis. 2022, 21, 30. [Google Scholar] [CrossRef]
- Ganjali, S.; Gotto, A.M.; Ruscica, M., Jr.; Atkin, S.L.; Butler, A.E.; Banach, M.; Sahebkar, A. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. Cell Physiol. 2018, 233, 9237–9246. [Google Scholar] [CrossRef]
- Akboga, M.K.; Balci, K.G.; Maden, O.; Ertem, A.G.; Kirbas, O.; Yayla, C.; Acar, B.; Aras, D.; Kisacik, H.; Aydogdu, S. Usefulness of monocyte to HDL-cholesterol ratio to predict high SYNTAX score in patients with stable coronary artery disease. Biomark Med. 2016, 10, 375–383. [Google Scholar] [CrossRef]
- Cetin, M.S.; Ozcan Cetin, E.H.; Kalender, E.; Aydin, S.; Topaloglu, S.; Kisacik, H.L.; Temizhan, A. Monocyte to HDL Cholesterol Ratio Predicts Coronary Artery Disease Severity and Future Major Cardiovascular Adverse Events in Acute Coronary Syndrome. Heart Lung Circ. 2016, 25, 1077–1086. [Google Scholar] [CrossRef]
- Liu, H.T.; Jiang, Z.H.; Yang, Z.B.; Quan, X.Q. Monocyte to high-density lipoprotein ratio predict long-term clinical outcomes in patients with coronary heart disease: A meta-analysis of 9 studies. Medicine 2022, 101, e30109. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Solak, Y.; Unal, H.U.; Kurt, Y.G.; Gok, M.; Cetinkaya, H.; Karaman, M.; Oguz, Y.; Eyileten, T.; Vural, A.; et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int. Urol. Nephrol. 2014, 46, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Tani, S.; Matsumoto, M.; Anazawa, T.; Kawamata, H.; Furuya, S.; Takahashi, H.; Iida, K.; Washio, T.; Kumabe, N.; Kobori, M.; et al. Development of a model for prediction of coronary atherosclerotic regression: Evaluation of high-density lipoprotein cholesterol level and peripheral blood monocyte count. Heart Vessels 2012, 27, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, P.; Ye, J. Lipid homeostasis and the formation of macrophage-derived foam cells in atherosclerosis. Protein Cell 2012, 3, 173–181. [Google Scholar] [CrossRef]
- Ghattas, A.; Griffiths, H.R.; Devitt, A.; Lip, G.Y.; Shantsila, E. Monocytes in coronary artery disease and atherosclerosis: Where are we now? J. Am. Coll. Cardiol. 2013, 62, 1541–1551. [Google Scholar] [CrossRef]
- Gratchev, A.; Sobenin, I.; Orekhov, A.; Kzhyshkowska, J. Monocytes as a diagnostic marker of cardiovascular diseases. Immunobiology 2012, 217, 476–482. [Google Scholar] [CrossRef]
- Toth, P.P.; Barter, P.J.; Rosenson, R.S.; Boden, W.E.; Chapman, M.J.; Cuchel, M.; D’Agostino, R.B.; Davidson, M.H., Sr.; Davidson, W.S.; Heinecke, J.W.; et al. High-density lipoproteins: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 484–525. [Google Scholar] [CrossRef]
- Tardif, J.C.; Grégoire, J.; L’Allier, P.L.; Ibrahim, R.; Lespérance, J.; Heinonen, T.M.; Kouz, S.; Berry, C.; Basser, R.; Lavoie, M.A.; et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. JAMA 2007, 297, 1675–1682. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Chin-Dusting, J.P.; Sviridov, D.; Woollard, K.J. The anti inflammatory effects of high density lipoproteins. Curr. Med. Chem. 2009, 16, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Woollard, K.J. High-density lipoprotein: A potent inhibitor of inflammation. Clin. Exp. Pharmacol. Physiol. 2010, 37, 710–718. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Jia, L.; Lu, B.; Gu, G.; Bai, L.; Cui, W. The Monocyte to High-Density Lipoprotein Cholesterol Ratio in the Prediction for Atherosclerosis: A Retrospective Study in Adult Chinese Participants. Lipids 2021, 56, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Sha, S.; Wang, D.; Li, S.; Jia, Y. Association between monocyte to high-density lipoprotein ratio and coronary heart disease in US adults in the National Health and Nutrition Examination Surveys 2009–2018. Coron. Artery Dis. 2023, 34, 111–118. [Google Scholar] [CrossRef]
- Kundi, H.; Kiziltunc, E.; Cetin, M.; Cicekcioglu, H.; Cetin, Z.G.; Cicek, G.; Ornek, E. Association of monocyte/HDL-C ratio with SYNTAX scores in patients with stable coronary artery disease. Zusammenhang des Monozyten-/HDL-C-Quotienten mit dem SYNTAX-Score bei Patienten mit stabiler koronarer Herzkrankheit. Herz 2016, 41, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Candemir, M.; Kiziltunç, E.; Nurkoç, S.; Şahinarslan, A. Relationship Between Systemic Immune-Inflammation Index (SII) and the Severity of Stable Coronary Artery Disease. Angiology 2021, 72, 575–581. [Google Scholar] [CrossRef]
- Erdoğan, M.; Erdöl, M.A.; Öztürk, S.; Durmaz, T. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark Med. 2020, 14, 1553–1561. [Google Scholar] [CrossRef]
- Wang, L.; Chen, F.; Xiaoqi, C.; Yujun, C.; Zijie, L. Atherogenic Index of Plasma Is an Independent Risk Factor for Coronary Artery Disease and a Higher SYNTAX Score. Angiology 2021, 72, 181–186. [Google Scholar] [CrossRef]
- Newman, R.J.; Darrow, M.; Cummings, D.M.; King, V.; Whetstone, L.; Kelly, S.; Jalonen, E. Predictive value of exercise stress testing in a family medicine population. J. Am. Board Fam. Med. 2008, 21, 531–538. [Google Scholar] [CrossRef]
- Taylor, C.A.; Fonte, T.A.; Min, J.K. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: Scientific basis. J. Am. Coll. Cardiol. 2013, 61, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiu, H.; Hou, Z.; Zheng, J.; Li, J.; Yin, Y.; Gao, R. Additional value of deep learning computed tomographic angiography-based fractional flow reserve in detecting coronary stenosis and predicting outcomes. Acta Radiol. 2022, 63, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.P.; Maredia, N.; Younger, J.F.; Brown, J.M.; Nixon, J.; Everett, C.C.; Bijsterveld, P.; Ridgway, J.P.; Radjenovic, A.; Dickinson, C.J.; et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet 2012, 379, 453–460. [Google Scholar] [CrossRef]
- Fathala, A.; Aboulkheir, M.; Shoukri, M.M.; Alsergani, H. Diagnostic accuracy of 13N-ammonia myocardial perfusion imaging with PET-CT in the detection of coronary artery disease. Cardiovasc. Diagn. Ther. 2019, 9, 35–42. [Google Scholar] [CrossRef]
- Woodward, W.; Dockerill, C.; McCourt, A.; Upton, R.; O’Driscoll, J.; Balkhausen, K.; Chandrasekaran, B.; Firoozan, S.; Kardos, A.; Wong, K.; et al. Real-world performance and accuracy of stress echocardiography: The EVAREST observational multi-centre study. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 689–698. [Google Scholar] [CrossRef]
- Molinaro, A.M. Diagnostic tests: How to estimate the positive predictive value. Neurooncol. Pract. 2015, 2, 162–166. [Google Scholar] [CrossRef]
- Froelicher, V.F.; Maron, D. Exercise testing and ancillary techniques to screen for coronary heart disease. Prog. Cardiovasc. Dis. 1981, 24, 261–274. [Google Scholar] [CrossRef]
- van de Sande, D.A.; Breuer, M.A.; Kemps, H.M. Utility of Exercise Electrocardiography in Pre-participation Screening in Asymptomatic Athletes: A Systematic Review. Sports Med. 2016, 46, 1155–1164. [Google Scholar] [CrossRef]
- Levisman, J.M.; Aspry, K.; Amsterdam, E.A. Improving the positive predictive value of exercise testing in women for coronary artery disease. Am. J. Cardiol. 2012, 110, 1619–1622. [Google Scholar] [CrossRef]

| Group 1 (n = 434) (NCA and n-obsCAD) | Group 2 (n = 106) (obsCAD) | p | |
|---|---|---|---|
| Age | 54.4 ± 9.6 | 59.4 ± 9.4 | <0.001 |
| Sex (F/M) | 190/244 (%43.8/%56.2) | 21/85 (%19.8/%80.2) | <0.001 |
| BMI | 30.1 ± 5.2 | 29.1 ± 4.4 | 0.067 |
| SBP | 117.6 ± 13.6 | 120.1 ± 15.1 | 0.128 |
| DBP | 76.5 ± 7.8 | 77.4 ± 7.3 | 0.292 |
| Heart rate. | 86.4 ± 15 | 87.3 ± 13.8 | 0.562 |
| HT | 88 (%20.3) | 16 (%15.1) | 0.282 |
| DM | 60 (%13.8) | 27 (%25.5) | 0.003 |
| Glucose | 102.5 [93–122] | 109.5 [95.5–146.8] | 0.006 |
| GFR | 95.3 ± 14.5 | 90.1 ± 13.5 | 0.001 |
| LDL-C | 117.7 ± 35.8 | 125.9 ± 34.6 | 0.033 |
| Triglyceride | 157 [112.8–223] | 163 [126.8–249.5] | 0.160 |
| HDL-C | 43 [37–51] | 40.5 [34–47] | 0.010 |
| WBC | 7254.8 ± 1823.8 | 7666.04 ± 2047.0 | 0.043 |
| Neutrophil | 4304.2 ± 1421.3 | 4541.23 ± 1542.2 | 0.131 |
| Lymphocyte | 2210.1 ± 627.9 | 2305.3 ± 748.7 | 0.179 |
| Monocyte | 545.1 ± 163.6 | 577.0 ± 193.5 | 0.120 |
| Hemoglobin | 14.6 ± 1.7 | 14.9 ± 1.7 | 0.162 |
| Platelet | 255.6 ± 55.5 | 261.3 ± 70.9 | 0.446 |
| MHR | 13.1 ± 5.5 | 14.7 ± 6.3 | 0.019 |
| SII | 479 [361.8–632.3] | 478 [345.3–660.8] | 0.848 |
| PAI | 0.21 ± 0.269 | 0.259 ± 0.274 | 0.094 |
| Factor | Odds Ratio (%95 CI) | p |
|---|---|---|
| Age | 1058 (1034–1084) | <0.001 |
| Sex (Male vs. Female) | 3652 (2137–6239) | <0.001 |
| DM | 2239 (1285–3903) | 0.004 |
| LDL-C | 1009 (1002–1015) | 0.007 |
| obsCAD | SYNTAX ≤ 22 (n = 82) | SYNTAX 23≤ (n = 24) | p |
|---|---|---|---|
| PAI | 0.251 ± 0.275 | 0.290 ± 0.273 | 0.543 |
| SII | 478 [369–652] | 471 [293.5–815.3] | 0.711 |
| MHR | 14.5 ± 6.1 | 15.3 ± 7.1 | 0.616 |
| (a) | |||||||
| Treatment Strategy n = 106 (%100) | OMT n = 14 (%13) | PCI n = 57 (%54) | CABG n = 35 (%33) | ||||
| (b) | |||||||
| Obstructed Vessel n = 189 (%100) | LAD n = 70 (%37) | CX n = 59 (%31) | RCA n = 49 (%26) | IMA n = 9 (%5) | LMCA n = 2 (%1) | ||
| Treatment Strategy | |||||||
| OMT | 8 | 6 | 5 | 2 | 0 | ||
| PCI | 29 | 26 | 24 | 2 | 0 | ||
| CABG | 33 | 27 | 20 | 5 | 2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergun, G.; Demirelli, S. Which Biomarker(s) Augment the Diagnostic Value of the Positive Exercise Electrocardiography Test: Systemic Inflammatory Index, Plasma Atherogenic Index, or Monocyte/HDL-C Ratio? J. Clin. Med. 2023, 12, 6440. https://doi.org/10.3390/jcm12206440
Ergun G, Demirelli S. Which Biomarker(s) Augment the Diagnostic Value of the Positive Exercise Electrocardiography Test: Systemic Inflammatory Index, Plasma Atherogenic Index, or Monocyte/HDL-C Ratio? Journal of Clinical Medicine. 2023; 12(20):6440. https://doi.org/10.3390/jcm12206440
Chicago/Turabian StyleErgun, Gokhan, and Selami Demirelli. 2023. "Which Biomarker(s) Augment the Diagnostic Value of the Positive Exercise Electrocardiography Test: Systemic Inflammatory Index, Plasma Atherogenic Index, or Monocyte/HDL-C Ratio?" Journal of Clinical Medicine 12, no. 20: 6440. https://doi.org/10.3390/jcm12206440





