Personalized Risk Assessment of Hepatic Fibrosis after Cholecystectomy in Metabolic-Associated Steatotic Liver Disease: A Machine Learning Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
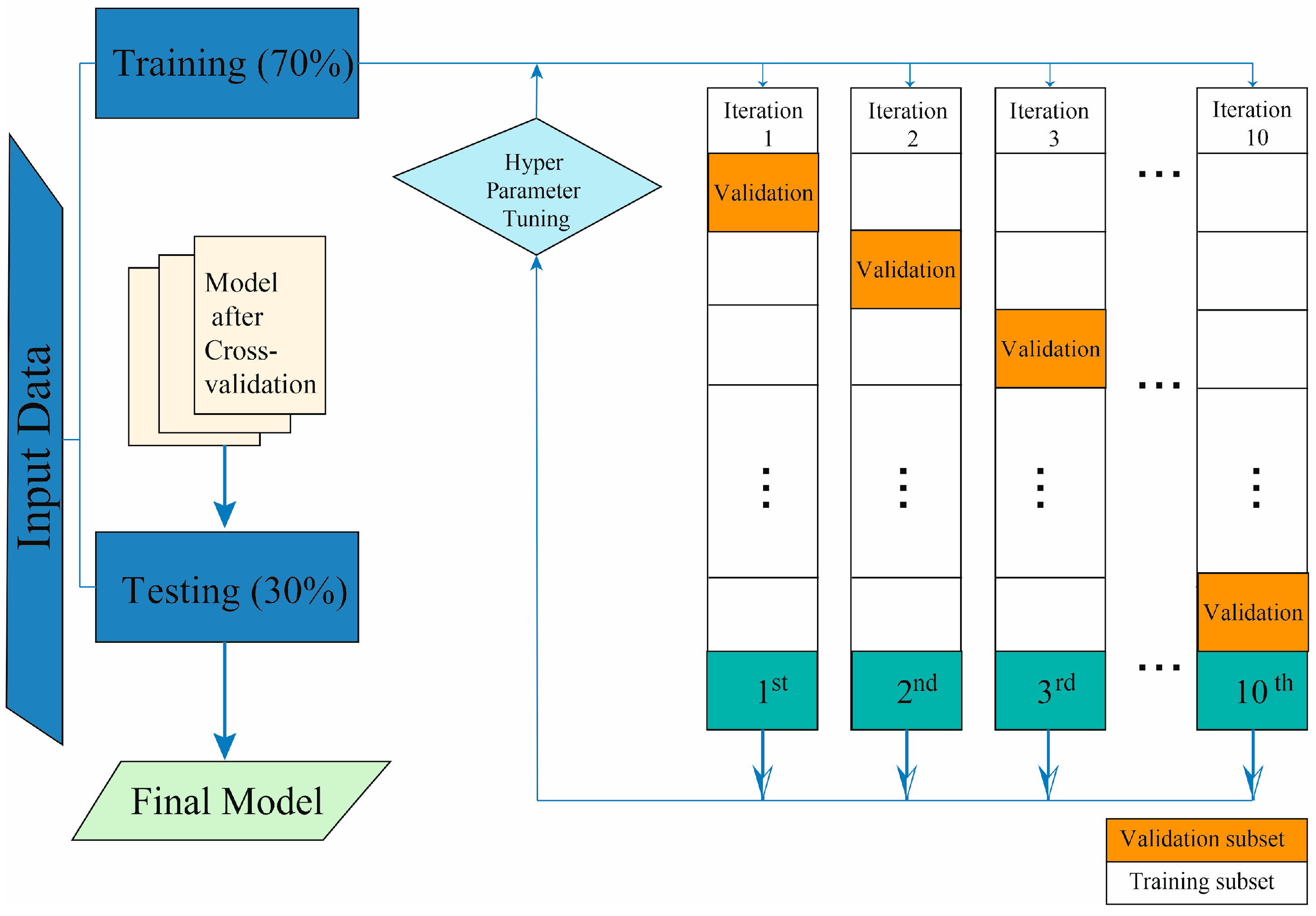
2.3. Model Development
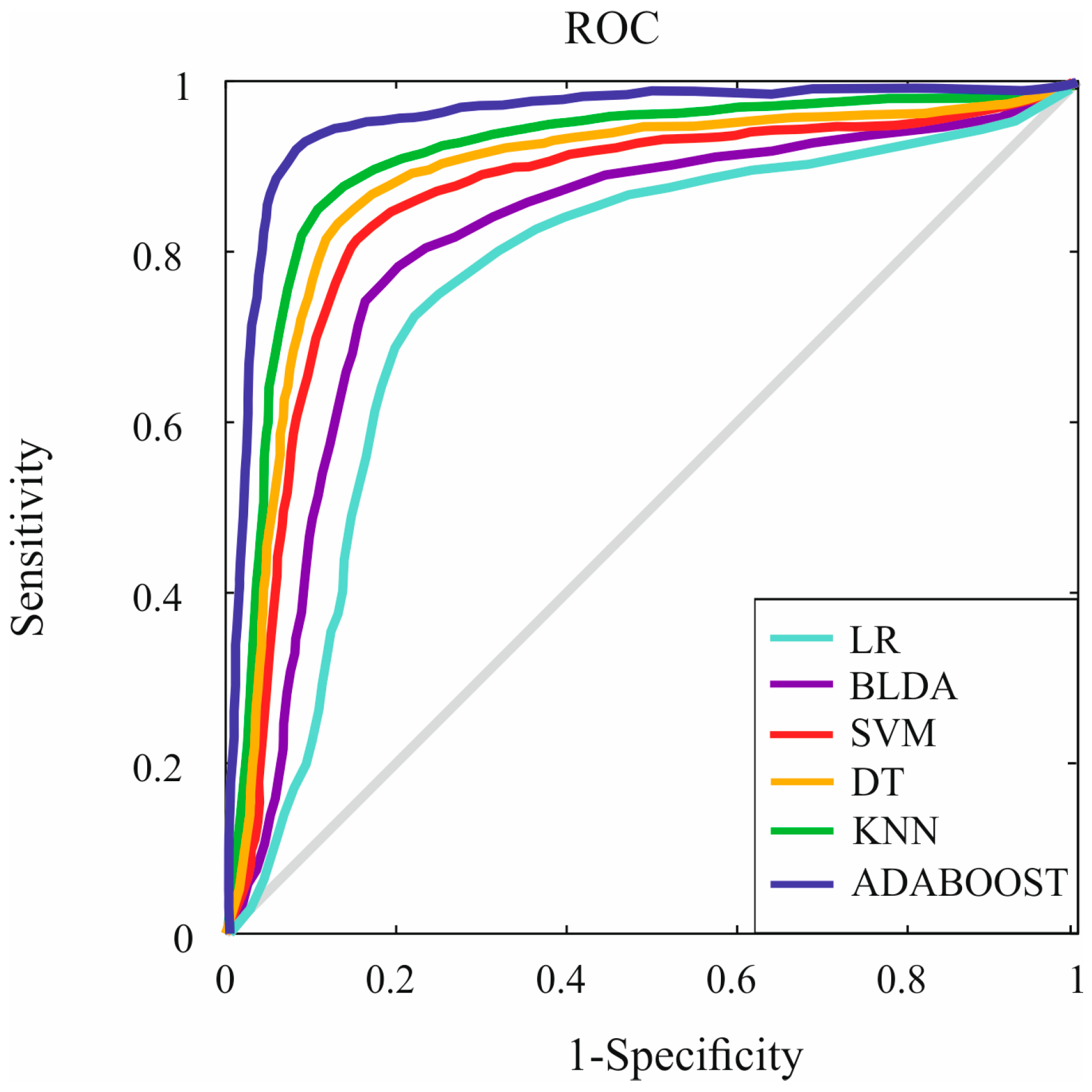
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 2023, 101133. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J. 2019 Global NAFLD prevalence: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2809–2817.e28. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Donghia, R.; Lampignano, L.; Castellana, F.; Zupo, R.; Sardone, R.; Pergola, G.D.; Giannelli, G. Prevalence of the Absence of Cirrhosis in Subjects with NAFLD-Associated Hepatocellular Carcinoma. J. Clin. Med. 2021, 10, 4638. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef]
- van de Graaf, F.W.; Zaïmi, I.; Stassen, L.P.; Lange, J.F. Safe laparoscopic cholecystectomy: A systematic review of bile duct injury prevention. Int. J. Surg. 2018, 60, 164–172. [Google Scholar] [CrossRef]
- Lamberts, M.P. Indications of cholecystectomy in gallstone disease. Curr. Opin. Gastroenterol. 2018, 34, 97–102. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, S.; Tian, Y. Cholecystectomy as a risk factor of metabolic syndrome: From epidemiologic clues to biochemical mechanisms. Lab. Investig. 2018, 98, 7–14. [Google Scholar] [CrossRef]
- Shen, C.; Wu, X.; Xu, C.; Yu, C.; Chen, P.; Li, Y. Association of cholecystectomy with metabolic syndrome in a Chinese population. PLoS ONE 2014, 9, e88189. [Google Scholar] [CrossRef]
- Alexander, H.C.; Bartlett, A.S.; Wells, C.I.; Hannam, J.A.; Moore, M.R.; Poole, G.H.; Merry, A.F. Reporting of complications after laparoscopic cholecystectomy: A systematic review. HPB 2018, 20, 786–794. [Google Scholar] [CrossRef]
- Donkervoort, S.; Kortram, K.; Dijksman, L.; Boermeester, M.; Van Ramshorst, B.; Boerma, D. Anticipation of complications after laparoscopic cholecystectomy: Prediction of individual outcome. Surg. Endosc. 2016, 30, 5388–5394. [Google Scholar] [CrossRef]
- Latenstein, C.S.; Alferink, L.J.; Murad, S.D.; Drenth, J.P.; van Laarhoven, C.J.; de Reuver, P.R. The association between cholecystectomy, metabolic syndrome, and nonalcoholic fatty liver disease: A population-based study. Clin. Transl. Gastroenterol. 2020, 11, e00170. [Google Scholar] [CrossRef]
- Luo, D.; Chen, X.-P.; Dai, Y.; Kuang, F.; Kang, M.-J.; Li, B.; Su, S. Cholecystectomy and risk of liver disease: A systematic review and meta-analysis of 27 million individuals. Int. J. Surg. 2023, 109, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Recent advances in understanding bile acid homeostasis. F1000Research 2017, 6, 2029. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-W.; Ge, T.-T.; Chen, S.-Z.; Wang, G.; Yang, Q.; Huang, C.-H.; Xu, L.-C.; Chen, Z. Role of bile acids in liver diseases mediated by the gut microbiome. World J. Gastroenterol. 2021, 27, 3010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gupte, J.; Gong, Y.; Weiszmann, J.; Zhang, Y.; Lee, K.J.; Richards, W.G.; Li, Y. Chronic over-expression of fibroblast growth factor 21 increases bile acid biosynthesis by opposing FGF15/19 action. EBioMedicine 2017, 15, 173–183. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.-W.; Targher, G. Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol. Metab. 2021, 32, 500–514. [Google Scholar] [CrossRef]
- Palma, R.; Pronio, A.; Romeo, M.; Scognamiglio, F.; Ventriglia, L.; Ormando, V.M.; Lamazza, A.; Pontone, S.; Federico, A.; Dallio, M. The role of insulin resistance in fueling NAFLD pathogenesis: From molecular mechanisms to clinical implications. J. Clin. Med. 2022, 11, 3649. [Google Scholar] [CrossRef]
- Hamamoto, R.; Komatsu, M.; Takasawa, K.; Asada, K.; Kaneko, S. Epigenetics analysis and integrated analysis of multiomics data, including epigenetic data, using artificial intelligence in the era of precision medicine. Biomolecules 2019, 10, 62. [Google Scholar] [CrossRef]
- Sarkate, P.A.; Deorankar, A. Classification of chemical medicine or drug using K nearest neighbor (KNN) and genetic algorithm. Int. Res. J. Eng. Technol. 2018, 5, 833–834. [Google Scholar]
- Ramón, A.; Zaragozá, M.; Torres, A.M.; Cascón, J.; Blasco, P.; Milara, J.; Mateo, J. Application of Machine Learning in Hospitalized Patients with Severe COVID-19 Treated with Tocilizumab. J. Clin. Med. 2022, 11, 4729. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-W.; Chen, C.-W.; Lin, W.-C.; Ke, S.-W.; Tsai, C.-F. SVM and SVM ensembles in breast cancer prediction. PLoS ONE 2017, 12, e0161501. [Google Scholar] [CrossRef] [PubMed]
- Althnian, A.; AlSaeed, D.; Al-Baity, H.; Samha, A.; Dris, A.B.; Alzakari, N.; Abou Elwafa, A.; Kurdi, H. Impact of dataset size on classification performance: An empirical evaluation in the medical domain. Appl. Sci. 2021, 11, 796. [Google Scholar] [CrossRef]
- Hosni, M.; Abnane, I.; Idri, A.; de Gea, J.M.C.; Alemán, J.L.F. Reviewing ensemble classification methods in breast cancer. Comput. Methods Programs Biomed. 2019, 177, 89–112. [Google Scholar] [CrossRef]
- Mirbabaie, M.; Stieglitz, S.; Frick, N.R. Artificial intelligence in disease diagnostics: A critical review and classification on the current state of research guiding future direction. Health Technol. 2021, 11, 693–731. [Google Scholar] [CrossRef]
- Valencia-Rodríguez, A. Long-Standing Effect of Cholecystectomy in Patients with Metabolic-Associated Fatty Liver Disease, V1 ed.; Harvard Dataverse: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Cassinotto, C.; Boursier, J.; de Lédinghen, V.; Lebigot, J.; Lapuyade, B.; Cales, P.; Hiriart, J.B.; Michalak, S.; Bail, B.L.; Cartier, V. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 2016, 63, 1817–1827. [Google Scholar] [CrossRef]
- Kleiner, D. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Eren, F.; Kaya, E.; Yilmaz, Y. Accuracy of Fibrosis-4 index and non-alcoholic fatty liver disease fibrosis scores in metabolic (dysfunction) associated fatty liver disease according to body mass index: Failure in the prediction of advanced fibrosis in lean and morbidly obese individuals. Eur. J. Gastroenterol. Hepatol. 2022, 34, 98–103. [Google Scholar] [CrossRef]
- Solomon, A.; Cipăian, C.R.; Negrea, M.O.; Boicean, A.; Mihaila, R.; Beca, C.; Popa, M.L.; Grama, S.M.; Teodoru, M.; Neamtu, B. Hepatic Involvement across the Metabolic Syndrome Spectrum: Non-Invasive Assessment and Risk Prediction Using Machine Learning. J. Clin. Med. 2023, 12, 5657. [Google Scholar] [CrossRef]
- Sudharson, D.; Divya, P.; Rajan, D.D.P.; Ratheeshkumar, A. Performance analysis of enhanced adaboost framework in multifacet medical dataset. NVEO-Nat. Volatiles Essent. Oils J. NVEO 2021, 8, 1752–1756. [Google Scholar]
- Schober, P.; Vetter, T.R. Logistic regression in medical research. Anesth. Analg. 2021, 132, 365. [Google Scholar] [CrossRef] [PubMed]
- Nour, M.; Cömert, Z.; Polat, K. A novel medical diagnosis model for COVID-19 infection detection based on deep features and Bayesian optimization. Appl. Soft. Comput. 2020, 97, 106580. [Google Scholar] [CrossRef]
- Wang, M.; Chen, H. Chaotic multi-swarm whale optimizer boosted support vector machine for medical diagnosis. Appl. Soft. Comput. 2020, 88, 105946. [Google Scholar] [CrossRef]
- Charbuty, B.; Abdulazeez, A. Classification based on decision tree algorithm for machine learning. J. Appl. Sci. Technol. Trends 2021, 2, 20–28. [Google Scholar] [CrossRef]
- Suárez, M.; Martínez, R.; Torres, A.M.; Torres, B.; Mateo, J. A Machine Learning Method to Identify the Risk Factors for Liver Fibrosis Progression in Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2023, 68, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Dai, W.; Kong, J.; Tian, Y.; Chen, Y. Cholecystectomy as a risk factor for metabolic dysfunction-associated fatty liver disease: Unveiling the metabolic and chronobiologic clues behind the bile acid enterohepatic circulation. J. Physiol. Biochem. 2021, 77, 497–510. [Google Scholar] [CrossRef]
- Lammert, F.; Gurusamy, K.; Ko, C.W.; Miquel, J.-F.; Méndez-Sánchez, N.; Portincasa, P.; van Erpecum, K.J.; van Laarhoven, C.J.; Wang, D.Q.H. Gallstones. Nat. Rev. Dis. Primers 2016, 2, 16024. [Google Scholar] [CrossRef]
- Murphy, P.B.; Vogt, K.N.; Winick-Ng, J.; McClure, J.A.; Welk, B.; Jones, S.A. The increasing incidence of gallbladder disease in children: A 20 year perspective. J. Pediatr. Surg. 2016, 51, 748–752. [Google Scholar] [CrossRef]
- Pogorelic, Z.; Aralica, M.; Jukic, M.; Zitko, V.; Despot, R.; Juric, I. Gallbladder disease in children: A 20-year single-center experience. Indian Pediatr. 2019, 56, 384–386. [Google Scholar] [CrossRef]
- Ruiz-Manriquez, J.; Olivas-Martinez, A.; Chávez-García, L.C.; Fernández-Ramírez, A.; Moctezuma-Velazquez, C.; Kauffman-Ortega, E.; Castro-Narro, G.; Astudillo-García, F.; Escalona-Nandez, I.; Aguilar-Salinas, C.A. Prevalence of metabolic-associated fatty liver disease in Mexico and development of a screening tool: The MAFLD-S score. Gastro Hep. Adv. 2022, 1, 352–358. [Google Scholar] [CrossRef]
- Méndez-Sánchez, N.; Díaz-Orozco, L.E.; Santamaría-Arza, C.; Orozco-Morales, J.A.; Medina-Bravo, P.G. Metabolic-associated fatty liver disease in children and adolescents: Mexican experience. Lancet Gastroenterol. Hepatol. 2021, 6, 986. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Cevallos, P.; Torre, A.; Mendez-Sanchez, N.; Uribe, M.; Chavez-Tapia, N.C. Epidemiological and Genetic Aspects of NAFLD and NASH in Mexico. Clin. Liver Dis. 2022, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Saavedra, G.; Lara-Lona, E.; Hidalgo-Valadez, C.; Romero-Salinas, N.; Méndez-Sashida, G.d.J. Experiencia en procedimientos laparoscópicos en México durante 2015:¿ dónde estamos? Cirugía y Cirujanos 2019, 87, 292–298. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The metabolic syndrome–What is it and how should it be managed? Eur. J. Prev. Cardiol. 2019, 26, 33–46. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Wang, D.Q.-H.; Portincasa, P. An update on the pathogenesis of cholesterol gallstone disease. Curr. Opin. Gastroenterol. 2018, 34, 71. [Google Scholar] [CrossRef] [PubMed]
- Cortés, V.A.; Barrera, F.; Nervi, F. Pathophysiological connections between gallstone disease, insulin resistance, and obesity. Obes. Rev. 2020, 21, e12983. [Google Scholar] [CrossRef]
- Yue, W.; Sun, X.; Du, T. Cholecystectomy versus central obesity or insulin resistance in relation to the risk of nonalcoholic fatty liver disease: The third US National Health and Nutrition Examination Survey. BMC Endocr. Disord. 2019, 19, 95. [Google Scholar] [CrossRef]
- Walters, J.R. Bile acid diarrhoea and FGF19: New views on diagnosis, pathogenesis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 426–434. [Google Scholar] [CrossRef]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.D.; Chen, W.D.; Wang, X.; Lou, G.; Liu, N.; Lin, M.; Forman, B.M.; Huang, W. Promotion of liver regeneration/repair by farnesoid X receptor in both liver and intestine in mice. Hepatology 2012, 56, 2336–2343. [Google Scholar] [CrossRef]
- Festa, C.; Renga, B.; D’Amore, C.; Sepe, V.; Finamore, C.; De Marino, S.; Carino, A.; Cipriani, S.; Monti, M.C.; Zampella, A. Exploitation of cholane scaffold for the discovery of potent and selective farnesoid X receptor (FXR) and G-protein coupled bile acid receptor 1 (GP-BAR1) ligands. J. Med. Chem. 2014, 57, 8477–8495. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Mangelsdorf, D.J.; Meyer, U.A. Pregnane X receptor is a target of farnesoid X receptor. J. Biol. Chem. 2006, 281, 19081–19091. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.G.; Greenberg, C.S.; Patton, H.M.; Caldwell, S.H. AGA clinical practice update: Coagulation in cirrhosis. Gastroenterology 2019, 157, 34–43.e1. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Castéra, L.; Wong, V.W.-S. Non-invasive assessment of liver fibrosis in NAFLD. Clin. Gastroenterol. Hepatol. 2023, 21, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Choi, D.; Lee, K.G.; Kim, H.J.; Kang, B.-K.; Kim, H.; Paik, S.S. Cholecystectomy causes ultrasound evidence of increased hepatic steatosis. World J. Surg. 2016, 40, 1412–1421. [Google Scholar] [CrossRef]
- Chang, Y.; Noh, Y.-H.; Suh, B.-S.; Kim, Y.; Sung, E.; Jung, H.-S.; Kim, C.-W.; Kwon, M.-J.; Yun, K.E.; Noh, J.-W. Bidirectional association between nonalcoholic fatty liver disease and gallstone disease: A cohort study. J. Clin. Med. 2018, 7, 458. [Google Scholar] [CrossRef]
- Jaruvongvanich, V.; Sanguankeo, A.; Jaruvongvanich, S.; Upala, S. Association between cholecystectomy and nonalcoholic fatty liver disease: A meta-analysis. World J. Surg. 2016, 40, 2816–2817. [Google Scholar] [CrossRef]
- Fernández-Delgado, M.; Cernadas, E.; Barro, S.; Amorim, D. Do we need hundreds of classifiers to solve real world classification problems? J. Mach. Learn. Res. 2014, 15, 3133–3181. [Google Scholar]
- Kallwitz, E.R.; Tayo, B.O.; Kuniholm, M.H.; Cai, J.; Daviglus, M.; Cooper, R.S.; Cotler, S.J. American ancestry is a risk factor for suspected nonalcoholic fatty liver disease in Hispanic/Latino adults. Clin. Gastroenterol. Hepatol. 2019, 17, 2301–2309. [Google Scholar] [CrossRef]
- Kanter Coronel, I. Magnitud del sobrepeso y la obesidad en México: Un cambio de estrategia para su erradicación. 2021. Available online: http://bibliodigitalibd.senado.gob.mx/handle/123456789/5127 (accessed on 9 February 2021).
- Basto-Abreu, A.; Barrientos-Gutiérrez, T.; Rojas-Martínez, R.; Aguilar-Salinas, C.A.; López-Olmedo, N.; Cruz-Góngora, V.D.l.; Rivera-Dommarco, J.; Shamah-Levy, T.; Romero-Martínez, M.; Barquera, S. Prevalencia de diabetes y descontrol glucémico en México: Resultados de la Ensanut 2016. Salud Pública México 2022, 62, 50–59. [Google Scholar] [CrossRef]
- Chinchilla-López, P.; Ramírez-Pérez, O.; Cruz-Ramón, V.; Canizales-Quinteros, S.; Domínguez-López, A.; Ponciano-Rodríguez, G.; Sánchez-Muñoz, F.; Méndez-Sánchez, N. More evidence for the genetic susceptibility of Mexican population to nonalcoholic fatty liver disease through PNPLA3. Ann. Hepatol. 2018, 17, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Pei, J.; Tong, H. Data Mining: Concepts and Techniques; Morgan Kaufmann: Burlington, MA, USA, 2022. [Google Scholar]


| Global Population (Mean and Standard Deviation) | Patients Diagnosed at least 6 Months after Cholecystectomy. (Mean and Standard Deviation) | Patients Diagnosed at the Moment of Cholecystectomy. (Mean and Standard Deviation) | |
|---|---|---|---|
| Sample (n) | 211 | 70 | 141 |
| Age (years) | 49.06 ± 15.15 | 53.15 ± 13.19 | 47.03 ± 15.69 |
| BMI (Kg/m2) | 29.19 ± 5.48 | 30.54 ± 5.37 | 28.52 ± 5.42 |
| Hemoglobin (g/dL) | 13.94 ± 1.88 | 13.64 ± 1.69 | 14.09 ± 1.95 |
| Platelet count (103/dL) | 256.61 ± 97.24 | 236.23 ± 110.19 | 266.72 ± 88.82 |
| INR | 1.07 ± 0.17 | 1.07 ± 0.17 | 1.08 ± 0.17 |
| Glucose (mg/dL) | 116.22 ± 67.76 | 115.37 ± 54.21 | 116.64 ± 69.59 |
| LDH (U/L) | 244.42 ± 112.03 | 197.36 ± 101.35 | 269.76 ± 109.62 |
| Cholesterol (mg/dL) | 188.17 ± 52.09 | 185.89 ± 49.23 | 189.4 ± 53.71 |
| HDL (mg/dL) | 39.6 ± 8.47 | 43.21 ± 9.49 | 37.65 ± 7.18 |
| LDL (mg/dL) | 102.23 ± 32.54 | 105.77 ± 32.67 | 100.33 ± 32.44 |
| Triglycerides (mg/dL) | 160.77 ± 77.37 | 164.56 ± 78.3 | 158.73 ± 77.09 |
| Albumin (mg/dL) | 3.96 ± 0.66 | 4.08 ± 0.57 | 3.9 ± 0.69 |
| ALT (U/L) | 82.63 ± 143.63 | 57.51 ± 71.46 | 95.09 ± 167.20 |
| AST (U/L) | 77.81 ± 151.36 | 77.46 ± 118.58 | 77.99 ± 165.64 |
| Total bilirubin (mg/dL) | 1.4 ± 1.68 | 1.27 ± 1.65 | 1.46 ± 1.69 |
| ALP (U/L) | 135.97 ± 104.78 | 121.59 ± 72.23 | 143.72 ± 117.24 |
| GGT (U/L) | 95.31 ± 87.64 | 107.67 ± 114.14 | 88.65 ± 68.95 |
| APRI | 2.11 ± 1.53 | 1.08 ± 1.57 | 0.89 ± 1.52 |
| FIB-4 | 2.11 ± 3.35 | 3 ± 4.92 | 1.66 ± 2.07 |
| NFS | 0.42 ± 4.39 | 0.97 ± 3.73 | 0.15 ± 4.67 |
| Methods | LR | BLDA | SVM | DT | KNN | Adaboost |
|---|---|---|---|---|---|---|
| Specificity | 75.23 ± 0.65 | 79.82 ± 0.94 | 82.29 ± 0.77 | 83.80 ± 0.73 | 84.37 ± 0.67 | 93.71 ± 0.48 |
| F1 score | 75.58 ± 0.67 | 79.67 ± 0.92 | 82.14 ± 0.75 | 83.68 ± 0.68 | 84.43 ± 0.64 | 92.95 ± 0.47 |
| Balanced Accuracy | 75.64 ± 0.68 | 79.92 ± 0.93 | 82.39 ± 0.78 | 83.89 ± 0.72 | 84.45 ± 0.65 | 93.53 ± 0.51 |
| MCC | 66.05 ± 0.67 | 70.91 ± 0.87 | 73.10 ± 0.74 | 74.50 ± 0.68 | 74.88 ± 0.64 | 84.69 ± 0.43 |
| Methods | LR | BLDA | SVM | DT | KNN | Adaboost |
|---|---|---|---|---|---|---|
| Kappa | 66.54 ± 0.64 | 71.00 ± 0.93 | 72.57 ± 0.74 | 74.02 ± 0.69 | 75.05 ± 0.63 | 83.98 ± 0.38 |
| AUC | 0.75 ± 0.02 | 0.79 ± 0.02 | 0.82 ± 0.02 | 0.83 ± 0.02 | 0.84 ± 0.01 | 0.93 ± 0.01 |
| DYI | 75.48 ± 0.69 | 79.92 ± 0.92 | 82.39 ± 0.75 | 83.89 ± 0.71 | 84.49 ± 0.65 | 93.45 ± 0.47 |
| Recall | 75.86 ± 0.73 | 80.02 ± 0.91 | 82.48 ± 0.73 | 83.99 ± 0.67 | 84.62 ± 0.62 | 93.32 ± 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, M.; Martínez, R.; Torres, A.M.; Ramón, A.; Blasco, P.; Mateo, J. Personalized Risk Assessment of Hepatic Fibrosis after Cholecystectomy in Metabolic-Associated Steatotic Liver Disease: A Machine Learning Approach. J. Clin. Med. 2023, 12, 6489. https://doi.org/10.3390/jcm12206489
Suárez M, Martínez R, Torres AM, Ramón A, Blasco P, Mateo J. Personalized Risk Assessment of Hepatic Fibrosis after Cholecystectomy in Metabolic-Associated Steatotic Liver Disease: A Machine Learning Approach. Journal of Clinical Medicine. 2023; 12(20):6489. https://doi.org/10.3390/jcm12206489
Chicago/Turabian StyleSuárez, Miguel, Raquel Martínez, Ana María Torres, Antonio Ramón, Pilar Blasco, and Jorge Mateo. 2023. "Personalized Risk Assessment of Hepatic Fibrosis after Cholecystectomy in Metabolic-Associated Steatotic Liver Disease: A Machine Learning Approach" Journal of Clinical Medicine 12, no. 20: 6489. https://doi.org/10.3390/jcm12206489
APA StyleSuárez, M., Martínez, R., Torres, A. M., Ramón, A., Blasco, P., & Mateo, J. (2023). Personalized Risk Assessment of Hepatic Fibrosis after Cholecystectomy in Metabolic-Associated Steatotic Liver Disease: A Machine Learning Approach. Journal of Clinical Medicine, 12(20), 6489. https://doi.org/10.3390/jcm12206489







