The Correct Indication to Induce Labour in a Swiss Cantonal Hospital
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Outcomes
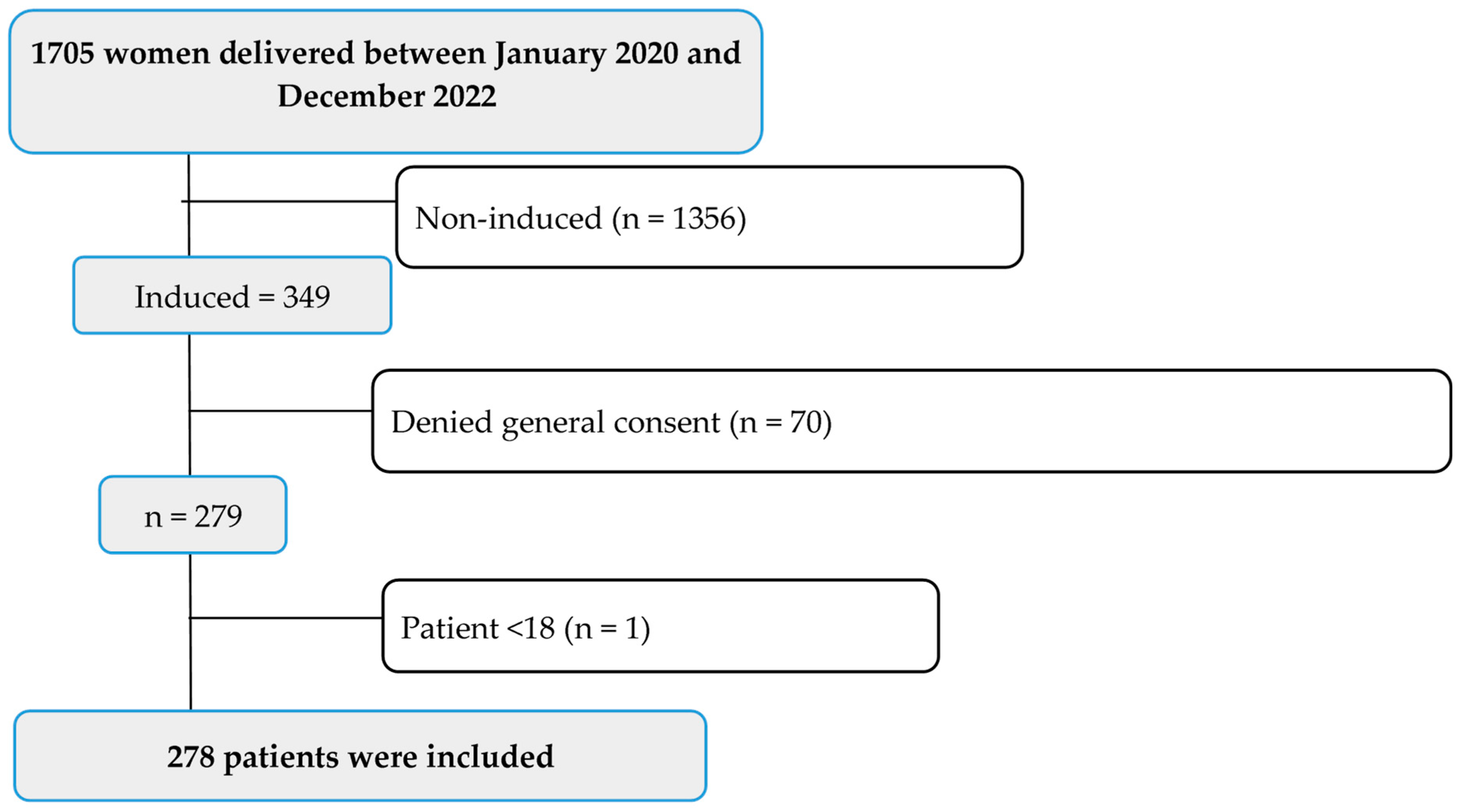
2.3. Patient Population: Inclusion and Exclusion Criteria
2.4. Data Collection Process
2.5. Statistical Analyses
3. Results
3.1. Patient-Based Baseline Characteristics
3.2. Implementation of the Guidelines in the Case of an Indication of IOL
3.3. Implementation of Guidelines Based on Other Recommendations
3.4. Factors and Outcome of Successful IOL
3.5. Maternal and Neonatal Outcomes
4. Discussion
4.1. Baseline Characteristics
4.2. Guideline Adherence
4.3. Factors Associated with Successful IOL
4.4. Maternal and Neonatal Outcomes
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gill, P.; Lende, M.N.; Van Hook, J.W. Induction of Labor; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kumar, B.; Kumari, S.; Hughes, S.; Savill, S. Prospective cohort study of induction of labor: Indications, outcome and postpartum hemorrhage. Eur. J. Midwifery 2021, 5, 53. [Google Scholar] [CrossRef]
- Swift, E.M.; Gunnarsdottir, J.; Zoega, H.; Bjarnadottir, R.I.; Steingrimsdottir, T.; Einarsdottir, K. Trends in labor induction indications: A 20-year population-based study. Acta Obstet. Gynecol. Scand. 2022, 101, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.R. Trends in Labor Induction in the United States, 1989 to 2020. MCN Am. J. Matern. Child Nurs. 2022, 47, 235. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, M.; Lidegaard, O.; Skovlund, C.W.; Morch, L.S.; Hedegaard, M. Reduction in stillbirths at term after new birth induction paradigm: Results of a national intervention. BMJ Open 2014, 4, e005785. [Google Scholar] [CrossRef] [PubMed]
- Queensland Clinical Guidelines. Induction of Labour. Available online: https://www.health.qld.gov.au/__data/assets/pdf_file/0020/641423/g-iol.pdf (accessed on 26 July 2023).
- World Health Organization. WHO Recommendations: Induction of Labour at or beyond Term; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- McCarthy, C.M.; Meaney, S.; McCarthy, M.; Conners, N.; Russell, N. Induction of labor: Reviewing the past to improve the future. AJOG Glob. Rep. 2022, 2, 100099. [Google Scholar] [CrossRef]
- Arage, M.W. Labor Induction. In New Aspects in Cesarean Sections; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Pinas-Carrillo, A.; Chandraharan, E. Induction and Augmentation of Labor. Contin. Textb. Women’s Med. Ser. Obstet. Modul. 2021, 11, 205–212. [Google Scholar] [CrossRef]
- Wormer, K.C.; Bauer, A.; Williford, A.E. Bishop Score; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lee, D.S.; Tandel, M.D.; Kwan, L.; Francoeur, A.A.; Duong, H.L.; Negi, M. Favorable Simplified Bishop Score after cervical ripening associated with decreased cesarean birth rate. Am. J. Obstet. Gynecol. MFM 2022, 4, 100534. [Google Scholar] [CrossRef] [PubMed]
- Laughon, S.K.; Zhang, J.; Troendle, J.; Sun, L.; Reddy, U.M. Using a simplified Bishop score to predict vaginal delivery. Obstet. Gynecol. 2011, 117, 805–811. [Google Scholar] [CrossRef]
- Morrish, D.; Hoskins, I.A. Induction of Labor: Review of Pros, Cons, and Controversies. In Childbirth; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- ACOG Committee on Obstetric Practice. ACOG committee opinion no. 561: Nonmedically indicated early-term deliveries. Obstet. Gynecol. 2013, 121, 911–915. [Google Scholar] [CrossRef]
- Geneen, L.J.; Gilbert, J.; Reeves, T.; Mainie, P.; Maresh, M.; Smith, L.; Wu, P.; Parisaei, M. Timing of induction of labour in the prevention of prolonged pregnancy: Systematic review with meta-analysis. Reprod. Female Child Health 2022, 1, 69–79. [Google Scholar] [CrossRef]
- Carlson, N.; Ellis, J.; Page, K.; Dunn Amore, A.; Phillippi, J. Review of Evidence-Based Methods for Successful Labor Induction. J. Midwifery Womens Health 2021, 66, 459–469. [Google Scholar] [CrossRef] [PubMed]
- de Aquino, M.M.; Cecatti, J.G. Misoprostol versus oxytocin for labor induction in term and post-term pregnancy: Randomized controlled trial. Sao Paulo Med. J. 2003, 121, 102–106. [Google Scholar] [CrossRef]
- Liu, A.; Lv, J.; Hu, Y.; Lang, J.; Ma, L.; Chen, W. Efficacy and safety of intravaginal misoprostol versus intracervical dinoprostone for labor induction at term: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2014, 40, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Kehl, S.; Hösli, I.; Pecks, U.; Reif, P.; Schild, R.L.; Schmidt, M.; Schmitz, D.; Schwarz, C.; Surbek, D.; Abou-Dakn, M. Induction of Labour. Guideline of the DGGG, OEGGG and SGGG (S2k, AWMF Registry No. 015-088, December 2020). Geburtshilfe Frauenheilkd. 2020, 81, 870–895. [Google Scholar] [CrossRef] [PubMed]
- de Vaan, M.D.; Ten Eikelder, M.L.; Jozwiak, M.; Palmer, K.R.; Davies-Tuck, M.; Bloemenkamp, K.W.; Mol, B.W.J.; Boulvain, M. Mechanical methods for induction of labour. Cochrane Database Syst. Rev. 2019, 10, CD001233. [Google Scholar] [CrossRef]
- ACOG Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 107: Induction of labor. Obstet. Gynecol. 2009, 114 Pt 1, 386–397. [Google Scholar] [CrossRef]
- Smyth, R.M.; Markham, C.; Dowswell, T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst. Rev. 2013, CD006167. [Google Scholar] [CrossRef]
- Ingvarsson, S.; Schildmeijer, K.; Oscarsson, M. Swedish midwives’ experiences and views of amniotomy: An interview study. Midwifery 2020, 91, 102840. [Google Scholar] [CrossRef]
- Al-Shaikh, G.K.; Wahabi, H.A.; Fayed, A.A.; Esmaeil, S.A.; Al-Malki, G.A. Factors associated with successful induction of labor. Saudi Med. J. 2012, 33, 298–303. [Google Scholar]
- Khan, N.B.; Ahmed, I.; Malik, A.; Sheikh, L. Factors associated with failed induction of labour in a secondary care hospital. J. Pak. Med. Assoc. 2012, 62, 6–10. [Google Scholar]
- Farah, F.Q.; Aynalem, G.L.; Seyoum, A.T.; Gedef, G.M. The prevalence and associated factors of success of labor induction in Hargeisa maternity hospitals, Hargeisa Somaliland 2022: A hospital-based cross-sectional study. BMC Pregnancy Childbirth 2023, 23, 437. [Google Scholar] [CrossRef]
- Banos, N.; Migliorelli, F.; Posadas, E.; Ferreri, J.; Palacio, M. Definition of Failed Induction of Labor and Its Predictive Factors: Two Unsolved Issues of an Everyday Clinical Situation. Fetal Diagn. Ther. 2015, 38, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.Z.; Cherkos, E.A.; Badi, M.B.; Anteneh, K.T.; Demssie, F.W.; Abdo, A.A.; Mihret, M.S. Factors and outcomes associated with the induction of labor in referral hospitals of Amhara regional state, Ethiopia: A multicenter study. BMC Pregnancy Childbirth 2021, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.E.; Grobman, W.A. When has an induction failed? Obstet. Gynecol. 2005, 105, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.G.; Rouse, D.J. What is a failed labor induction? Clin. Obstet. Gynecol. 2006, 49, 585–593. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Marconi, A.M. Recent advances in the induction of labor. F1000Research 2019, 8, F1000 Faculty Rev-1829. [Google Scholar] [CrossRef]
- Sydsjo, G.; Lindell Pettersson, M.; Bladh, M.; Skoog Svanberg, A.; Lampic, C.; Nedstrand, E. Evaluation of risk factors’ importance on adverse pregnancy and neonatal outcomes in women aged 40 years or older. BMC Pregnancy Childbirth 2019, 19, 92. [Google Scholar] [CrossRef]
- Federal Office for Statistics Switzerland. Reproductive Health. 16 December 2021. Available online: https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitszustand/reproduktive.html (accessed on 14 August 2023).
- Bouzaglou, A.; Aubenas, I.; Abbou, H.; Rouanet, S.; Carbonnel, M.; Pirtea, P.; Ayoubi, J.M.B. Pregnancy at 40 years Old and Above: Obstetrical, Fetal, and Neonatal Outcomes. Is Age an Independent Risk Factor for Those Complications? Front. Med. 2020, 7, 208. [Google Scholar] [CrossRef]
- Declercq, E.; Wolterink, A.; Rowe, R.; de Jonge, A.; De Vries, R.; Nieuwenhuijze, M.; Verhoeven, C.; Shah, N. The natural pattern of birth timing and gestational age in the U.S. compared to England, and the Netherlands. PLoS ONE 2023, 18, e0278856. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.; Naqvi, S.; Khanam, M.; Jafry, H.F. Primiparity as an intrapartum obstetric risk factor. J. Pak. Med. Assoc. 2012, 62, 694–698. [Google Scholar] [PubMed]
- Hassan, B.; Mandar, O.; Alhabardi, N.; Adam, I. Length of Hospital Stay After Cesarean Delivery and Its Determinants Among Women in Eastern Sudan. Int. J. Womens Health 2022, 14, 731–738. [Google Scholar] [CrossRef]
- Ghaffari, P.; Vanda, R.; Aramesh, S.; Jamali, L.; Bazarganipour, F.; Ghatee, M.A. Hospital discharge on the first compared with the second day after a planned cesarean delivery had equivalent maternal postpartum outcomes: A randomized single-blind controlled clinical trial. BMC Pregnancy Childbirth 2021, 21, 466. [Google Scholar] [CrossRef]
- Liu, S.; Heaman, M.; Kramer, M.S.; Demissie, K.; Wen, S.W.; Marcoux, S.; Maternal Health Study Group of the Canadian Perinatal Surveillance System. Length of hospital stay, obstetric conditions at childbirth, and maternal readmission: A population-based cohort study. Am. J. Obstet. Gynecol. 2002, 187, 681–687. [Google Scholar] [CrossRef]
- Lame, G.; Liberati, E.; Burt, J.; Draycott, T.; Winter, C.; Ward, J.; Dixon-Woods, M. IMproving the practice of intrapartum electronic fetal heart rate MOnitoring with cardiotocography for safer childbirth (the IMMO programme): Protocol for a qualitative study. BMJ Open 2019, 9, e030271. [Google Scholar] [CrossRef]
- German Society of Gynecology and Obstetrics (DGGG); Maternal Fetal Medicine Study Group (AGMFM); German Society of Prenatal Medicine and Obstetrics (DGPGM); German Society of Perinatal Medicine (DGPM). S1-Guideline on the Use of CTG During Pregnancy and Labor: Long version—AWMF Registry No. 015/036. Geburtshilfe Frauenheilkd. 2014, 74, 721–732. [Google Scholar] [CrossRef]
- Alfirevic, Z.; Devane, D.; Gyte, G.M.; Cuthbert, A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst. Rev. 2017, 2, CD006066. [Google Scholar] [CrossRef]
- O’Connell, M.A.; Leahy-Warren, P.; Kenny, L.C.; O’Neill, S.M.; Khashan, A.S. The prevalence and risk factors of fear of childbirth among pregnant women: A cross-sectional study in Ireland. Acta Obstet. Gynecol. Scand. 2019, 98, 1014–1023. [Google Scholar] [CrossRef]
- Green, G.; Tesler, R.; Marques, A. Primiparous and Multiparous Women’s Mode of Birth and Negative Emotions. Int. J. Environ. Res. Public Health 2022, 19, 5189. [Google Scholar] [CrossRef]
- Fenwick, J.; Toohill, J.; Creedy, D.K.; Smith, J.; Gamble, J. Sources, responses and moderators of childbirth fear in Australian women: A qualitative investigation. Midwifery 2015, 31, 239–246. [Google Scholar] [CrossRef]
- Ellis, J.A.; Brown, C.M.; Barger, B.; Carlson, N.S. Influence of Maternal Obesity on Labor Induction: A Systematic Review and Meta-Analysis. J. Midwifery Womens Health 2019, 64, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Jansson, M.H.; Franzen, K.; Hiyoshi, A.; Tegerstedt, G.; Dahlgren, H.; Nilsson, K. Risk factors for perineal and vaginal tears in primiparous women—The prospective POPRACT-cohort study. BMC Pregnancy Childbirth 2020, 20, 749. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists and American Academy of Pediatrics. The Apgar score. Committee Opinion No. 644. Obstet. Gynecol. 2015, 126, e52–e55. [Google Scholar] [CrossRef]
- Lin, X.S.; Peng, X.Y.; Yang, M.M.; Ning, L.L.; Shao, Y.W.; Jiang, Y.; Feng, S.W.; Luo, Q. The single pregnancy predicting model of 1 minute Apgar score less than 7 after preterm birth: A retrospective study. PLoS ONE 2022, 17, e0279385. [Google Scholar] [CrossRef] [PubMed]
- Workineh, Y.A.; Workie, H.M. Adverse Neonatal Outcomes and Associated Risk Factors: A Case-Control Study. Glob. Pediatr. Health 2022, 9, 2333794X221084070. [Google Scholar] [CrossRef]
- Tavares, V.B.; JS, E.S.; Affonso, M.V.G.; Da Rocha, E.S.; Rodrigues, L.F.G.; da Costa Moraes, L.F.; Dos Santos Coelho, G.C.; Araujo, S.S.; das Neves, P.F.M.; Gomes, F.C.; et al. Factors associated with 5-min APGAR score, death and survival in neonatal intensive care: A case-control study. BMC Pediatr. 2022, 22, 560. [Google Scholar] [CrossRef]

| Baseline Characteristics | n = 278 |
|---|---|
| Age (years), mean ± SD | 32.18 ± 4.75 |
| 19–34, n (%) | 198 (71.2) |
| ≥35, n (%) | 80 (28.8) |
| BMI (kg/m2), mean ± SD | 29.83 ± 6.48 |
| LOS (days) median (IQR) | 5 (4–6) |
| Duration of induction, mean ± SD (days) | 2.36 ± 1.58 |
| Gravida | |
| GA (weeks), median (IQR) | 40.4 (39.4–41.3) |
| Primigravida, n (%) | 104 (37.4) |
| Multigravida, n (%) | 171 (62.5) |
| Grandmultigravida, n (%) | 3 (1.1) |
| Parity | |
| Primipara, n (%) | 157 (56) |
| Multipara, n (%) | 119 (43) |
| Grand multipara, n (%) | 2 (1) |
| Pre-existing comorbidities, n (%) | 77 (27.7) |
| Bronchial asthma, n (%) | 12 (4.3) |
| Hypertension, n (%) | 8 (2.9) |
| Endometriosis, n (%) | 2 (0.7) |
| Hypothyroidism, n (%) | 18 (6.5) |
| Iron deficiency anaemia, n (%) | 31 (11.1) |
| Fibroid, n (%) | 6 (2.2) |
| Indications | Guideline | KSBL IOL According to Guideline |
|---|---|---|
| Missed deadline, n (%) | 41+0–42+0 | 56 (20.1) |
| PROM, n (%) | 37+0 | 46 (16.5) |
| GDM, n (%) | 40+0 | 26 (9.3) |
| Oligohydramnios, n (%) | 37+0 | 3 (1.1) |
| Polyhydramnios, n (%) | 38+0 with additional risks | 3 (1.1) |
| Intrahepatic cholestasis, n (%) | 37+0 | 5 (1.8) |
| Preeclampsia, n (%) | ≤37+0 | 9 (3.2) |
| HELLP syndrome, n (%) | 34+0 | 0 |
| Macrosomia, n (%) | 39+0 | 9 (3.2) |
| IUGR/SGA, n (%) | 26+0–38+0 | 9 (3.2) |
| Without medical reason, n (%) | ≥39+0 | 3 (1.1) |
| Not documented, n (%) | - | 4 (1.4) |
| Multiple indications, n (%) | Recommended | 105 (38) |
| Other Recommendations | Guideline | KSBL IOL According to Guideline |
|---|---|---|
| Amniotomy alone, n (%) | Not recommended | 0 |
| Balloon catheter + Oxytocin, n (%) | Recommended | 10 (3.6) |
| Balloon catheter + PGE1, n (%) | Recommended | 0 |
| Balloon catheter + PGE2, n (%) | Recommended | 0 |
| PGE2 alone, n (%) | Recommended alone | 102 (36.7) |
| PGE1 + Oxytocin, n (%) | Recommended | 24 (8.6) |
| PGE2 + PGE1, n (%) | Recommended | 9 (3.2) |
| Use of multiple IOL methods, n (%) | Recommended | 133 (47.8) |
| CTG monitoring, n (%) | Recommended | 278 (100) |
| Education about the procedure, n (%) | Recommended | 278 (100) |
| Variables | Total | Successful IOL | p-Value | |
|---|---|---|---|---|
| Yes | No | |||
| 278 (100) | 197 (70.9) | 81 (29.1) | ||
| Maternal age | 0.956 | |||
| <35, n (%) | 198 (71.2) | 141 (71.2) | 57 (28.8) | |
| ≥35, n (%) | 80 (28.8) | 56 (70.0) | 24 (30.0) | |
| Parity | 0.001 * | |||
| Primiparous, n (%) | 157 (56.5) | 98 (62.4) | 59 (37.6) | |
| Multiparous, n (%) | 121 (43.5) | 99 (81.8) | 22 (18.2) | |
| Bishop score | 0.645 | |||
| Documented (unfavourable), n (%) | 114 (41) | 83 (72.8) | 31 (27.2) | |
| Undocumented, n (%) | 164 (59) | 114 (69.5) | 50 (30.5) | |
| GA (weeks) | 0.511 ± | |||
| <37, n (%) | 6 (2.2) | 4 (66.7) | 2 (33.3) | |
| 37–41, n (%) | 261 (93.9) | 187 (71.6) | 74 (28.4) | |
| ≥42, n (%) | 11 (4) | 6 (54.5) | 5 (45.5) | |
| Birth weight (g) | 0.839 ± | |||
| <2500, n (%) | 12 (4.3) | 8 (66.7) | 4 (33.3) | |
| 2500–4000, n (%) | 217 (78.1) | 153 (70.5) | 64 (29.5) | |
| >4000, n (%) | 49 (17.6) | 36 (73.5) | 13 (26.5) | |
| Risks | ||||
| No GBS, n (%) | 239 (86) | 173 (72.4) | 66 (27.6) | 0.233 |
| GBS, n (%) | 39 (14) | 24 (61.5) | 15 (38.5) | |
| Non-obese, n (%) | 241 (86.7) | 177 (73.4) | 64 (26.6) | 0.026 * |
| Obesity, n (%) | 37 (13.3) | 20 (54.1) | 17 (45.9) | |
| No preeclampsia, n (%) | 260 (93.5) | 187 (71.9) | 73 (28.1) | 0.226 |
| Preeclampsia, n (%) | 18 (6.5) | 10 (55.6) | 8 (44.4) | |
| NO PROM, n (%) | 219 (78.8) | 154 (70.3) | 65 (29.7) | 0.824 |
| PROM, n (%) | 59 (21.2) | 43 (72.9) | 16 (27.1) | |
| No GDM, n (%) | 226 (81.3) | 157 (69.5) | 69 (30.5) | 0.37 |
| GDM, n (%) | 52 (18.7) | 40 (76.9) | 12 (23.1) | |
| No IUGR, n (%) | 254 (91.4) | 179 (70.5) | 75 (29.5) | 0.817 |
| IUGR, n (%) | 24 (8.6) | 18 (75.0) | 6 (25.0) | |
| Outcomes of Induced Deliveries | |
|---|---|
| Mode of delivery | |
| Vaginal, n (%) | 197 (70.9) |
| Caesarean section, n (%) | 81 (29.1) |
| Birth injuries, n (%) | 152 (54.7) |
| Episiotomy, n (%) | 31 (11.2) |
| Vaginal tear, n (%) | 74 (26.6) |
| Perineal tear, n (%) | 80 (28.8) |
| Labia tear, n (%) | 44 (15.8) |
| Paraurethral tear, n (%) | 2 (0.7) |
| Cervical tear, n (%) | 1 (0.4) |
| Postpartum haemorrhage | 266 (95.7) a |
| ≤500 mL, n (%) | 184 (66.2) |
| >500 mL, n (%) | 58 (20.9) |
| ≥1000 mL, n (%) | 24 (8.6) |
| Infections | 25 (9) |
| Amniotic infection syndrome, n (%) | 13 (4.6) |
| Genital warts, n (%) | 1 (0.4) |
| Candidiasis, n (%) | 2 (0.7) |
| Chlamydia, n (%) | 1 (0.4) |
| Herpes, n (%) | 1 (0.4) |
| Active HBV, HCV, HEV, n (%) | 7 (2.5) |
| Apgar at 1 min | |
| ≤3, n (%) | 13 (4.7) |
| 4–6, n (%) | 16 (5.8) |
| ≥7, n (%) | 249 (89.5) |
| Apgar at 5 min | |
| ≤3, n (%) | 3 (1.1) |
| 4–6, n (%) | 7 (2.5) |
| ≥7, n (%) | 268 (96.4) |
| Umbilical cord artery pH | 215 (77.3) b |
| 7.20–7.40, n (%) | 168 (60.4) |
| <7.20, n (%) | 45 (16.2) |
| >7.40, n (%) | 2 (0.7) |
| Resuscitation, n (%) | 12 (4.3) |
| Livebirth, n (%) | 272 (98) c |
| Abortion induction (14–26 GW), n (%) | 2 (0.7) |
| Birth weight | |
| SGA (1500–2500), n (%) | 12 (4.3) |
| Normal birth weight (2500–4000), n (%) | 217 (78.1) |
| LGA (>4000), n (%) | 49 (17.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbata, M.K.; Boesing, M.; Lüthi-Corridori, G.; Jaun, F.; Vetter, G.; Gröbli-Stäheli, J.; Leuppi-Taegtmeyer, A.B.; Frey Tirri, B.; Leuppi, J.D. The Correct Indication to Induce Labour in a Swiss Cantonal Hospital. J. Clin. Med. 2023, 12, 6515. https://doi.org/10.3390/jcm12206515
Mbata MK, Boesing M, Lüthi-Corridori G, Jaun F, Vetter G, Gröbli-Stäheli J, Leuppi-Taegtmeyer AB, Frey Tirri B, Leuppi JD. The Correct Indication to Induce Labour in a Swiss Cantonal Hospital. Journal of Clinical Medicine. 2023; 12(20):6515. https://doi.org/10.3390/jcm12206515
Chicago/Turabian StyleMbata, Munachimso Kizito, Maria Boesing, Giorgia Lüthi-Corridori, Fabienne Jaun, Grit Vetter, Jeanette Gröbli-Stäheli, Anne B. Leuppi-Taegtmeyer, Brigitte Frey Tirri, and Jörg D. Leuppi. 2023. "The Correct Indication to Induce Labour in a Swiss Cantonal Hospital" Journal of Clinical Medicine 12, no. 20: 6515. https://doi.org/10.3390/jcm12206515
APA StyleMbata, M. K., Boesing, M., Lüthi-Corridori, G., Jaun, F., Vetter, G., Gröbli-Stäheli, J., Leuppi-Taegtmeyer, A. B., Frey Tirri, B., & Leuppi, J. D. (2023). The Correct Indication to Induce Labour in a Swiss Cantonal Hospital. Journal of Clinical Medicine, 12(20), 6515. https://doi.org/10.3390/jcm12206515





