The Sympathetic Nervous System in Hypertensive Heart Failure with Preserved LVEF
Abstract
1. Introduction
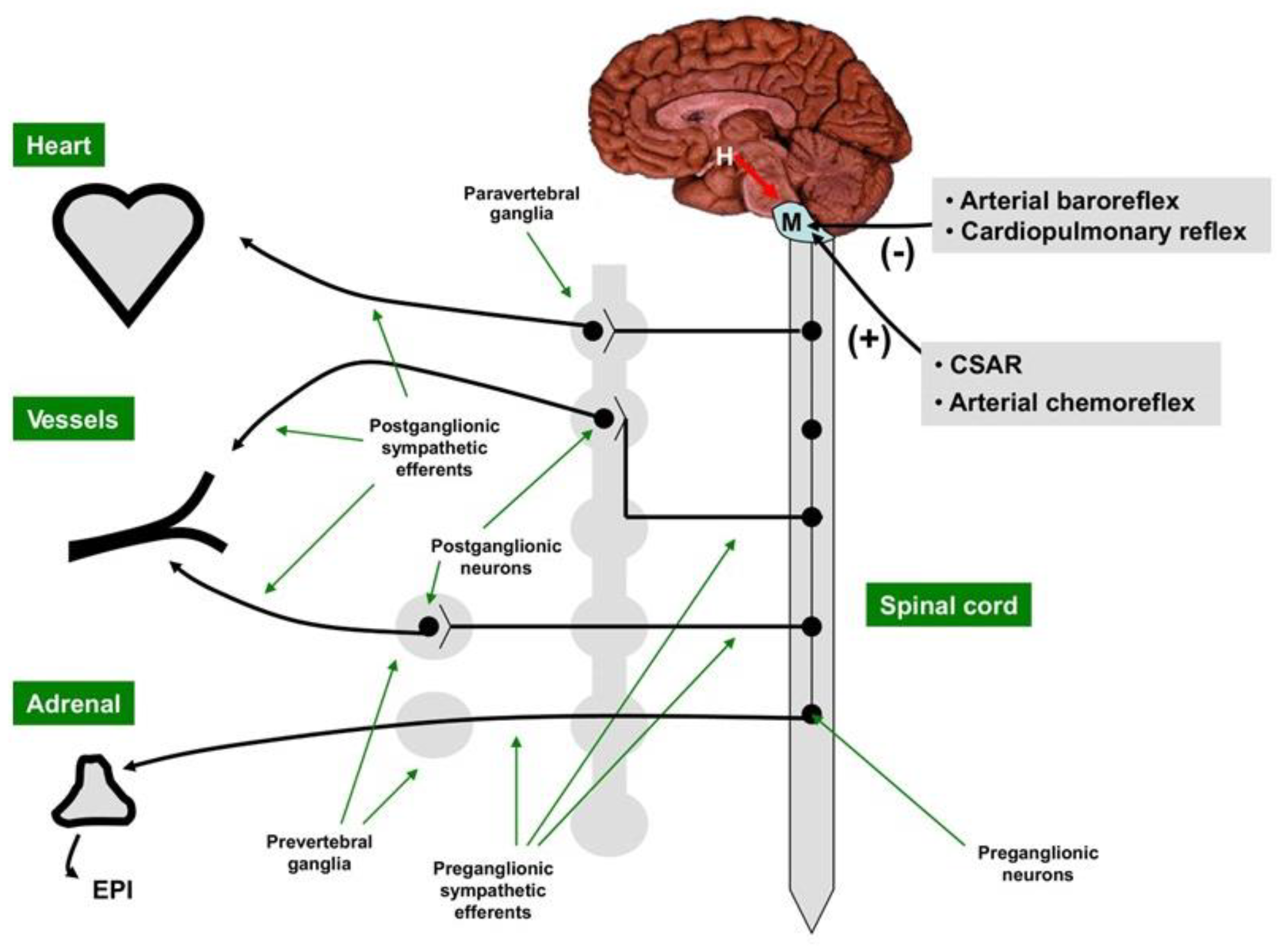
2. Cardiac Sympathetic Control
3. SNS Overactivity in Hypertension
4. SNS Overactivity in Hypertensive Heart Failure with Preserved LVEF
5. Evaluation of SNS Activity in Heart Failure
6. Therapeutic Implications
6.1. Medical Neuromodulation
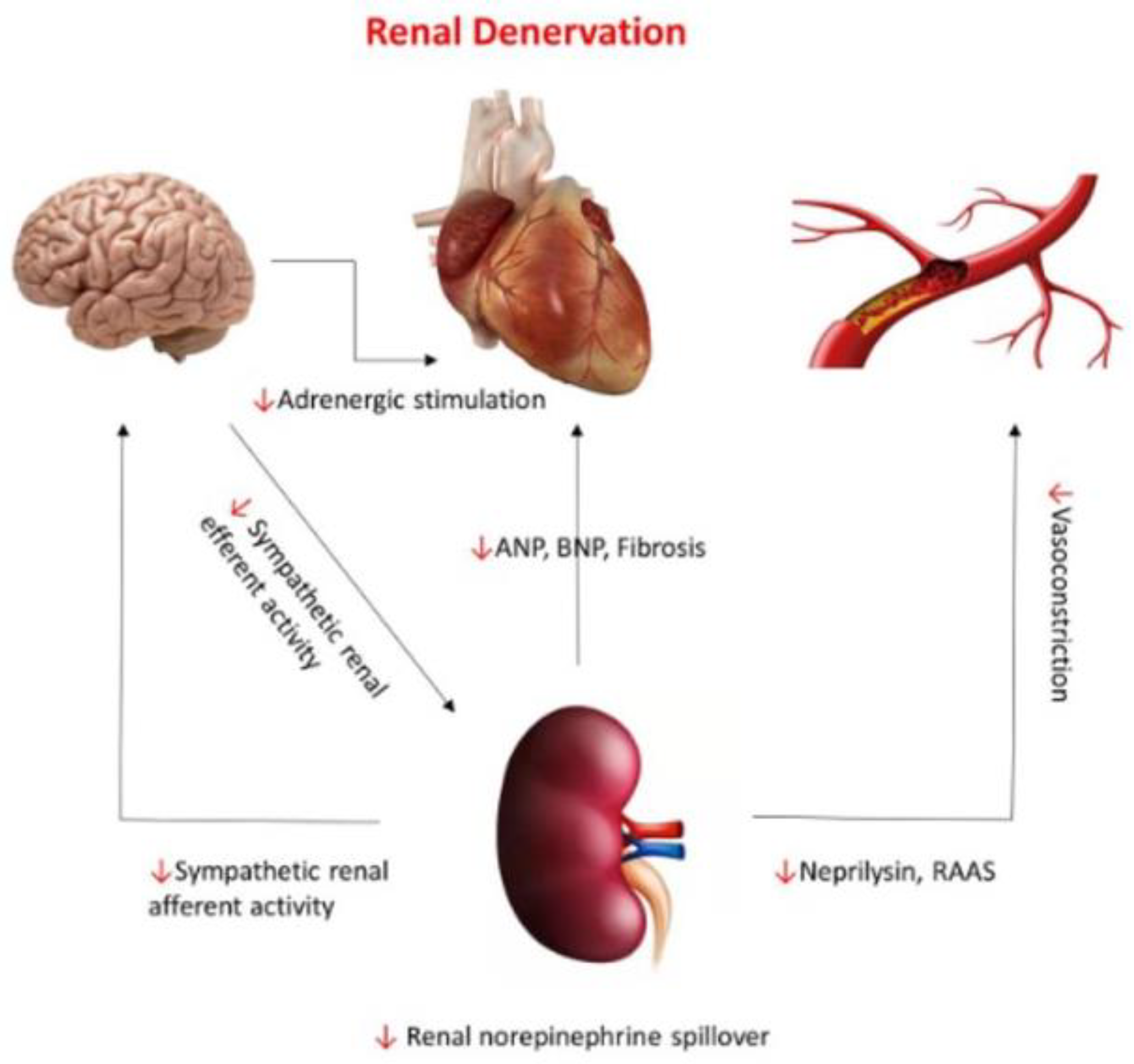
6.2. Nonpharmacological Neuromodulation
7. GAPS in Evidence and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Triposkiadis, F.; Karayannis, G.; Giamouzis, G.; Skoularigis, J.; Louridas, G.; Butler, J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J. Am. Coll. Cardiol. 2009, 54, 1747–1762. [Google Scholar] [CrossRef] [PubMed]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2017, 14, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Hogg, K.; McMurray, J. Neurohumoral pathways in heart failure with preserved systolic function. Prog. Cardiovasc. Dis. 2005, 47, 357–366. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Levy, D.; Larson, M.G.; Vasan, R.S.; Kannel, W.B.; Ho, K.K. The progression from hypertension to congestive heart failure. JAMA 1996, 275, 1557–1562. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Sympathetic nervous system and hypertension: New evidences. Auton. Neurosci. 2022, 238, 102954. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Sarafidis, P.; Briasoulis, A.; Magouliotis, D.E.; Athanasiou, T.; Skoularigis, J.; Xanthopoulos, A. Hypertensive Heart Failure. J. Clin. Med. 2023, 12, 5090. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Triposkiadis, F.; Starling, R.C. Heart failure with preserved ejection fraction: Classification based upon phenotype is essential for diagnosis and treatment. Trends Cardiovasc. Med. 2018, 28, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Sevre, K.; Rist, A.; Wachtell, K.; Devereux, R.B.; Aurigemma, G.P.; Smiseth, O.A.; Kjeldsen, S.E.; Julius, S.; Pitt, B.; Burnier, M.; et al. What is the Current Best Drug Treatment for Hypertensive Heart Failure with Preserved Ejection Fraction? Review of the Totality of Evidence. Am. J. Hypertens. 2023, 2023, hpad073. [Google Scholar] [CrossRef] [PubMed]
- Karamichalakis, N.; Xanthopoulos, A.; Triposkiadis, F.; Paraskevaidis, I.; Tsougos, E. Reshaping Treatment of Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2022, 11, 3706. [Google Scholar] [CrossRef]
- Karemaker, J.M. An introduction into autonomic nervous function. Physiol. Meas. 2017, 38, R89–R118. [Google Scholar] [CrossRef]
- Bankenahally, R.; Kovvidi, H. Autonomic nervous system: Anatomy, physiology, and relevance in anaesthesia and critical care medicine. BJA Educ. 2016, 16, 381–387. [Google Scholar] [CrossRef]
- Savic, B.; Murphy, D.; Japundzic-Zigon, N. The Paraventricular Nucleus of the Hypothalamus in Control of Blood Pressure and Blood Pressure Variability. Front. Physiol. 2022, 13, 858941. [Google Scholar] [CrossRef]
- Zoccal, D.B.; Furuya, W.I.; Bassi, M.; Colombari, D.S.; Colombari, E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front. Physiol. 2014, 5, 238. [Google Scholar] [CrossRef]
- de Diego, A.M.; Gandia, L.; Garcia, A.G. A physiological view of the central and peripheral mechanisms that regulate the release of catecholamines at the adrenal medulla. Acta Physiol. 2008, 192, 287–301. [Google Scholar] [CrossRef]
- Motiejunaite, J.; Amar, L.; Vidal-Petiot, E. Adrenergic receptors and cardiovascular effects of catecholamines. Ann. Endocrinol. 2021, 82, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Brodde, O.E. Beta-adrenoceptors in cardiac disease. Pharmacol. Ther. 1993, 60, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Leblais, V.; Kobzik, L.; Trochu, J.N.; Khandoudi, N.; Bril, A.; Balligand, J.L.; Le Marec, H. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J. Clin. Investig. 1998, 102, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- de Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac beta-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 904. [Google Scholar] [CrossRef]
- Engelhardt, S.; Hein, L.; Wiesmann, F.; Lohse, M.J. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc. Natl. Acad. Sci. USA 1999, 96, 7059–7064. [Google Scholar] [CrossRef]
- Chesley, A.; Lundberg, M.S.; Asai, T.; Xiao, R.P.; Ohtani, S.; Lakatta, E.G.; Crow, M.T. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ. Res. 2000, 87, 1172–1179. [Google Scholar] [CrossRef]
- Sato, P.Y.; Chuprun, J.K.; Schwartz, M.; Koch, W.J. The evolving impact of of g-protein coupled kinases in cardiac health and disease. Physiol. Rev. 2015, 95, 377–404. [Google Scholar] [CrossRef]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef]
- Duraes Campos, I.; Pinto, V.; Sousa, N.; Pereira, V.H. A brain within the heart: A review on the intracardiac nervous system. J. Mol. Cell Cardiol. 2018, 119, 1–9. [Google Scholar] [CrossRef]
- Stavrakis, S.; Po, S. Ganglionated Plexi Ablation: Physiology and Clinical Applications. Arrhythm. Electrophysiol. Rev. 2017, 6, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Keir, D.A.; Badrov, M.B.; Tomlinson, G.; Notarius, C.F.; Kimmerly, D.S.; Millar, P.J.; Shoemaker, J.K.; Floras, J.S. Influence of Sex and Age on Muscle Sympathetic Nerve Activity of Healthy Normotensive Adults. Hypertension 2020, 76, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G. Adrenergic overdrive as the link among hypertension, obesity, and impaired thermogenesis: Lights and shadows. Hypertension 2007, 49, 5–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guber, K.; Kirtane, A.J. Renal Sympathetic Denervation for Hypertension. Kidney Int. Rep. 2022, 7, 2129–2140. [Google Scholar] [CrossRef]
- Julius, S.; Krause, L.; Schork, N.J.; Mejia, A.D.; Jones, K.A.; van de Ven, C.; Johnson, E.H.; Sekkarie, M.A.; Kjeldsen, S.E.; Petrin, J.; et al. Hyperkinetic borderline hypertension in Tecumseh, Michigan. J. Hypertens. 1991, 9, 77–84. [Google Scholar] [CrossRef]
- Grassi, G.; Esler, M. How to assess sympathetic activity in humans. J. Hypertens. 1999, 17, 719–734. [Google Scholar] [CrossRef]
- Meredith, I.T.; Esler, M.D.; Cox, H.S.; Lambert, G.W.; Jennings, G.L.; Eisenhofer, G. Biochemical evidence of sympathetic denervation of the heart in pure autonomic failure. Clin. Auton. Res. 1991, 1, 187–194. [Google Scholar] [CrossRef]
- Ferrier, C.; Esler, M.D.; Eisenhofer, G.; Wallin, B.G.; Horne, M.; Cox, H.S.; Lambert, G.; Jennings, G.L. Increased norepinephrine spillover into the jugular veins in essential hypertension. Hypertension 1992, 19, 62–69. [Google Scholar] [CrossRef]
- Kannan, A.; Medina, R.I.; Nagajothi, N.; Balamuthusamy, S. Renal sympathetic nervous system and the effects of denervation on renal arteries. World J. Cardiol. 2014, 6, 814–823. [Google Scholar] [CrossRef]
- Kopp, U.C. Role of renal sensory nerves in physiological and pathophysiological conditions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R79–R95. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Lampropoulos, K.; Briasoulis, A.; Sarafidis, P.; Skoularigis, J.; Boudoulas, H. Aortic Stiffness: A Major Risk Factor for Multimorbidity in the Elderly. J. Clin. Med. 2023, 12, 2321. [Google Scholar] [CrossRef] [PubMed]
- Nardone, M.; Floras, J.S.; Millar, P.J. Sympathetic neural modulation of arterial stiffness in humans. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1338–H1346. [Google Scholar] [CrossRef] [PubMed]
- Petrak, O.; Strauch, B.; Zelinka, T.; Rosa, J.; Holaj, R.; Vrankova, A.; Kasalicky, M.; Kvasnicka, J.; Pacak, K.; Widimsky, J., Jr. Factors influencing arterial stiffness in pheochromocytoma and effect of adrenalectomy. Hypertens. Res. 2010, 33, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Floras, J.S.; Ponikowski, P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur. Heart J. 2015, 36, 1974–1982. [Google Scholar] [CrossRef]
- Gronda, E.; Dusi, V.; D’Elia, E.; Iacoviello, M.; Benvenuto, E.; Vanoli, E. Sympathetic activation in heart failure. Eur. Heart J. Suppl. 2022, 24, E4–E11. [Google Scholar] [CrossRef]
- Bencivenga, L.; Palaia, M.E.; Sepe, I.; Gambino, G.; Komici, K.; Cannavo, A.; Femminella, G.D.; Rengo, G. Why Do We Not Assess Sympathetic Nervous System Activity in Heart Failure Management: Might GRK2 Serve as a New Biomarker? Cells 2021, 10, 457. [Google Scholar] [CrossRef]
- Mayor, F., Jr.; Murga, C. G Protein-Coupled Receptor Kinases Take Central Stage. Cells 2022, 12, 23. [Google Scholar] [CrossRef]
- Liu, H.; Ma, H.; Zeng, X.; Wu, C.; Acharya, S.; Sudan, S.K.; Zhang, X. Ubiquitination of GRK2 Is Required for the beta-Arrestin-Biased Signaling Pathway of Dopamine D2 Receptors to Activate ERK Kinases. Int. J. Mol. Sci. 2023, 24, 10031. [Google Scholar] [CrossRef]
- Floras, J.S. The 2021 Carl Ludwig Lecture. Unsympathetic autonomic regulation in heart failure: Patient-inspired insights. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R338–R351. [Google Scholar] [CrossRef]
- Tavi, P.; Laine, M.; Weckstrom, M.; Ruskoaho, H. Cardiac mechanotransduction: From sensing to disease and treatment. Trends Pharmacol. Sci. 2001, 22, 254–260. [Google Scholar] [CrossRef]
- Kulej-Lyko, K.; Niewinski, P.; Tubek, S.; Ponikowski, P. Contribution of Peripheral Chemoreceptors to Exercise Intolerance in Heart Failure. Front. Physiol. 2022, 13, 878363. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.; Teschemacher, A.G.; Kasparov, S.; Gourine, A.V. Glia, sympathetic activity and cardiovascular disease. Exp. Physiol. 2016, 101, 565–576. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Quarti-Trevano, F.; Dell’Oro, R.; Arenare, F.; Spaziani, D.; Mancia, G. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension 2009, 53, 205–209. [Google Scholar] [CrossRef]
- Vergaro, G.; Aimo, A.; Prontera, C.; Ghionzoli, N.; Arzilli, C.; Zyw, L.; Taddei, C.; Gabutti, A.; Poletti, R.; Giannoni, A.; et al. Sympathetic and renin-angiotensin-aldosterone system activation in heart failure with preserved, mid-range and reduced ejection fraction. Int. J. Cardiol. 2019, 296, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Quarti-Trevano, F.; Dell’Oro, R.; Gronda, E.; Spaziani, D.; Facchetti, R.; Cuspidi, C.; Mancia, G.; Grassi, G. Sympathetic and baroreflex alterations in congestive heart failure with preserved, midrange and reduced ejection fraction. J. Hypertens. 2019, 37, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Yamada, T.; Tamaki, S.; Watanabe, T.; Morita, T.; Furukawa, Y.; Kawasaki, M.; Kikuchi, A.; Kawai, T.; Abe, M.; et al. Prognostic significance of cardiac I-123-metaiodobenzylguanidine imaging in patients with reduced, mid-range, and preserved left ventricular ejection fraction admitted for acute decompensated heart failure: A prospective study in Osaka Prefectural Acute Heart Failure Registry (OPAR). Eur. Heart J. Cardiovasc. Imaging 2021, 22, 58–66. [Google Scholar] [CrossRef]
- Seo, M.; Yamada, T.; Tamaki, S.; Watanabe, T.; Morita, T.; Furukawa, Y.; Kawasaki, M.; Kikuchi, A.; Kawai, T.; Nakamura, J.; et al. Prognostic Significance of Cardiac (123)I-MIBG SPECT Imaging in Heart Failure Patients with Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2022, 15, 655–668. [Google Scholar] [CrossRef]
- Kaye, D.M.; Nanayakkara, S.; Wang, B.; Shihata, W.; Marques, F.Z.; Esler, M.; Lambert, G.; Mariani, J. Characterization of Cardiac Sympathetic Nervous System and Inflammatory Activation in HFpEF Patients. JACC Basic Transl. Sci. 2022, 7, 116–127. [Google Scholar] [CrossRef]
- Badrov, M.B.; Notarius, C.F.; Keys, E.; Floras, J.S. Muscle Sympathetic Excitatory Response to Dynamic 1-Leg Cycling in Heart Failure With Preserved Ejection Fraction. JACC Case Rep. 2022, 4, 1501–1503. [Google Scholar] [CrossRef]
- Manabe, K.; D’Souza, A.W.; Washio, T.; Takeda, R.; Hissen, S.L.; Akins, J.D.; Fu, Q. Sympathetic and hemodynamic responses to exercise in heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 2023, 10, 1148324. [Google Scholar] [CrossRef]
- Florea, V.G.; Cohn, J.N. The autonomic nervous system and heart failure. Circ. Res. 2014, 114, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.; Swedberg, K.; Komajda, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L.; Investigators, S. Heart rate as a risk factor in chronic heart failure (SHIFT): The association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010, 376, 886–894. [Google Scholar] [CrossRef]
- Bohm, M.; Butler, J.; Mahfoud, F.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Slawik, J.; Brueckmann, M.; Linetzky, B.; Schuler, E.; et al. Heart failure outcomes according to heart rate and effects of empagliflozin in patients of the EMPEROR-Preserved trial. Eur. J. Heart Fail. 2022, 24, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Zeid, S.; Buch, G.; Velmeden, D.; Sohne, J.; Schulz, A.; Schuch, A.; Trobs, S.O.; Heidorn, M.W.; Muller, F.; Strauch, K.; et al. Heart rate variability: Reference values and role for clinical profile and mortality in individuals with heart failure. Clin. Res. Cardiol. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Baig, M.; Moafi-Madani, M.; Qureshi, R.; Roberts, M.B.; Allison, M.; Manson, J.E.; LaMonte, M.J.; Liu, S.; Eaton, C.B. Heart rate variability and the risk of heart failure and its subtypes in post-menopausal women: The Women’s Health Initiative study. PLoS ONE 2022, 17, e0276585. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Stein, P.K. Heart rate variability in risk stratification of cardiac patients. Prog. Cardiovasc. Dis. 2013, 56, 153–159. [Google Scholar] [CrossRef]
- Toschi-Dias, E.; Rondon, M.; Cogliati, C.; Paolocci, N.; Tobaldini, E.; Montano, N. Contribution of Autonomic Reflexes to the Hyperadrenergic State in Heart Failure. Front. Neurosci. 2017, 11, 162. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Robbi, E.; Caporotondi, A.; Guazzotti, G.; Sleight, P.; Febo, O. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J. Am. Coll. Cardiol. 2009, 53, 193–199. [Google Scholar] [CrossRef]
- Esler, M.; Kaye, D.; Lambert, G.; Esler, D.; Jennings, G. Adrenergic nervous system in heart failure. Am. J. Cardiol. 1997, 80, 7L–14L. [Google Scholar] [CrossRef] [PubMed]
- Ramchandra, R.; Barrett, C.J. Regulation of the renal sympathetic nerves in heart failure. Front. Physiol. 2015, 6, 238. [Google Scholar] [CrossRef]
- Ramchandra, R.; Hood, S.G.; Xing, D.; Lambert, G.W.; May, C.N. Mechanisms underlying the increased cardiac norepinephrine spillover in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H340–H347. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Cattaneo, B.M.; Lanfranchi, A.; Vailati, S.; Giannattasio, C.; Del Bo, A.; Sala, C.; Bolla, G.B.; Pozzi, M. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 1995, 92, 3206–3211. [Google Scholar] [CrossRef]
- Leimbach, W.N., Jr.; Wallin, B.G.; Victor, R.G.; Aylward, P.E.; Sundlof, G.; Mark, A.L. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 1986, 73, 913–919. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Bertinieri, G.; Turri, C.; Stella, M.L.; Scopelliti, F.; Mancia, G. Sympathetic and reflex abnormalities in heart failure secondary to ischaemic or idiopathic dilated cardiomyopathy. Clin. Sci. 2001, 101, 141–146. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Dell’Oro, R.; Facchini, A.; Ilardo, V.; Mancia, G. Sympathetic and baroreflex function in hypertensive or heart failure patients with ventricular arrhythmias. J. Hypertens. 2004, 22, 1747–1753. [Google Scholar] [CrossRef]
- Brunner-La Rocca, H.P.; Esler, M.D.; Jennings, G.L.; Kaye, D.M. Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur. Heart J. 2001, 22, 1136–1143. [Google Scholar] [CrossRef]
- Zelt, J.G.E.; deKemp, R.A.; Rotstein, B.H.; Nair, G.M.; Narula, J.; Ahmadi, A.; Beanlands, R.S.; Mielniczuk, L.M. Nuclear Imaging of the Cardiac Sympathetic Nervous System: A Disease-Specific Interpretation in Heart Failure. JACC Cardiovasc. Imaging 2020, 13, 1036–1054. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.S. Cardiac sympathetic nerve terminal function in congestive heart failure. Acta Pharmacol. Sin. 2007, 28, 921–927. [Google Scholar] [CrossRef]
- Verschure, D.O.; Nakajima, K.; Verberne, H.J. Cardiac (123)I-mIBG Imaging in Heart Failure. Pharmaceuticals 2022, 15, 656. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, T.; Naya, M.; Obara, M.; Oyama-Manabe, N.; Manabe, O.; Magota, K.; Ito, Y.M.; Katoh, C.; Tamaki, N. Regional interaction between myocardial sympathetic denervation, contractile dysfunction, and fibrosis in heart failure with preserved ejection fraction: (11)C-hydroxyephedrine PET study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1897–1905. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Butler, J.; Abboud, F.M.; Armstrong, P.W.; Adamopoulos, S.; Atherton, J.J.; Backs, J.; Bauersachs, J.; Burkhoff, D.; Bonow, R.O.; et al. The continuous heart failure spectrum: Moving beyond an ejection fraction classification. Eur. Heart J. 2019, 40, 2155–2163. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Starling, R.C. Medical Treatment of Heart Failure: Ignore the Ejection Fraction and Treat All? J. Card. Fail. 2021, 27, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Fici, F.; Robles, N.R.; Tengiz, I.; Grassi, G. Beta-Blockers and Hypertension: Some Questions and Answers. High Blood Press. Cardiovasc. Prev. 2023, 30, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Kjeldsen, S.E.; Pathak, A.; Grassi, G.; Kreutz, R.; Mancia, G. Diverse pharmacological properties, trial results, comorbidity prescribing and neural pathophysiology suggest European hypertension guideline downgrading of beta-blockers is not justified. Blood Press. 2022, 31, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Uijl, A.; Koudstaal, S.; Stolfo, D.; Dahlstrom, U.; Vaartjes, I.; Grobbee, R.E.; Asselbergs, F.W.; Lund, L.H.; Savarese, G. Does Heterogeneity Exist in Treatment Associations with Renin-Angiotensin-System Inhibitors or Beta-blockers According to Phenotype Clusters in Heart Failure with Preserved Ejection Fraction? J. Card. Fail. 2023. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, E.R.; Kubo, T.; Mak, S.; Al-Hesayen, A.; Schofield, A.; Allan, R.; Kelly, S.; Newton, G.E.; Floras, J.S.; Parker, J.D. Nonselective versus selective beta-adrenergic receptor blockade in congestive heart failure: Differential effects on sympathetic activity. Circulation 2001, 104, 2194–2199. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.; Jordan, J. Norepinephrine transporter function and human cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1273–H1282. [Google Scholar] [CrossRef]
- Lund, L.H.; Claggett, B.; Liu, J.; Lam, C.S.; Jhund, P.S.; Rosano, G.M.; Swedberg, K.; Yusuf, S.; Granger, C.B.; Pfeffer, M.A.; et al. Heart failure with mid-range ejection fraction in CHARM: Characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur. J. Heart Fail. 2018, 20, 1230–1239. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; Gordeev, I.; et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015, 131, 34–42. [Google Scholar] [CrossRef]
- Rohde, L.E.; Claggett, B.L.; Wolsk, E.; Packer, M.; Zile, M.; Swedberg, K.; Rouleau, J.; Pfeffer, M.A.; Desai, A.S.; Lund, L.H.; et al. Cardiac and Noncardiac Disease Burden and Treatment Effect of Sacubitril/Valsartan: Insights from a Combined PARAGON-HF and PARADIGM-HF Analysis. Circ. Heart Fail. 2021, 14, e008052. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Butler, J. From PARADIGM to PARAGON further evidence supporting continuous heart failure spectrum. Eur. J. Heart Fail. 2020, 22, 1536–1539. [Google Scholar] [CrossRef]
- Singh, R.B.; Hristova, K.; Fedacko, J.; El-Kilany, G.; Cornelissen, G. Chronic heart failure: A disease of the brain. Heart Fail. Rev. 2019, 24, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Yu, Y.; Wei, S.G.; Felder, R.B. Aldosterone-induced brain MAPK signaling and sympathetic excitation are angiotensin II type-1 receptor dependent. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H742–H751. [Google Scholar] [CrossRef] [PubMed]
- Sarrias, A.; Bayes-Genis, A. Is Sacubitril/Valsartan (Also) an Antiarrhythmic Drug? Circulation 2018, 138, 551–553. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Matthews, V.B.; Elliot, R.H.; Rudnicka, C.; Hricova, J.; Herat, L.; Schlaich, M.P. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J. Hypertens. 2017, 35, 2059–2068. [Google Scholar] [CrossRef]
- Jordan, J.; Tank, J.; Heusser, K.; Heise, T.; Wanner, C.; Heer, M.; Macha, S.; Mattheus, M.; Lund, S.S.; Woerle, H.J.; et al. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J. Am. Soc. Hypertens. 2017, 11, 604–612. [Google Scholar] [CrossRef]
- Sano, M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J. Cardiol. 2018, 71, 471–476. [Google Scholar] [CrossRef]
- Perrone, V.; Veronesi, C.; Gambera, M.; Nati, G.; Perone, F.; Tagliabue, P.F.; Degli Esposti, L.; Volpe, M. Treatment with Free Triple Combination Therapy of Atorvastatin, Perindopril, Amlodipine in Hypertensive Patients: A Real-World Population Study in Italy. High Blood Press. Cardiovasc. Prev. 2019, 26, 399–404. [Google Scholar] [CrossRef]
- Gaciong, Z. Preference and Adherence to a Fixed-Dose Combination of Bisoprolol-Aspirin and Blood Pressure Control: Results of an Open-Label, Multicentre Study. J. Clin. Med. 2022, 12, 17. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Perrone, V.; Veronesi, C.; Gambera, M.; Nati, G.; Perone, F.; Tagliabue, P.F.; Buda, S.; Borghi, C. Modifications in drug adherence after switch to fixed-dose combination of perindopril/amlodipine in clinical practice. Results of a large-scale Italian experience. The amlodipine-perindopril in real settings (AMPERES) study. Curr. Med. Res. Opin. 2018, 34, 1571–1577. [Google Scholar] [CrossRef]
- Kiuchi, M.G.; Esler, M.D.; Fink, G.D.; Osborn, J.W.; Banek, C.T.; Bohm, M.; Denton, K.M.; DiBona, G.F.; Everett, T.H.t.; Grassi, G.; et al. Renal Denervation Update From the International Sympathetic Nervous System Summit: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 3006–3017. [Google Scholar] [CrossRef]
- Kassab, K.; Soni, R.; Kassier, A.; Fischell, T.A. The Potential Role of Renal Denervation in the Management of Heart Failure. J. Clin. Med. 2022, 11, 4147. [Google Scholar] [CrossRef]
- Fudim, M.; Sobotka, P.A.; Piccini, J.P.; Patel, M.R. Renal Denervation for Patients With Heart Failure: Making a Full Circle. Circ. Heart Fail. 2021, 14, e008301. [Google Scholar] [CrossRef]
- Fudim, M.; Ponikowski, P.P.; Burkhoff, D.; Dunlap, M.E.; Sobotka, P.A.; Molinger, J.; Patel, M.R.; Felker, G.M.; Hernandez, A.F.; Litwin, S.E.; et al. Splanchnic nerve modulation in heart failure: Mechanistic overview, initial clinical experience, and safety considerations. Eur. J. Heart Fail. 2021, 23, 1076–1084. [Google Scholar] [CrossRef]
- Malek, F.; Gajewski, P.; Zymlinski, R.; Janczak, D.; Chabowski, M.; Fudim, M.; Martinca, T.; Neuzil, P.; Biegus, J.; Mates, M.; et al. Surgical ablation of the right greater splanchnic nerve for the treatment of heart failure with preserved ejection fraction: First-in-human clinical trial. Eur. J. Heart Fail. 2021, 23, 1134–1143. [Google Scholar] [CrossRef]
- Fudim, M.; Fail, P.S.; Litwin, S.E.; Shaburishvili, T.; Goyal, P.; Hummel, S.L.; Borlaug, B.A.; Mohan, R.C.; Patel, R.B.; Mitter, S.S.; et al. Endovascular ablation of the right greater splanchnic nerve in heart failure with preserved ejection fraction: Early results of the REBALANCE-HF trial roll-in cohort. Eur. J. Heart Fail. 2022, 24, 1410–1414. [Google Scholar] [CrossRef]
- Capilupi, M.J.; Kerath, S.M.; Becker, L.B. Vagus Nerve Stimulation and the Cardiovascular System. Cold Spring Harb. Perspect. Med. 2020, 10, a034173. [Google Scholar] [CrossRef]
- Stavrakis, S.; Elkholey, K.; Morris, L.; Niewiadomska, M.; Asad, Z.U.A.; Humphrey, M.B. Neuromodulation of Inflammation to Treat Heart Failure With Preserved Ejection Fraction: A Pilot Randomized Clinical Trial. J. Am. Heart Assoc. 2022, 11, e023582. [Google Scholar] [CrossRef]
- Rengo, G.; Pagano, G.; Filardi, P.P.; Femminella, G.D.; Parisi, V.; Cannavo, A.; Liccardo, D.; Komici, K.; Gambino, G.; D’Amico, M.L.; et al. Prognostic Value of Lymphocyte G Protein-Coupled Receptor Kinase-2 Protein Levels in Patients With Heart Failure. Circ. Res. 2016, 118, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Rengo, G.; Lymperopoulos, A.; Leosco, D.; Koch, W.J. GRK2 as a novel gene therapy target in heart failure. J. Mol. Cell Cardiol. 2011, 50, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Shan, S.; Li, X.Q.; Chen, T.T.; Qi, M.; Zhang, S.N.; Wang, Z.Y.; Zhang, L.L.; Wei, W.; Sun, W.Y. G Protein-Coupled Receptor Kinase 2 as Novel Therapeutic Target in Fibrotic Diseases. Front. Immunol. 2021, 12, 822345. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triposkiadis, F.; Briasoulis, A.; Sarafidis, P.; Magouliotis, D.; Athanasiou, T.; Paraskevaidis, I.; Skoularigis, J.; Xanthopoulos, A. The Sympathetic Nervous System in Hypertensive Heart Failure with Preserved LVEF. J. Clin. Med. 2023, 12, 6486. https://doi.org/10.3390/jcm12206486
Triposkiadis F, Briasoulis A, Sarafidis P, Magouliotis D, Athanasiou T, Paraskevaidis I, Skoularigis J, Xanthopoulos A. The Sympathetic Nervous System in Hypertensive Heart Failure with Preserved LVEF. Journal of Clinical Medicine. 2023; 12(20):6486. https://doi.org/10.3390/jcm12206486
Chicago/Turabian StyleTriposkiadis, Filippos, Alexandros Briasoulis, Pantelis Sarafidis, Dimitrios Magouliotis, Thanos Athanasiou, Ioannis Paraskevaidis, John Skoularigis, and Andrew Xanthopoulos. 2023. "The Sympathetic Nervous System in Hypertensive Heart Failure with Preserved LVEF" Journal of Clinical Medicine 12, no. 20: 6486. https://doi.org/10.3390/jcm12206486
APA StyleTriposkiadis, F., Briasoulis, A., Sarafidis, P., Magouliotis, D., Athanasiou, T., Paraskevaidis, I., Skoularigis, J., & Xanthopoulos, A. (2023). The Sympathetic Nervous System in Hypertensive Heart Failure with Preserved LVEF. Journal of Clinical Medicine, 12(20), 6486. https://doi.org/10.3390/jcm12206486










