Testosterone and the Amygdala’s Functional Connectivity in Women and Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Saliva Samples
2.3. Data and Statistical Analysis of Behavioral and Hormone Data
2.4. Resting-State Functional Connectivity Analysis
2.4.1. Acquisition and Preprocessing
2.4.2. Functional Connectivity Analyses
2.5. Functional Characterization
2.6. Voxel-Based Morphometry (VBM) Analysis
3. Results
3.1. Sample Description
3.2. Amygdala rsFC and Testosterone: Whole Group
3.3. Amygdala rsFC and Testosterone: Men vs. Women
3.4. Exploratory Regression Analyses: Impact on Social Behavior
3.5. VBM Analysis
4. Discussion
4.1. Testosterone and Functional Connectivity of the Right Amygdala
4.2. Sex Differences
4.3. Future Directions and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeDoux, J.E. Emotion Circuits in the Brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Veer, I.M.; Oei, N.Y.L.; Spinhoven, P.; van Buchem, M.A.; Elzinga, B.M.; Rombouts, S.A.R.B. Beyond Acute Social Stress: Increased Functional Connectivity between Amygdala and Cortical Midline Structures. Neuroimage 2011, 57, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, N.W.; Herman, M.A.; Roberto, M. The Central Amygdala as an Integrative Hub for Anxiety and Alcohol Use Disorders. Biol. Psychiatry 2015, 77, 859–869. [Google Scholar] [CrossRef]
- Stevens, J.S.; Hamann, S. Sex Differences in Brain Activation to Emotional Stimuli: A Meta-Analysis of Neuroimaging Studies. Neuropsychologia 2012, 50, 1578–1593. [Google Scholar] [CrossRef]
- Domes, G.; Schulze, L.; Böttger, M.; Grossmann, A.; Hauenstein, K.; Wirtz, P.H.; Heinrichs, M.; Herpertz, S.C. The Neural Correlates of Sex Differences in Emotional Reactivity and Emotion Regulation. Hum. Brain Mapp. 2010, 31, 758–769. [Google Scholar] [CrossRef]
- Kogler, L.; Gur, R.C.; Derntl, B. Sex Differences in Cognitive Regulation of Psychosocial Achievement Stress: Brain and Behavior. Hum. Brain Mapp. 2015, 36, 1028–1042. [Google Scholar] [CrossRef]
- McRae, K.; Ochsner, K.N.; Mauss, I.B.; Gabrieli, J.J.D.; Gross, J.J. Gender Differences in Emotion Regulation: An FMRI Study of Cognitive Reappraisal. Group Process. Intergroup Relat. 2008, 11, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Simerly, R.B.; Chang, C.; Muramatsu, M.; Swanson, L.W. Distribution of Androgen and Estrogen Receptor MRNA-Containing Cells in the Rat Brain: An In Situ Hybridization Study. J. Comp. Neurol. 1990, 294, 76–95. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Vreugdenhil, E.; Oitzl, M.S.; Joëls, M. Brain Corticosteroid Receptor Balance in Health and Disease. Endocr. Rev. 1998, 19, 269–301. [Google Scholar] [PubMed]
- Eisenegger, C.; Haushofer, J.; Fehr, E. The Role of Testosterone in Social Interaction. Trends Cogn. Sci. 2011, 15, 263–271. [Google Scholar] [CrossRef]
- Dickerson, S.S.; Kemeny, M.E. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychol. Bull. 2004, 130, 355–391. [Google Scholar] [CrossRef]
- Derntl, B.; Windischberger, C.; Robinson, S.; Kryspin-Exner, I.; Gur, R.C.; Moser, E.; Habel, U. Amygdala Activity to Fear and Anger in Healthy Young Males Is Associated with Testosterone. Psychoneuroendocrinology 2009, 34, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Afrisham, R.; Sadegh-Nejadi, S.; SoliemaniFar, O.; Kooti, W.; Ashtary-Larky, D.; Alamiri, F.; Aberomand, M.; Najjar-Asl, S.; Khaneh-Keshi, A. Salivary Testosterone Levels under Psychological Stress and Its Relationship with Rumination and Five Personality Traits in Medical Students. Psychiatry Investig. 2016, 13, 637–643. [Google Scholar] [CrossRef]
- Smeets-Janssen, M.M.J.; Roelofs, K.; Van Pelt, J.; Spinhoven, P.; Zitman, F.G.; Penninx, B.W.J.H.; Giltay, E.J. Salivary Testosterone Is Consistently and Positively Associated with Extraversion: Results from the Netherlands Study of Depression and Anxiety. Neuropsychobiology 2015, 71, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liao, J.; Zilioli, S.; Wu, Y.; Deng, H.; Li, H.; Tobler, P.N. Testosterone Administration Increases Social Discounting in Healthy Males. Psychoneuroendocrinology 2019, 108, 127–134. [Google Scholar] [CrossRef]
- Nave, G.; Nadler, A.; Zava, D.; Camerer, C. Single-Dose Testosterone Administration Impairs Cognitive Reflection in Men. Psychol. Sci. 2017, 28, 1398–1407. [Google Scholar] [CrossRef]
- Metzger, N.Y.; Boettger, S. The Effect of Testosterone Therapy on Personality Traits of Trans Men: A Controlled Prospective Study in Germany and Switzerland. Psychiatry Res. 2019, 276, 31–38. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Müller, V.I. Functional Connectivity. In Brain Mapping—An Encyclopedic Reference. Volume 2: Anatomy and Physiology, Systems; Academic Press Elsevier: Cambridge, MA, USA, 2015; pp. 187–201. [Google Scholar]
- Eickhoff, S.B.; Grefkes, C. Approaches for the Integrated Analysis of Structure, Function and Connectivity of the Human Brain. Clin. EEG Neurosci. 2011, 42, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Functional and Effective Connectivity: A Review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef]
- Buckner, R.L.; Vincent, J.L. Unrest at Rest: Default Activity and Spontaneous Network Correlations. Neuroimage 2007, 37, 1091–1096. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional Connectivity in the Resting Brain: A Network Analysis of the Default Mode Hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Laird, A.R.; Glahn, D.C.; Lovallo, W.R.; Fox, P.T. Meta-Analytic Connectivity Modeling: Delineating the Functional of the Human Amygdala. Hum. Brain Mapp. 2011, 31, 173–184. [Google Scholar] [CrossRef]
- Roy, A.K.; Shehzad, Z.; Margulies, D.S.; Kelly, A.M.C.; Uddin, L.Q.; Gotimer, K.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Functional Connectivity of the Human Amygdala Using Resting State FMRI. Neuroimage 2010, 45, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.L.; Wiedholz, L.M.; Bassett, D.S.; Weinberger, D.R.; Zink, C.F.; Mattay, V.S.; Meyer-Lindenberg, A. A Validated Network of Effective Amygdala Connectivity. Neuroimage 2007, 36, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.B.; Mennes, M.; Zuo, X.-N.; Gohel, S.; Kelly, C.; Smith, S.M.; Beckmann, C.F.; Adelstein, J.S.; Buckner, R.L.; Colcombe, S.; et al. Toward Discovery Science of Human Brain Function. Proc. Natl. Acad. Sci. USA 2010, 107, 4734–4739. [Google Scholar] [CrossRef]
- Satterthwaite, T.D.; Wolf, D.H.; Roalf, D.R.; Ruparel, K.; Erus, G.; Vandekar, S.; Gennatas, E.D.; Elliott, M.A.; Smith, A.; Hakonarson, H.; et al. Linked Sex Differences in Cognition and Functional Connectivity in Youth. Cereb. Cortex 2014, 25, 2383–2394. [Google Scholar] [CrossRef]
- Wu, K.; Taki, Y.; Sato, K.; Hashizume, H.; Sassa, Y.; Takeuchi, H.; Thyreau, B.; He, Y.; Evans, A.C.; Li, X.; et al. Topological Organization of Functional Brain Networks in Healthy Children: Differences in Relation to Age, Sex, and Intelligence. PLoS ONE 2013, 8, e55347. [Google Scholar] [CrossRef]
- Wang, L.; Shen, H.; Tang, F.; Zang, Y.; Hu, D. Combined Structural and Resting-State Functional MRI Analysis of Sexual Dimorphism in the Young Adult Human Brain: An MVPA Approach. Neuroimage 2012, 61, 931–940. [Google Scholar] [CrossRef]
- Kogler, L.; Müller, V.I.; Seidel, E.-M.; Boubela, R.; Kalcher, K.; Moser, E.; Habel, U.; Gur, R.C.; Eickhoff, S.B.; Derntl, B. Sex Differences in the Functional Connectivity of the Amygdalae in Association with Cortisol. Neuroimage 2016, 134, 410–423. [Google Scholar] [CrossRef]
- Fareri, D.S.; Gabard-Durnam, L.; Goff, B.; Flannery, J.; Gee, D.G.; Lumian, D.S.; Caldera, C.; Tottenham, N. Normative Development of Ventral Striatal Resting State Connectivity in Humans. Neuroimage 2015, 118, 422–437. [Google Scholar] [CrossRef]
- Vaisvaser, S.; Lin, T.; Admon, R.; Podlipsky, I.; Greenman, Y.; Stern, N.; Fruchter, E.; Wald, I.; Pine, D.S.; Tarrasch, R.; et al. Neural Traces of Stress: Cortisol Related Sustained Enhancement of Amygdala-Hippocampal Functional Connectivity. Front. Hum. Neurosci. 2013, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Sripada, R.K.; Swain, J.E.; Evans, G.W.; Welsh, R.C.; Liberzon, I. Childhood Poverty and Stress Reactivity Are Associated with Aberrant Functional Connectivity in Default Mode Network. Neuropsychopharmacology 2014, 39, 2244–2251. [Google Scholar] [CrossRef]
- Buades-Rotger, M.; Engelke, C.; Krämer, U.M. Trait and State Patterns of Basolateral Amygdala Connectivity at Rest Are Related to Endogenous Testosterone and Aggression in Healthy Young Women. Brain Imaging Behav. 2019, 13, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, G.; Cservenka, A.; Rudolph, M.D.; Fair, D.A.; Nagel, B.J. Developmental Sex Differences in Resting State Functional Connectivity of Amygdala Sub-Regions. Neuroimage 2015, 115, 235–244. [Google Scholar] [CrossRef]
- Shansky, R.M. Sex Differences in Amygdala Structure and Function: From Rodents to Humans. In Handbook of Behavioral Neuroscience; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 26, pp. 275–284. [Google Scholar]
- Lauretta, R.; Sansone, M.; Sansone, A.; Romanelli, F.; Appetecchia, M. Gender in Endocrine Diseases: Role of Sex Gonadal Hormones. Int. J. Endocrinol. 2018, 2018, 4847376. [Google Scholar] [CrossRef]
- Peper, J.S.; van den Heuvel, M.P.; Mandl, R.C.W.; Hulshoff Pol, H.E.; van Honk, J. Sex Steroids and Connectivity in the Human Brain: A Review of Neuroimaging Studies. Psychoneuroendocrinology 2011, 36, 1101–1113. [Google Scholar] [CrossRef]
- Bos, P.A.; Hermans, E.J.; Ramsey, N.F.; van Honk, J. The Neural Mechanisms by Which Testosterone Acts on Interpersonal Trust. Neuroimage 2012, 61, 730–737. [Google Scholar] [CrossRef]
- Votinov, M.; Wagels, L.; Hoffstaedter, F.; Kellermann, T.; Goerlich, K.S.; Eickhoff, S.B.; Habel, U. Effects of Exogenous Testosterone Application on Network Connectivity within Emotion Regulation Systems. Sci. Rep. 2020, 10, 2352. [Google Scholar] [CrossRef]
- Van Wingen, G.; Mattern, C.; Verkes, R.J.; Buitelaar, J.; Fernández, G. Testosterone Reduces Amygdala-Orbitofrontal Cortex Coupling. Psychoneuroendocrinology 2010, 35, 105–113. [Google Scholar] [CrossRef]
- Heany, S.J.; Bethlehem, R.A.I.; van Honk, J.; Bos, P.A.; Stein, D.J.; Terburg, D. Effects of Testosterone Administration on Threat and Escape Anticipation in the Orbitofrontal Cortex. Psychoneuroendocrinology 2018, 96, 42–51. [Google Scholar] [CrossRef]
- Peters, S.; Jolles, D.J.; Duijvenvoorde, A.C.K.V.; Crone, E.A.; Peper, J.S. The Link between Testosterone and Amygdala-Orbitofrontal Cortex Connectivity in Adolescent Alcohol Use. Psychoneuroendocrinology 2015, 53, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Volman, I.; Toni, I.; Verhagen, L.; Roelofs, K. Endogenous Testosterone Modulates Prefrontal-Amygdala Connectivity during Social Emotional Behavior. Cereb. Cortex 2011, 21, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L. Why Sex Matters for Neuroscience. Nat. Rev. Neurosci. 2006, 7, 477–484. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Arnold, A.P.; Ball, G.F.; Blaustein, J.D.; De Vries, G.J. Sex Differences in the Brain: The Not so Inconvenient Truth. J. Neurosci. 2012, 32, 2241–2247. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and Gender: Modifiers of Health, Disease, and Medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Cowell, P.E.; Kostianovsky, D.J.; Gur, R.C.; Turetsky, B.I.; Gur, R.E. Sex Differences in Neuroanatomical and Clinical Correlations in Schizophrenia. Am. J. Psychiatry 1996, 153, 799–805. [Google Scholar] [CrossRef]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but Systematic Correlations in Functional Connectivity MRI Networks Arise from Subject Motion. Neuroimage 2012, 59, 2142–2154. [Google Scholar] [CrossRef]
- Satterthwaite, T.D.; Elliott, M.; Gerraty, R.; Ruparel, K.; Loughead, J.; Calkin, M.E.; Eickhoff, S.B.; Hakonarson, H.; Gur, R.C.; Gur, R.E.; et al. An Improved Framework for Confound Regression and Filtering for Control of Motion Artifact in the Preprocessing of Resting-State Functional Connectivity Data. Neuroimage 2013, 64, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Radke, S.; Seidel, E.M.; Boubela, R.N.; Thaler, H.; Metzler, H.; Kryspin-Exner, I.; Moser, E.; Habel, U.; Derntl, B. Immediate and Delayed Neuroendocrine Responses to Social Exclusion in Males and Females. Psychoneuroendocrinology 2018, 93, 56–64. [Google Scholar] [CrossRef]
- Kogler, L.; Seidel, E.-M.; Metzler, H.; Thaler, H.; Boubela, R.N.; Pruessner, J.C.; Kryspin-Exner, I.; Gur, R.C.; Windischberger, C.; Moser, E.; et al. Impact of Self-Esteem and Sex on Stress Reactions. Sci. Rep. 2017, 7, 17210. [Google Scholar] [CrossRef] [PubMed]
- Bürger, Z.; Müller, V.I.; Hoffstaedter, F.; Habel, U.; Gur, R.C.; Windischberger, C.; Moser, E.; Derntl, B.; Kogler, L. Stressor-Specific Sex Differences in Amygdala-Frontal Cortex Networks. J. Clin. Med. 2023, 12, 865. [Google Scholar] [CrossRef]
- McCrae, R.R.; Costa, P.T. A Contemplated Revision of the NEO Five-Factor Inventory. Pers. Individ. Dif. 2004, 36, 587–596. [Google Scholar] [CrossRef]
- Bem, S.L. The Measurement of Psychological Androgyny. J. Consult. Clin. Psychol. 1974, 42, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Arregger, A.L.; Contreras, L.N.; Tumilasci, O.R.; Aquilano, D.R.; Cardoso, E.M.L. Salivary Testosterone: A Reliable Approach to the Diagnosis of Male Hypogonadism. Clin. Endocrinol. 2007, 67, 656–662. [Google Scholar] [CrossRef]
- Vittek, J.; Hommedieu, D.; Gordon, G.G.; Rappaport, S.C.; Southren, A.L. Direct Radioimmunoassay (RIA) of Salivary Testosterone: Correlation with Free and Total Serum Testosterone. Life Sci. 1985, 37, 711–716. [Google Scholar] [CrossRef]
- Holmes, C.J.; Hoge, R.; Collins, L.; Woods, R.; Toga, A.W. Enhancement of MR Images Using Registration for Signal Averaging. J. Comput. Assist. Tomogr. 1998, 22, 324–333. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified Segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Weissenbacher, A.; Kasess, C.; Gerstl, F.; Lanzenberger, R.; Moser, E.; Windischberger, C. Correlations and Anticorrelations in Resting-State Functional Connectivity MRI: A Quantitative Comparison of Preprocessing Strategies. Neuroimage 2009, 47, 1408–1416. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Stephan, K.E.; Mohlberg, H.; Grefkes, C.; Fink, G.R.; Amunts, K.; Zilles, K. A New SPM Toolbox for Combining Probabilistic Cytoarchitectonic Maps and Functional Imaging Data. Neuroimage 2005, 25, 1325–1335. [Google Scholar] [CrossRef]
- Laird, A.R.; Lancaster, J.L.; Fox, P.T. BrainMap: The Social Evolution of a Human Brain Mapping Database. Neuroinformatics 2005, 3, 65–77. [Google Scholar] [CrossRef]
- Turner, J.; Laird, A. The Cognitive Paradigm Ontology: Design and Application. Neuroinformatics 2012, 10, 57–66. [Google Scholar] [CrossRef]
- Müller, V.I.; Cieslik, E.C.; Laird, A.R.; Fox, P.T.; Eickhoff, S.B. Dysregulated Left Inferior Parietal Activity in Schizophrenia and Depression: Functional Connectivity and Characterization. Front. Hum. Neurosci. 2013, 7, 268. [Google Scholar] [CrossRef]
- Kogler, L.; Müller, V.I.; Chang, A.; Eickhoff, S.B.; Fox, P.T.; Gur, R.C.; Derntl, B. Psychosocial versus Physiological Stress—Meta-Analyses on the Deactivations and Activations of the Neural Correlates of Stress Reactions. Neuroimage 2015, 119, 235–251. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Wüst, S.; Hellhammer, D. Consistent Sex Differences in Cortisol Responses to Psychological Stress. Psychosom. Med. 1992, 54, 648–657. [Google Scholar] [CrossRef]
- Vermeersch, H.; T’Sjoen, G.; Kaufman, J.M.; Vincke, J.; Van Houtte, M. Gender Ideology, Same-Sex Peer Group Affiliation and the Relationship between Testosterone and Dominance in Adolescent Boys and Girls. J. Biosoc. Sci. 2010, 42, 463–475. [Google Scholar] [CrossRef]
- Seidel, E.; Silani, G.; Metzler, H.; Thaler, H.; Lamm, C.; Gur, R.; Kryspin-Exner, I.; Habel, U.; Derntl, B. The Impact of Social Exclusion vs. Inclusion on Subjective and Hormonal Reactions in Females and Males. Psychoneuroendocrinology 2013, 38, 2925–2932. [Google Scholar] [CrossRef]
- Fernández-Guasti, A.; Kruijver, F.P.M.; Fodor, M.; Swaab, D.F. Sex Differences in the Distribution of Androgen Receptors in the Human Hypothalamus. J. Comp. Neurol. 2000, 425, 422–435. [Google Scholar] [CrossRef]
- Celec, P.; Ostatníková, D.; Hodosy, J. On the Effects of Testosterone on Brain Behavioral Functions. Front. Neurosci. 2015, 9, 12. [Google Scholar] [CrossRef]
- Höfer, P.; Lanzenberger, R.; Kasper, S. Testosterone in the Brain: Neuroimaging Findings and the Potential Role for Neuropsychopharmacology. Eur. Neuropsychopharmacol. 2013, 23, 79–88. [Google Scholar] [CrossRef]
- Güntürkün, O.; Ströckens, F.; Ocklenburg, S. Brain Lateralization: A Comparative Perspective. Physiol. Rev. 2020, 100, 1019–1063. [Google Scholar] [CrossRef]
- Pletzer, B.; Harris, T. Sex Hormones Modulate the Relationship between Global Advantage, Lateralization, and Interhemispheric Connectivity in a Navon Paradigm. Brain Connect. 2018, 8, 106–118. [Google Scholar] [CrossRef]
- Toga, A.W.; Thompson, P.M. Mapping Brain Asymmetry. Nat. Rev. Neurosci. 2003, 4, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Li, X.; Wu, Y.; Peng, W. Single Dose Testosterone Administration Modulates the Temporal Dynamics of Distractor Processing. Psychoneuroendocrinology 2020, 121, 104838. [Google Scholar] [CrossRef] [PubMed]
- Pintzka, C.W.S.; Evensmoen, H.R.; Lehn, H.; Håberg, A.K. Changes in Spatial Cognition and Brain Activity after a Single Dose of Testosterone in Healthy Women. Behav. Brain Res. 2016, 298, 78–90. [Google Scholar] [CrossRef]
- Janowsky, J.S. Thinking with Your Gonads: Testosterone and Cognition. Trends Cogn. Sci. 2006, 10, 77–82. [Google Scholar] [CrossRef]
- Schöning, S.; Engelien, A.; Kugel, H.; Schäfer, S.; Schiffbauer, H.; Zwitserlood, P.; Pletziger, E.; Beizai, P.; Kersting, A.; Ohrmann, P.; et al. Functional Anatomy of Visuo-Spatial Working Memory during Mental Rotation Is Influenced by Sex, Menstrual Cycle, and Sex Steroid Hormones. Neuropsychologia 2007, 45, 3203–3214. [Google Scholar] [CrossRef] [PubMed]
- Pletzer, B.; Petasis, O.; Cahill, L. Switching between Forest and Trees: Opposite Relationship of Progesterone and Testosterone to Global-Local Processing. Horm. Behav. 2014, 66, 257–266. [Google Scholar] [CrossRef]
- Schutter, D.J.L.G.; Meuwese, R.; Bos, M.G.N.; Crone, E.A.; Peper, J.S. Exploring the Role of Testosterone in the Cerebellum Link to Neuroticism: From Adolescence to Early Adulthood. Psychoneuroendocrinology 2017, 78, 203–212. [Google Scholar] [CrossRef]
- Courvoisier, D.S.; Renaud, O.; Geiser, C.; Paschke, K.; Gaudy, K.; Jordan, K. Sex Hormones and Mental Rotation: An Intensive Longitudinal Investigation. Horm. Behav. 2013, 63, 345–351. [Google Scholar] [CrossRef]
- Genon, S.; Li, H.; Fan, L.; Müller, V.I.; Cieslik, E.C.; Hoffstaedter, F.; Reid, A.T.; Langner, R.; Grefkes, C.; Fox, P.T.; et al. The Right Dorsal Premotor Mosaic: Organization, Functions, and Connectivity. Cereb. Cortex 2017, 27, 2095–2110. [Google Scholar] [CrossRef]
- Choi, J.C.; Yi, D.J.; Han, B.S.; Lee, P.H.; Kim, J.H.; Kim, B.H. Placebo Effects on Analgesia Related to Testosterone and Premotor Activation. Neuroreport 2011, 22, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Glinka, K.; Staudinger, U.M.; Voelcker-Rehage, C.; Godde, B. Neural Processing of Arousing Emotional Information Is Associated with Executive Functioning in Older Adults. Emotion 2020, 20, 541. [Google Scholar] [CrossRef] [PubMed]
- Faul, L.; Knight, L.K.; Espay, A.J.; Depue, B.E.; LaFaver, K. Neural Activity in Functional Movement Disorder after Inpatient Rehabilitation. Psychiatry Res. Neuroimaging 2020, 303, 111125. [Google Scholar] [CrossRef] [PubMed]
- Bos, P.A.; Panksepp, J.; Bluthe, R.M.; van Honk, J. Acute Effects of Steroid Hormones and Neuropeptides on Human Social-Emotional Behavior: A Review of Single Administration Studies. Front. Neuroendocrinol. 2012, 33, 17–35. [Google Scholar] [CrossRef]
- Ulrich, M.; Niemann, J.; Boland, M.; Kammer, T.; Niemann, F.; Grön, G. The Neural Correlates of Flow Experience Explored with Transcranial Direct Current Stimulation. Exp. Brain Res. 2018, 236, 3223–3237. [Google Scholar] [CrossRef]
- Dedoncker, J.; Brunoni, A.R.; Baeken, C.; Vanderhasselt, M.A. A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain Stimulat. 2016, 9, 501–517. [Google Scholar] [CrossRef]
- Veldema, J. Non-Invasive Brain Stimulation and Sex/Polypeptide Hormones in Reciprocal Interactions: A Systematic Review. Biomedicines 2023, 11, 1981. [Google Scholar] [CrossRef]
- Nguyen, T.V.; McCracken, J.T.; Albaugh, M.D.; Botteron, K.N.; Hudziak, J.J.; Ducharme, S. A testosterone-related structural brain phenotype predicts aggressive behavior from childhood to adulthood. Psychoneuroendocrinology 2016, 63, 109–118. [Google Scholar] [CrossRef]
- Zuloaga, D.G.; Puts, D.A.; Jordan, C.L.; Breedlove, S.M. The role of androgen receptors in the masculinization of brain and behavior: What we’ve learned from the testicular feminization mutation. Horm. Behav. 2008, 53, 613–626. [Google Scholar] [CrossRef]
- Pletzer, B. Sex-Specific Strategy Use and Global-Local Processing: A Perspective toward Integrating Sex Differences in Cognition. Front. Neurosci. 2014, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Wüstenberg, T.; Heinze, H.-J.; Peters, M.; Jäncke, L. Women and Men Exhibit Different Cortical Activation Patterns during Mental Rotation Tasks. Neuropsychologia 2002, 40, 2397–2408. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.; Imperato-McGinley, J.; Pan, H.; Voyer, D.; Cordero, J.; Zhu, Y.S.; Stern, E.; Silbersweig, D. Sex Differences in Mental Rotation: Top-down versus Bottom-up Processing. Neuroimage 2006, 32, 445–456. [Google Scholar] [CrossRef]
- Hausmann, M.; Schoofs, D.; Rosenthal, H.E.S.; Jordan, K. Interactive Effects of Sex Hormones and Gender Stereotypes on Cognitive Sex Differences—A Psychobiosocial Approach. Psychoneuroendocrinology 2009, 34, 389–401. [Google Scholar] [CrossRef]
- Zell, E.; Krizan, Z.; Teeter, S.R. Evaluating Gender Similarities and Differences Using Metasynthesis. Am. Psychol. 2015, 70, 10–20. [Google Scholar] [CrossRef]
- Finkelstein, M.; Weidenfeld, J.; Ne’eman, Y.; Samuni, A.; Mizrachi, Y.; Ben-Uzilio, R. Comparative Studies of the Aromatization of Testosterone and Epitestosterone by Human Placental Aromatase. Endocrinology 1981, 108, 943–947. [Google Scholar] [CrossRef]
- Wright, F.; Giacomini, M.; Riahi, M.; Mowszowicz, I. Antihormone Activity of Progesterone and Progestins. In Progesterone and Progestins; Bardin, C.W., Milgröm, E., Mauvais-Jarvis, P., Eds.; Raven Press: New York, NY, USA, 1983; pp. 121–134. [Google Scholar]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-Analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Sebastián-Tirado, A.; Félix-Esbrí, S.; Forn, C.; Sanchis-Segura, C. Are Gender-Science Stereotypes Barriers for Women in Science, Technology, Engineering, and Mathematics? Exploring When, How, and to Whom in an Experimentally-Controlled Setting. Front. Psychol. 2023, 14, 1219012. [Google Scholar] [CrossRef]
- Stoevenbelt, A.H.; Wicherts, J.M.; Flore, P.C.; Phillips, L.A.T.; Pietschnig, J.; Verschuere, B.; Voracek, M.; Schwabe, I. Are Speeded Tests Unfair? Modeling the Impact of Time Limits on the Gender Gap in Mathematics. Educ. Psychol. Meas. 2022, 83, 684–709. [Google Scholar] [CrossRef]
- Engman, J.; Sundström-Poromaa, I.; Moby, L.; Wikström, J.; Fredrikson, M.; Gingnell, M. Hormonal Cycle and Contraceptive Effects on Amygdala and Salience Resting-State Networks in Women with Previous Affective Side Effects on the Pill. Neuropsychopharmacology 2018, 43, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Engman, J.; Linnman, C.; Van Dijk, K.R.; Milad, M.R. Amygdala subnuclei resting-state functional connectivity sex and estrogen differences. Psychoneuroendocrinology 2016, 63, 34–42. [Google Scholar] [CrossRef]
- Hidalgo-Lopez, E.; Noachtar, I.; Pletzer, B. Hormonal contraceptive exposure relates to changes in resting state functional connectivity of anterior cingulate cortex and amygdala. Front. Endocrinol. 2023, 14, 1131995. [Google Scholar] [CrossRef]
- Cooper, M.A.; Clinard, C.T.; Dulka, B.N.; Grizzell, J.A.; Loewen, A.L.; Campbell, A.V.; Adler, S.G. Gonadal Steroid Hormone Receptors in the Medial Amygdala Contribute to Experience-Dependent Changes in Stress Vulnerability. Psychoneuroendocrinology 2021, 129, 105249. [Google Scholar] [CrossRef] [PubMed]
- Josephs, R.A.; Telch, M.J.; Hixon, J.G.; Evans, J.J.; Lee, H.; Knopik, V.S.; McGeary, J.E.; Hariri, A.R.; Beevers, C.G. Genetic and hormonal sensitivity to threat: Testing a serotonin transporter genotype × testosterone interaction. Psychoneuroendocrinology 2012, 37, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Maseroli, E.; Vignozzi, L. Are Endogenous Androgens Linked to Female Sexual Function? A Systemic Review and Meta-Analysis. J. Sex. Med. 2022, 19, 553–568. [Google Scholar] [CrossRef] [PubMed]
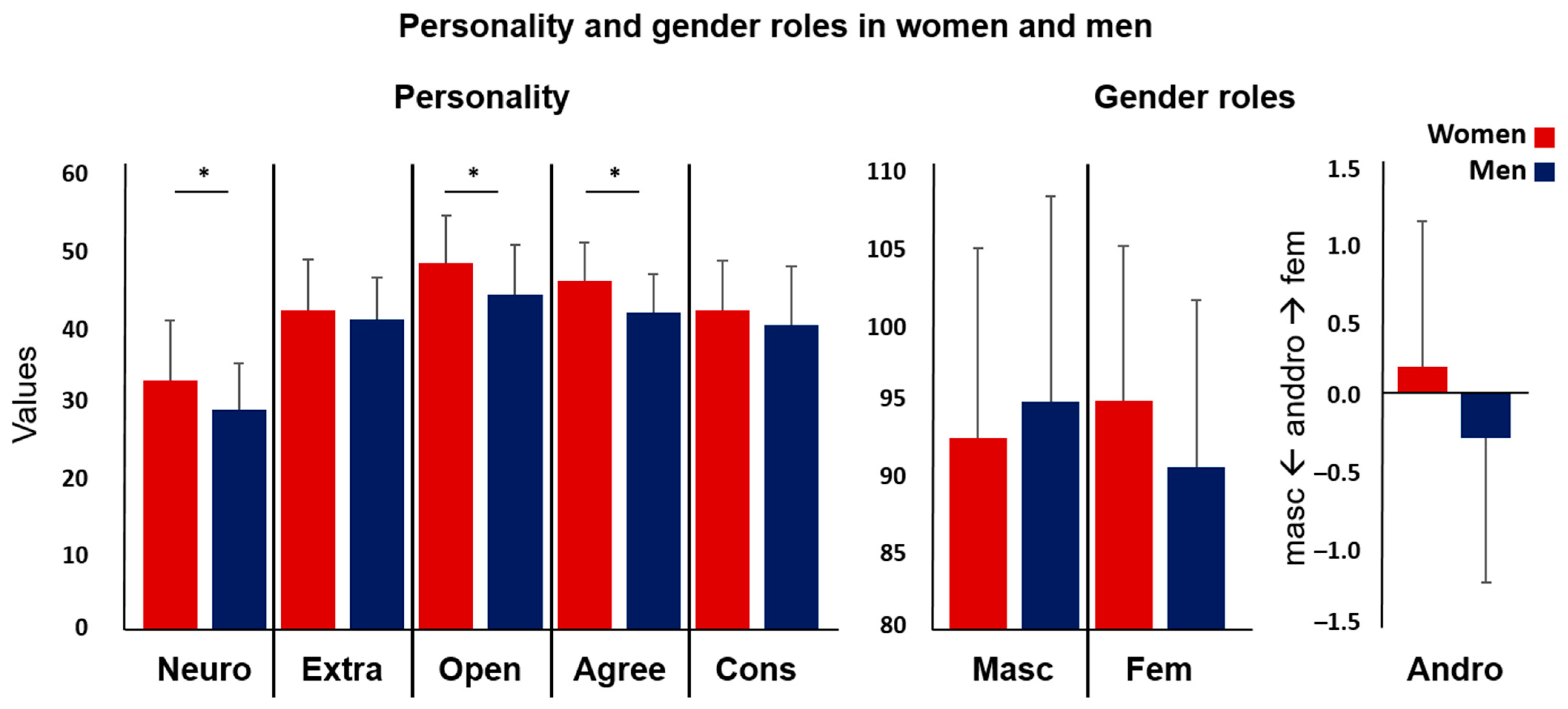


| Women | Men | ||||
|---|---|---|---|---|---|
| Mean | STD | Mean | STD | p-Value | |
| Age | 24.31 | 3.942 | 24.00 | 3.052 | 0.695 |
| Testosterone (pg/mL, log-transformed) | 1.37 | 0.257 | 1.86 | 0.267 | <0.001 * |
| Testosterone (pg/mL) | 26.63 | 17.26 | 84.45 | 56.45 | <0.001 * |
| Affect (PANAS, mean scores) | |||||
| Positive affect | 2.63 | 0.708 | 2.64 | 0.625 | 0.952 |
| Negative affect | 1.25 | 0.355 | 1.19 | 0.222 | 0.362 |
| Personality (NEO-FFI) | |||||
| Neuroticism | 32.83 | 7.880 | 28.95 | 6.177 | 0.016 * |
| Extraversion | 42.10 | 6.585 | 40.82 | 5.562 | 0.351 |
| Openness | 48.33 | 6.234 | 44.15 | 6.576 | 0.004 * |
| Agreeableness | 45.98 | 5.033 | 41.77 | 5.029 | 0.000 * |
| Conscientiousness | 42.07 | 6.535 | 40.08 | 7.727 | 0.212 |
| Gender roles (BSRI) | |||||
| Masculinity | 92.67 | 12.491 | 95.05 | 13.504 | 0.412 |
| Femininity | 95.14 | 10.166 | 90.79 | 10.931 | 0.067 |
| Androgyny | 0.18 | 0.979 | −0.30 | 0.974 | 0.030 + |
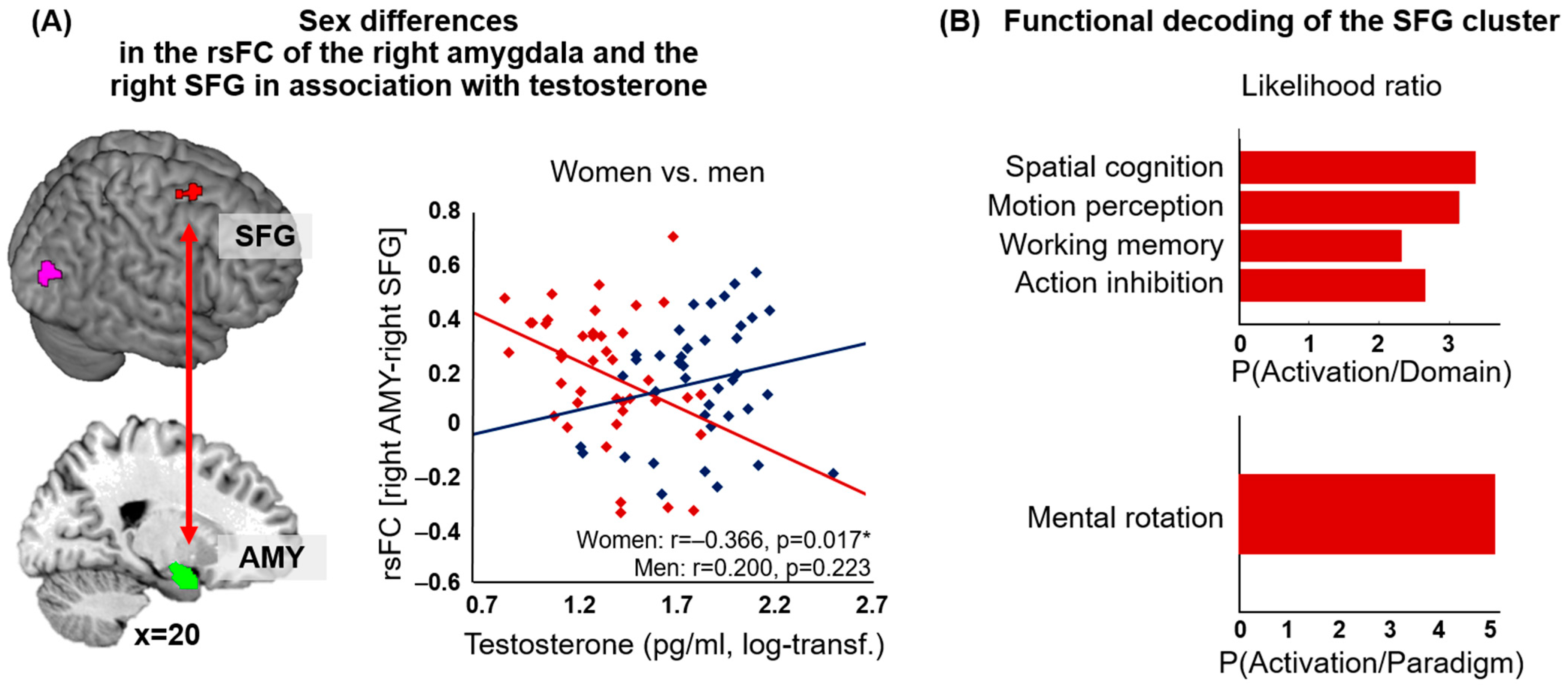
| t Value | X | Y | Z | Macroanatomical Location | |
|---|---|---|---|---|---|
| (A) rsFC of the right amygdala in correlation with testosterone | |||||
| Whole group (n = 81) | |||||
| Cluster 1 (k = 98) | |||||
| 4.29 | 38 | −82 | 14 | R Middle occipital gyrus | |
| 4.16 | 34 | −74 | 10 | ||
| 3.91 | 42 | −86 | 10 | R Middle occipital gyrus | |
| (B) rsFC of the right amygdala in correlation with testosterone | |||||
| Men (n = 39) > women (n = 42) | |||||
| Cluster 1 (k = 86) | |||||
| 4.90 | 24 | 6 | 62 | R Superior frontal gyrus | |
| 4.29 | 20 | 8 | 58 | ||
| 3.83 | 28 | −2 | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogler, L.; Müller, V.I.; Moser, E.; Windischberger, C.; Gur, R.C.; Habel, U.; Eickhoff, S.B.; Derntl, B. Testosterone and the Amygdala’s Functional Connectivity in Women and Men. J. Clin. Med. 2023, 12, 6501. https://doi.org/10.3390/jcm12206501
Kogler L, Müller VI, Moser E, Windischberger C, Gur RC, Habel U, Eickhoff SB, Derntl B. Testosterone and the Amygdala’s Functional Connectivity in Women and Men. Journal of Clinical Medicine. 2023; 12(20):6501. https://doi.org/10.3390/jcm12206501
Chicago/Turabian StyleKogler, Lydia, Veronika I. Müller, Ewald Moser, Christian Windischberger, Ruben C. Gur, Ute Habel, Simon B. Eickhoff, and Birgit Derntl. 2023. "Testosterone and the Amygdala’s Functional Connectivity in Women and Men" Journal of Clinical Medicine 12, no. 20: 6501. https://doi.org/10.3390/jcm12206501
APA StyleKogler, L., Müller, V. I., Moser, E., Windischberger, C., Gur, R. C., Habel, U., Eickhoff, S. B., & Derntl, B. (2023). Testosterone and the Amygdala’s Functional Connectivity in Women and Men. Journal of Clinical Medicine, 12(20), 6501. https://doi.org/10.3390/jcm12206501







