The Surreptitious Burden of Nonalcoholic Fatty Liver Disease in the Elderly in the Asia-Pacific Region: An Insight from the Global Burden of Disease Study 2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Estimation Methods
2.3. Data and Statistical Analysis
3. Results
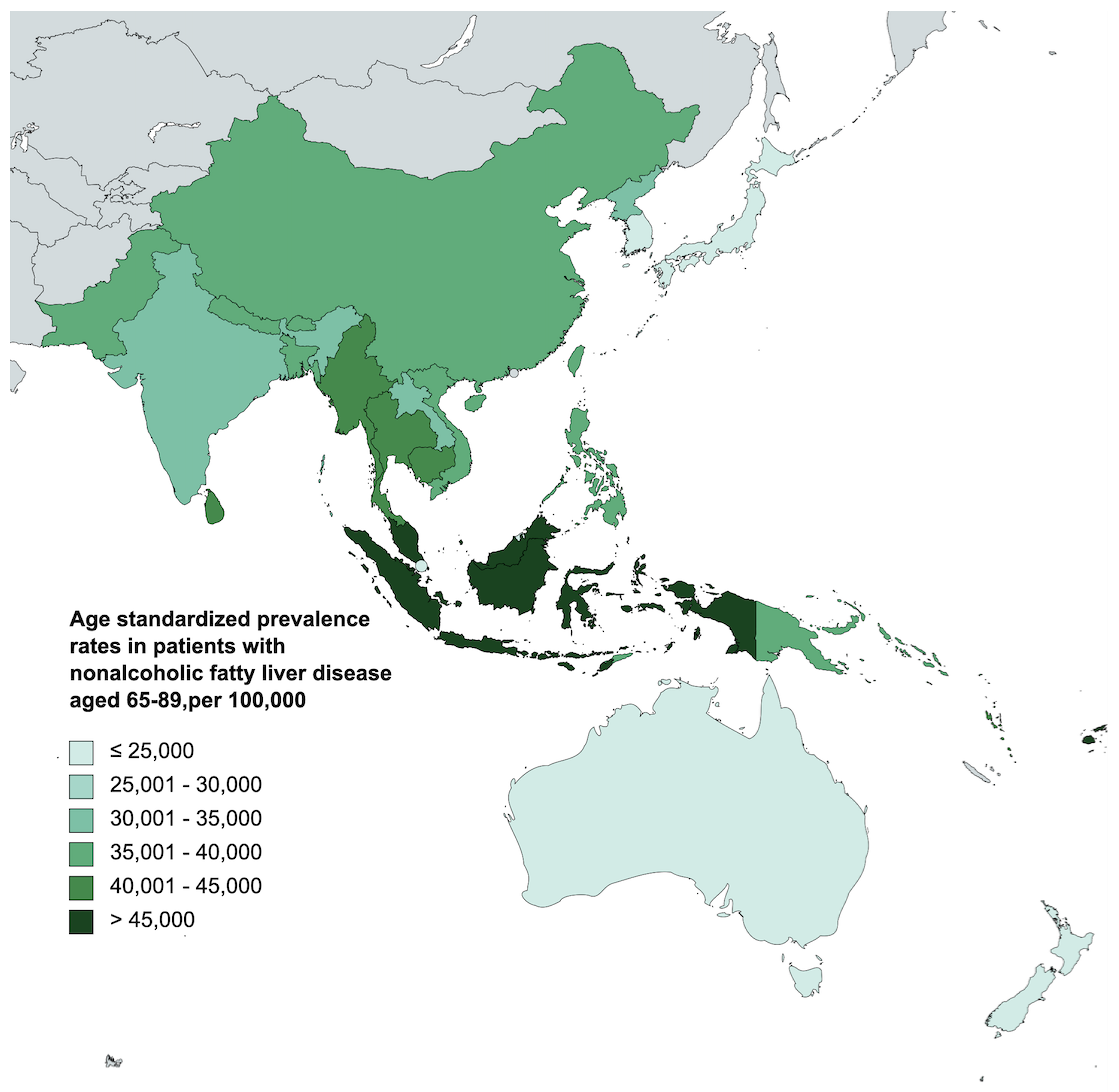
3.1. Prevalence of Nonalcoholic Fatty Liver Disease in the Elderly in Asia-Pacific
3.2. Disability-Adjusted Life Years of Nonalcoholic Fatty Liver Disease in the Elderly in Asia-Pacific
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, V.W.; Ekstedt, M.; Wong, G.L.; Hagstrom, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Danpanichkul, P.; Ng, C.H.; Muthiah, M.D.; Duangsonk, K.; Yong, J.N.; Tan, D.J.H.; Lim, W.H.; Wong, Z.Y.; Syn, N.; Tsusumi, T.; et al. The silent burden of non-alcoholic fatty liver disease in the elderly: A global burden of disease analysis. Aliment. Pharmacol. Ther. 2023. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.A.; Schattenberg, J.M. NAFLD in the Elderly. Clin. Interv. Aging 2021, 16, 1633–1649. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Yates, K.P.; Vaughn, I.A.; Neuschwander-Tetri, B.A.; Sanyal, A.J.; McCullough, A.; Merriman, R.; Hameed, B.; Doo, E.; Kleiner, D.E.; et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology 2013, 58, 1644–1654. [Google Scholar] [CrossRef]
- Hartleb, M.; Baranski, K.; Zejda, J.; Chudek, J.; Wiecek, A. Non-alcoholic fatty liver and advanced fibrosis in the elderly: Results from a community-based Polish survey. Liver Int. 2017, 37, 1706–1714. [Google Scholar] [CrossRef]
- Fan, J.G.; Kim, S.U.; Wong, V.W. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017, 67, 862–873. [Google Scholar] [CrossRef]
- Goh, V.H. Aging in Asia: A cultural, socio-economical and historical perspective. Aging Male 2005, 8, 90–96. [Google Scholar] [CrossRef]
- Yip, T.C.; Lee, H.W.; Chan, W.K.; Wong, G.L.; Wong, V.W. Asian perspective on NAFLD-associated HCC. J. Hepatol. 2022, 76, 726–734. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Song, S.J.; Che-To Lai, J.; Lai-Hung Wong, G.; Wai-Sun Wong, V.; Cheuk-Fung Yip, T. Can we use old NAFLD data under the new MASLD definition? J. Hepatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 101133. [Google Scholar] [CrossRef]
- Paik, J.M.; Kabbara, K.; Eberly, K.E.; Younossi, Y.; Henry, L.; Younossi, Z.M. Global burden of NAFLD and chronic liver disease among adolescents and young adults. Hepatology 2022, 75, 1204–1217. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, M.; Lonardo, A.; Mussi, C.; Baldelli, E.; Pellegrini, E.; Ballestri, S.; Romagnoli, D.; Loria, P. Nonalcoholic fatty liver disease and aging: Epidemiology to management. World J. Gastroenterol. 2014, 20, 14185–14204. [Google Scholar] [CrossRef]
- Li, Y.; Adeniji, N.T.; Fan, W.; Kunimoto, K.; Torok, N.J. Non-alcoholic Fatty Liver Disease and Liver Fibrosis during Aging. Aging Dis. 2022, 13, 1239–1251. [Google Scholar] [CrossRef]
- Kim, D.; Ahmed, A. Nonalcoholic fatty liver disease in early life and all-cause and cause-specific mortality. Hepatobiliary Surg. Nutr. 2022, 11, 317–319. [Google Scholar] [CrossRef]
- Targher, G.; Corey, K.E.; Byrne, C.D. NAFLD, and cardiovascular and cardiac diseases: Factors influencing risk, prediction and treatment. Diabetes Metab. 2021, 47, 101215. [Google Scholar] [CrossRef]
- Venetsanaki, V.; Polyzos, S.A. Menopause and Non-Alcoholic Fatty Liver Disease: A Review Focusing on Therapeutic Perspectives. Curr. Vasc. Pharmacol. 2019, 17, 546–555. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Ohbora, A.; Takeda, N.; Fukui, M.; Kato, T. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World J. Gastroenterol. 2012, 18, 237–243. [Google Scholar] [CrossRef]
- Cao, W.; Xu, Y.; Shen, Y.; Wang, Y.; Ma, X.; Bao, Y. Associations between sex hormones and metabolic-associated fatty liver disease in a middle-aged and elderly community. Endocr. J. 2022, 69, 1007–1014. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Lomas-Soria, C.; Cox, L.A.; Nathanielsz, P.W.; Zambrano, E. Sexual dimorphism in liver cell cycle and senescence signalling pathways in young and old rats. J. Physiol. 2021, 599, 4309–4320. [Google Scholar] [CrossRef] [PubMed]
- Pramfalk, C.; Pavlides, M.; Banerjee, R.; McNeil, C.A.; Neubauer, S.; Karpe, F.; Hodson, L. Sex-Specific Differences in Hepatic Fat Oxidation and Synthesis May Explain the Higher Propensity for NAFLD in Men. J. Clin. Endocrinol. Metab. 2015, 100, 4425–4433. [Google Scholar] [CrossRef] [PubMed]
- Rostad, B.; Deeg, D.J.H.; Schei, B. Socioeconomic inequalities in health in older women. Eur. J. Ageing 2009, 6, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.A.; Song, J.; Manheim, L.M.; Dunlop, D.D. Gender disparities in health and healthcare use among older adults. J. Womens Health 2010, 19, 1643–1650. [Google Scholar] [CrossRef]
- Claramita, M.; Nugraheni, M.D.; van Dalen, J.; van der Vleuten, C. Doctor-patient communication in Southeast Asia: A different culture? Adv. Health Sci. Educ. Theory Pract. 2013, 18, 15–31. [Google Scholar] [CrossRef]
- Jiang, W.; Mao, X.; Liu, Z.; Zhang, T.; Jin, L.; Chen, X. Global Burden of Nonalcoholic Fatty Liver Disease, 1990 to 2019: Findings From the Global Burden of Disease Study 2019. J. Clin. Gastroenterol. 2022, 57, 631–639. [Google Scholar] [CrossRef]
- Nanditha, A.; Ma, R.C.; Ramachandran, A.; Snehalatha, C.; Chan, J.C.; Chia, K.S.; Shaw, J.E.; Zimmet, P.Z. Diabetes in Asia and the Pacific: Implications for the Global Epidemic. Diabetes Care 2016, 39, 472–485. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Zhu, J.; Gao, L.; Wang, Y. Growing fast food consumption and obesity in Asia: Challenges and implications. Soc. Sci. Med. 2021, 269, 113601. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Zhu, Z.; Li, Y.; Li, X.; Li, Y.; Shen, H.; Wu, W.; Liu, Y.; Han, C. Changes in the global, regional, and national burdens of NAFLD from 1990 to 2019: A systematic analysis of the global burden of disease study 2019. Front. Nutr. 2022, 9, 1047129. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kutting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Tilg, H. NAFLD and increased risk of cardiovascular disease: Clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 2020, 69, 1691–1705. [Google Scholar] [CrossRef]
- Lu, F.B.; Zheng, K.I.; Rios, R.S.; Targher, G.; Byrne, C.D.; Zheng, M.H. Global epidemiology of lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2020, 35, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Panjawatanan, P.; Aby, E.; Ahmed, A.; Kim, D. Nonalcoholic fatty liver disease in the over-60s: Impact of sarcopenia and obesity. Maturitas 2019, 124, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Scafato, E.; Custodero, C.; Loparco, F.; Ciavarella, A.; Panza, F.; Seripa, D.; Imbimbo, B.P.; Lozupone, M.; Napoli, N.; et al. Liver fibrosis score, physical frailty, and the risk of dementia in older adults: The Italian Longitudinal Study on Aging. Alzheimers Dement 2020, 6, e12065. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Li, F.; Lundin, S.K.; Suresh, D.; Song, M.W.; Tao, C.; Chen, V.L.; Lok, A.S.F. Higher mortality among lean patients with non-alcoholic fatty liver disease despite fewer metabolic comorbidities. Aliment. Pharmacol. Ther. 2023, 57, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.M.; Henry, L.; Younossi, Z.M. Nonalcoholic fatty liver disease mortality may not be decreasing: A need for careful interpretation of GBD 2019 estimates of liver deaths. Cell Metab. 2023, 35, 1087–1088. [Google Scholar] [CrossRef]
- Herrera, A.P.; Snipes, S.A.; King, D.W.; Torres-Vigil, I.; Goldberg, D.S.; Weinberg, A.D. Disparate inclusion of older adults in clinical trials: Priorities and opportunities for policy and practice change. Am. J. Public Health 2010, 100 (Suppl. 1), S105–S112. [Google Scholar] [CrossRef]
- Eslam, M.; Sarin, S.K.; Wong, V.W.; Fan, J.G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef]
- Lonardo, A.; Arab, J.P.; Arrese, M. Perspectives on Precision Medicine Approaches to NAFLD Diagnosis and Management. Adv. Ther. 2021, 38, 2130–2158. [Google Scholar] [CrossRef]
- Forlano, R.; Sigon, G.; Mullish, B.H.; Yee, M.; Manousou, P. Screening for NAFLD—Current Knowledge and Challenges. Metabolites 2023, 13, 536. [Google Scholar] [CrossRef]
- Gu, Y.; Luo, J.; Chen, Q.; Qiu, Y.; Zhou, Y.; Wang, X.; Qian, X.; Liu, Y.; Xie, J.; Xu, Z.; et al. Inverse Association of Serum Adipsin with the Remission of Nonalcoholic Fatty-Liver Disease: A 3-Year Community-Based Cohort Study. Ann. Nutr. Metab. 2022, 78, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Pipitone, R.M.; Camma, C.; Di Marco, V.; Di Martino, V.; Spatola, F.; Zito, R.; Craxi, A.; Grimaudo, S.; Petta, S. PNPLA3 rs738409 C>G Variant Predicts Fibrosis Progression by Noninvasive Tools in Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 1979–1981. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Imajo, K.; Kobayashi, T.; Kessoku, T.; Ogawa, Y.; Tomeno, W.; Yoneda, M.; Kobayashi, N.; Saito, S.; Nakajima, A. Autotaxin is a valuable biomarker for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2019, 49, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.L.; Oliveri, A.; Miller, M.J.; Wijarnpreecha, K.; Du, X.; Chen, Y.; Cushing, K.C.; Lok, A.S.; Speliotes, E.K. PNPLA3 Genotype and Diabetes Identify Patients with Nonalcoholic Fatty Liver Disease at High Risk of Incident Cirrhosis. Gastroenterology 2023, 164, 966–977.e1. [Google Scholar] [CrossRef]
- Cvitanovic, T.; Reichert, M.C.; Moskon, M.; Mraz, M.; Lammert, F.; Rozman, D. Large-scale computational models of liver metabolism: How far from the clinics? Hepatology 2017, 66, 1323–1334. [Google Scholar] [CrossRef]
- Cvitanovic Tomas, T.; Moskon, M.; Mraz, M.; Rozman, D. Computational Modelling of Liver Metabolism and its Applications in Research and the Clinics. Acta Chim. Slov. 2018, 65, 253–265. [Google Scholar] [CrossRef]
- Skubic, C.; Drakulic, Z.; Rozman, D. Personalized therapy when tackling nonalcoholic fatty liver disease: A focus on sex, genes, and drugs. Expert Opin. Drug Metab. Toxicol. 2018, 14, 831–841. [Google Scholar] [CrossRef]
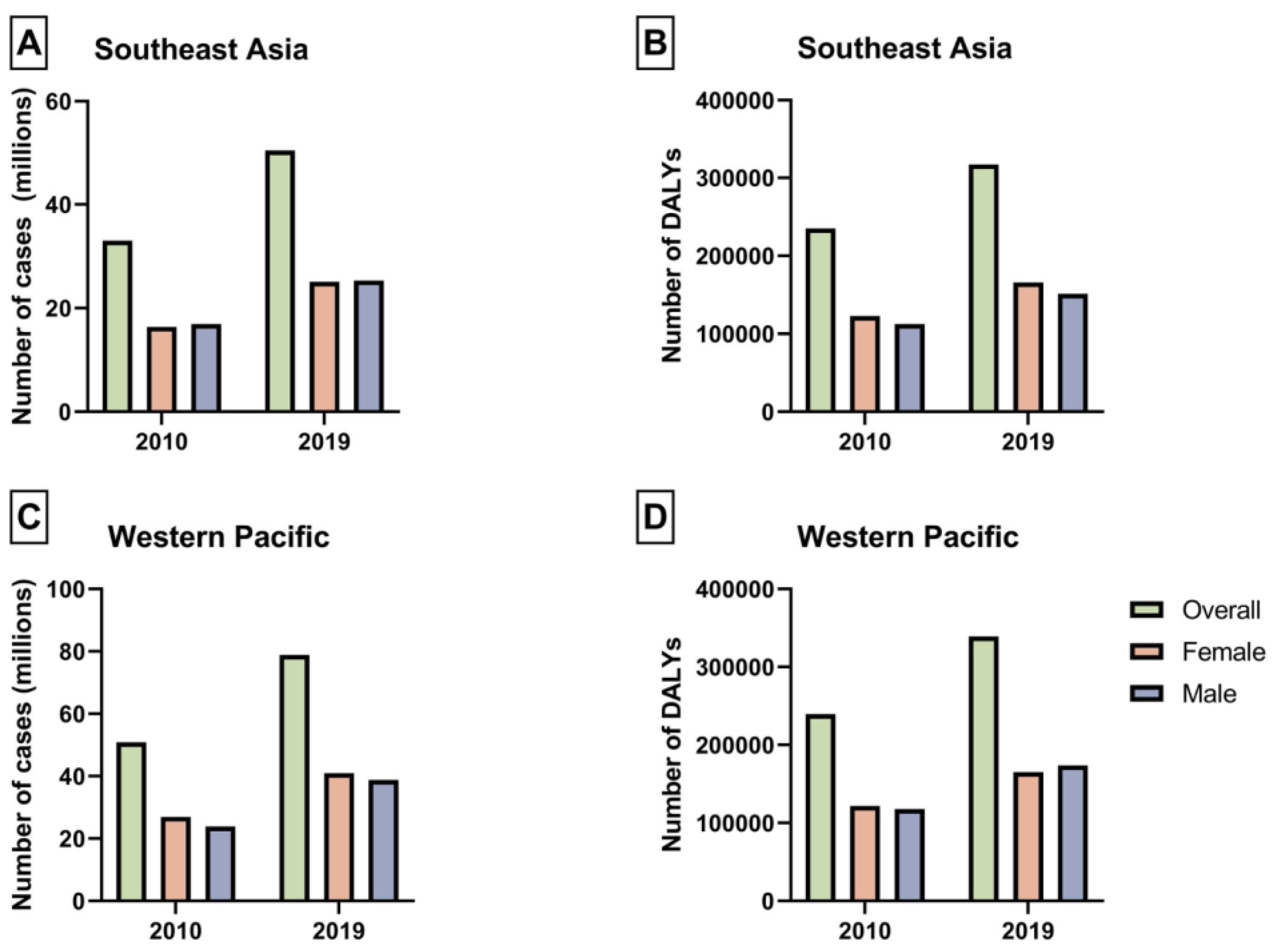
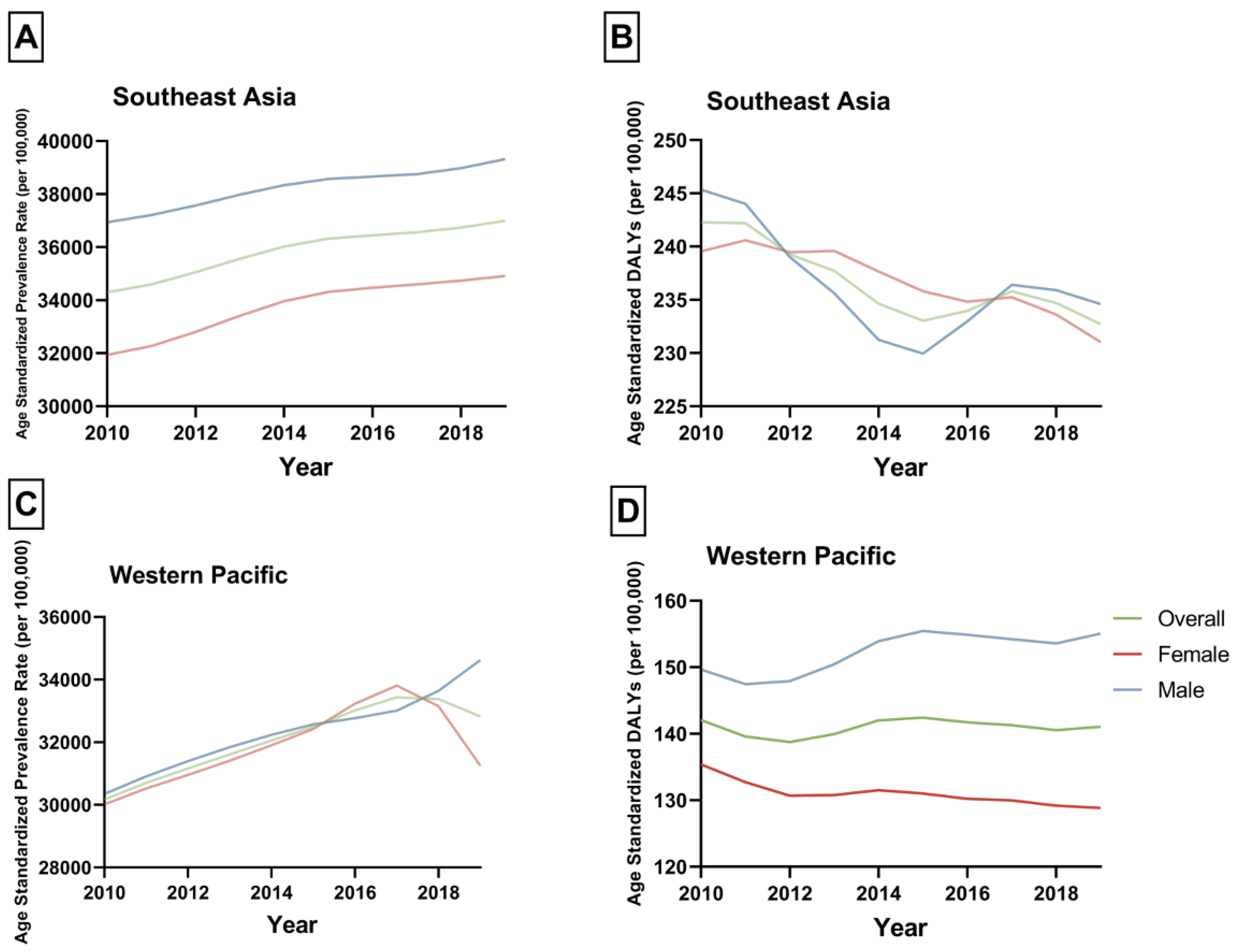
| 2010 Prevalence (95% UI) | 2010 ASPR, per 100,000 (95% UI) | 2019 Prevalence (95% UI) | 2019 ASPR, per 100,000 (95% UI) | APC (95% CI) | p | |
|---|---|---|---|---|---|---|
| Western Pacific | ||||||
| Overall | 50,872,617.88 (42,425,025.12 to 60,674,143.65) | 30,171.62 (25,161.51 to 35,984.73) | 78,901,129.54 (66,357,352.99 to 92,968,439.78) | 32,821.78 (27,603.74 to 38,673.58) | 0.95 (0.87 to 1.03) | <0.001 |
| Female | 26,989,633.59 (22,468,540.21 to 32,562,717.23) | 30,017.44 (24,989.15 to 36,215.73) | 40,095,703.93 (33,317,904.88 to 48,768,977.91) | 31,247.77 (25,965.63 to 38,007.11) | 0.59 (0.35 to 0.83) | <0.001 |
| Male | 23,882,984.29 (19,905,539.39 to 28,371,702.26) | 30,347.79 (25,293.7 to 36,051.54) | 38,805,425.61 (32,892,635.6 to 45,127,982.77) | 34,623.83 (29,348.19 to 40,265.09) | 1.3 (1.12 to 1.48) | <0.001 |
| Southeast Asia | ||||||
| Overall | 33,306,919.91 (27,504,062.2 to 40,512,069.6) | 34,295.03 (28,320.02 to 41,713.93) | 50,406,719.89 (41,956,546.18 to 60,400,342.45) | 36,995.37 (30,793.47 to 44,330.06) | 0.87 (0.78 to 0.95) | <0.001 |
| Female | 16,388,617.1 (13,407,026.03 to 20,105,405.24) | 31,935.36 (26,125.34 to 39,178.01) | 25,081,401.08 (20,780,159.13 to 30,536,791.54) | 34,912.59 (28,925.38 to 42,506.33) | 1.01 (0.9 to 1.11) | <0.001 |
| Male | 16,918,302.81 (14,079,388.13 to 20,414,306.75) | 36,938.96 (30,740.55 to 44,572.04) | 25,325,318.8 (21,207,566.73 to 30,006,548.71) | 39,318.41 (32,925.46 to 46,586.17) | 0.68 (0.57 to 0.79) | <0.001 |
| 2010 DALYs (95% UI) | 2010 ASDALYs, per 100,000 (95% UI) | 2019 DALYs (95% UI) | 2019 ASDALYs, per 100,000 (95% UI) | APC (95% CI) | p | |
|---|---|---|---|---|---|---|
| Western Pacific | ||||||
| Overall | 239,498.28 (181,736.79 to 306,983.96) | 142.04 (107.78 to 182.07) | 339,101.73 (259,107.34 to 435,733.54) | 141.06 (107.79 to 181.26) | 0.0 (−0.15 to 0.3) | 0.485 |
| Female | 121,739.38 (93,158.61 to 154,664.67) | 135.4 (103.61 to 172.02) | 165,325.16 (124,489.45 to 214,151.49) | 128.84 (97.02 to 166.89) | −0.5 (−0.73 to −0.26) | <0.001 |
| Male | 117,758.9 (88,314.9 to 153,076.43) | 149.63 (112.22 to 194.51) | 173,776.58 (130,264.88 to 228,502.19) | 155.05 (116.23 to 203.88) | 0.55 (0.24 to 0.87) | 0.003 |
| Southeast Asia | ||||||
| Overall | 235,306.26 (167,996.37 to 319,919.62) | 242.29 (172.98 to 329.41) | 317,070.93 (224,777.19 to 432,256.57) | 232.71 (164.97 to 317.25) | −0.41 (−0.66 to −0.17) | 0.001 |
| Female | 122,934.42 (86,730.17 to 167,482.56) | 239.55 (169.01 to 326.36) | 165,970.08 (117,185.61 to 225,328.43) | 231.03 (163.12 to 313.65) | −0.42 (−0.53 to −0.31) | <0.001 |
| Male | 112,371.83 (78,463.65 to 153,263.92) | 245.35 (171.32 to 334.63) | 151,100.85 (105,089.3 to 210,042.2) | 234.59 (163.15 to 326.1) | −0.48 (−0.86 to −0.11) | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danpanichkul, P.; Kongarin, S.; Permpatdechakul, S.; Polpichai, N.; Duangsonk, K.; Manosroi, W.; Chaiyakunapruk, N.; Mousa, O.Y.; Kim, D.; Chen, V.L.; et al. The Surreptitious Burden of Nonalcoholic Fatty Liver Disease in the Elderly in the Asia-Pacific Region: An Insight from the Global Burden of Disease Study 2019. J. Clin. Med. 2023, 12, 6456. https://doi.org/10.3390/jcm12206456
Danpanichkul P, Kongarin S, Permpatdechakul S, Polpichai N, Duangsonk K, Manosroi W, Chaiyakunapruk N, Mousa OY, Kim D, Chen VL, et al. The Surreptitious Burden of Nonalcoholic Fatty Liver Disease in the Elderly in the Asia-Pacific Region: An Insight from the Global Burden of Disease Study 2019. Journal of Clinical Medicine. 2023; 12(20):6456. https://doi.org/10.3390/jcm12206456
Chicago/Turabian StyleDanpanichkul, Pojsakorn, Siwanart Kongarin, Sarunpakorn Permpatdechakul, Natchaya Polpichai, Kwanjit Duangsonk, Worapaka Manosroi, Nathorn Chaiyakunapruk, Omar Y. Mousa, Donghee Kim, Vincent L. Chen, and et al. 2023. "The Surreptitious Burden of Nonalcoholic Fatty Liver Disease in the Elderly in the Asia-Pacific Region: An Insight from the Global Burden of Disease Study 2019" Journal of Clinical Medicine 12, no. 20: 6456. https://doi.org/10.3390/jcm12206456
APA StyleDanpanichkul, P., Kongarin, S., Permpatdechakul, S., Polpichai, N., Duangsonk, K., Manosroi, W., Chaiyakunapruk, N., Mousa, O. Y., Kim, D., Chen, V. L., & Wijarnpreecha, K. (2023). The Surreptitious Burden of Nonalcoholic Fatty Liver Disease in the Elderly in the Asia-Pacific Region: An Insight from the Global Burden of Disease Study 2019. Journal of Clinical Medicine, 12(20), 6456. https://doi.org/10.3390/jcm12206456






