Predictors of Atrial Fibrillation in Patients with Embolic Stroke of Unknown Etiology and Implantable Loop Recorders—Further Insights of the TRACK AF Study on the Role of ECG and Echocardiography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
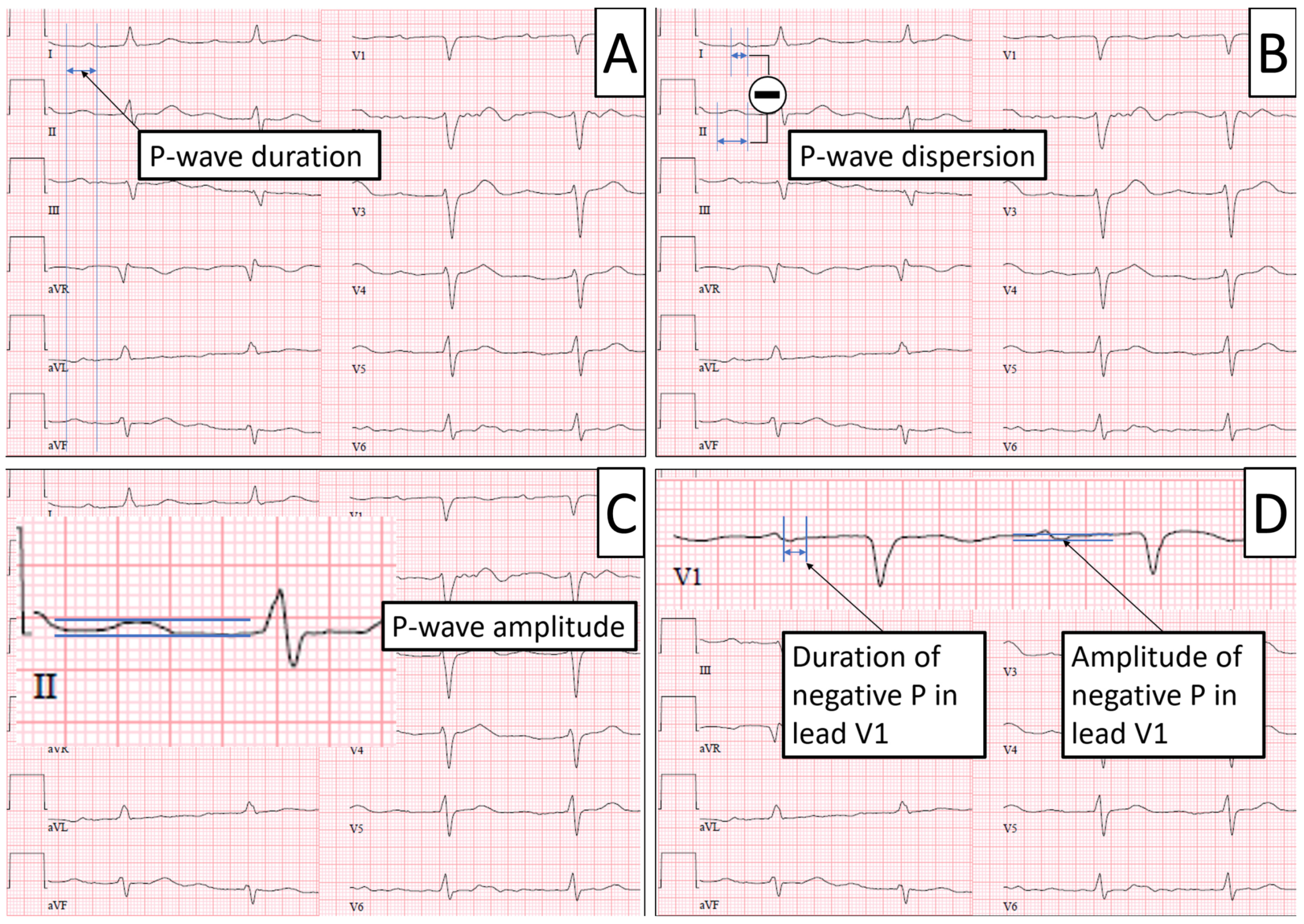
2.2. ECG Parameters
2.3. Echocardiographic Parameters
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Univariate Analysis
3.2.1. Baseline Parameters
3.2.2. Echocardiographic Parameters
3.2.3. ECG Parameters
3.3. Multivariate Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziegler, P.D.; Koehler, J.L.; Mehra, R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm 2006, 3, 1445–1452. [Google Scholar] [CrossRef]
- Buck, B.H.; Hill, M.D.; Quinn, F.R.; Butcher, K.S.; Menon, B.K.; Gulamhusein, S.; Siddiqui, M.; Coutts, S.B.; Jeerakathil, T.; Smith, E.E.; et al. Effect of Implantable vs Prolonged External Electrocardiographic Monitoring on Atrial Fibrillation Detection in Patients with Ischemic Stroke: The PER DIEM Randomized Clinical Trial. JAMA 2021, 325, 2160–2168. [Google Scholar] [CrossRef]
- Cotter, P.E.; Martin, P.J.; Ring, L.; Warburton, E.A.; Belham, M.; Pugh, P.J. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013, 80, 1546–1550. [Google Scholar] [CrossRef]
- Reinke, F.; Bettin, M.; Ross, L.S.; Kochhäuser, S.; Kleffner, I.; Ritter, M.; Minnerup, J.; Dechering, D.; Eckardt, L.; Dittrich, R. Refinement of detecting atrial fibrillation in stroke patients: Results from the TRACK-AF Study. Eur. J. Neurol. 2018, 25, 631–636. [Google Scholar] [CrossRef]
- Brachmann, J.; Morillo, C.A.; Sanna, T.; Di Lazzaro, V.; Diener, H.-C.; Bernstein, R.A.; Rymer, M.; Ziegler, P.D.; Liu, S.; Passman, R.S. Uncovering Atrial Fibrillation Beyond Short-Term Monitoring in Cryptogenic Stroke Patients: Three-Year Results from the Cryptogenic Stroke and Underlying Atrial Fibrillation Trial. Circ. Arrhythm. Electrophysiol. 2016, 9, e003333. [Google Scholar] [CrossRef]
- Sposato, L.A.; Cipriano, L.E.; Saposnik, G.; Ruíz Vargas, E.; Riccio, P.M.; Hachinski, V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 377–387. [Google Scholar] [CrossRef]
- Svendsen, J.H.; Diederichsen, S.Z.; Højberg, S.; Krieger, D.W.; Graff, C.; Kronborg, C.; Olesen, M.S.; Nielsen, J.B.; Holst, A.G.; Brandes, A.; et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): A randomised controlled trial. Lancet 2021, 398, 1507–1516. [Google Scholar] [CrossRef]
- Hart, R.G.; Sharma, M.; Mundl, H.; Kasner, S.E.; Bangdiwala, S.I.; Berkowitz, S.D.; Swaminathan, B.; Lavados, P.; Wang, Y.; Wang, Y.; et al. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. N. Engl. J. Med. 2018, 378, 2191–2201. [Google Scholar] [CrossRef]
- Diener, H.-C.; Sacco, R.L.; Easton, J.D.; Granger, C.B.; Bernstein, R.A.; Uchiyama, S.; Kreuzer, J.; Cronin, L.; Cotton, D.; Grauer, C.; et al. Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source. N. Engl. J. Med. 2019, 380, 1906–1917. [Google Scholar] [CrossRef]
- Edhouse, J.; Thakur, R.K.; Khalil, J.M. ABC of clinical electrocardiography. Conditions affecting the left side of the heart. BMJ 2002, 324, 1264–1267. [Google Scholar] [CrossRef]
- Dilaveris, P.E.; Gialafos, E.J.; Sideris, S.K.; Theopistou, A.M.; Andrikopoulos, G.K.; Kyriakidis, M.; Gialafos, J.E.; Toutouzas, P.K. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am. Heart J. 1998, 135, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-M.; Lee, S.-H.; Lu, M.-J.; Lin, C.-H.; Chao, H.-H.; Cheng, J.-J.; Kuan, P.; Hung, C.-R. The role of P wave in prediction of atrial fibrillation after coronary artery surgery. Int. J. Cardiol. 1999, 68, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Kühl, J.T.; Pietersen, A.; Graff, C.; Lind, B.; Struijk, J.J.; Olesen, M.S.; Sinner, M.F.; Bachmann, T.N.; Haunsø, S.; et al. P-wave duration and the risk of atrial fibrillation: Results from the Copenhagen ECG Study. Heart Rhythm 2015, 12, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Sugiyama, Y.; Ohara, N.; Ikegami, T.; Watanabe, K.; Kobayashi, J.; Takahashi, D. P-Wave Terminal Force in Lead V 1 Predicts Paroxysmal Atrial Fibrillation in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 1912–1915. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, S.M.; Larson, M.G.; Benjamin, E.J.; Levy, D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994, 89, 724–730. [Google Scholar] [CrossRef]
- Mont, L.; Tamborero, D.; Elosua, R.; Molina, I.; Coll-Vinent, B.; Sitges, M.; Vidal, B.; Scalise, A.; Tejeira, A.; Berruezo, A.; et al. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace 2008, 10, 15–20. [Google Scholar] [CrossRef]
- Ritter, M.A.; Kochhäuser, S.; Duning, T.; Reinke, F.; Pott, C.; Dechering, D.G.; Eckardt, L.; Ringelstein, E.B. Occult atrial fibrillation in cryptogenic stroke: Detection by 7-day electrocardiogram versus implantable cardiac monitors. Stroke 2013, 44, 1449–1452. [Google Scholar] [CrossRef]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Hindricks, G.; Pokushalov, E.; Urban, L.; Taborsky, M.; Kuck, K.-H.; Lebedev, D.; Rieger, G.; Pürerfellner, H.; XPECT Trial Investigators. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: Results of the XPECT trial. Circ. Arrhythm. Electrophysiol. 2010, 3, 141–147. [Google Scholar] [CrossRef]
- Kochhäuser, S.; Dechering, D.G.; Dittrich, R.; Reinke, F.; Ritter, M.A.; Ramtin, S.; Duning, T.; Frommeyer, G.; Eckardt, L. Supraventricular premature beats and short atrial runs predict atrial fibrillation in continuously monitored patients with cryptogenic stroke. Stroke 2014, 45, 884–886. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Dorian, P.; Spring, M.; Panzov, V.; Mamdani, M.; Healey, J.S.; Thorpe, K.E.; EMBRACE Steering Committee and Investigators. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: Results from the EMBRACE trial. Stroke 2015, 46, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef]
- Kirchhof, P.; Toennis, T.; Goette, A.; Camm, A.J.; Diener, H.C.; Becher, N.; Bertaglia, E.; Blomstrom Lundqvist, C.; Borlich, M.; Brandes, A.; et al. Anticoagulation with Edoxaban in Patients with Atrial High-Rate Episodes. N. Engl. J. Med. 2023, NEJMoa2303062. [Google Scholar] [CrossRef]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic Therapy to Prevent Stroke in Patients Who Have Nonvalvular Atrial Fibrillation. Ann. Intern. Med. 2007, 146, 857. [Google Scholar] [CrossRef]
| Characteristics | All (n = 104) | Atrial Fibrillation (n = 20) | No Atrial Fibrillation (n = 84) | p-Value |
|---|---|---|---|---|
| Age, years, median (IQR) | 63 (52–74) | 72 (67–75) | 60 (52–72) | 0.001 |
| Female, n (%) | 45 (43.3) | 10 (53) | 35 (41) | 0.62 |
| Body mass index, kg/m2, median (IQR) | 27 (24–29) | 26 (24–29) | 27 (24–29) | 0.89 |
| CHA2DS2-Vasc Score (IQR) | 4 (3–6) | 5 (4–6) | 4 (3–5) | 0.02 |
| Arterial hypertension, n (%) | 77 (74) | 16 (84) | 61 (72) | 0.39 |
| Diabetes, n (%) | 24 (23) | 4 (21) | 20 (24) | 0.82 |
| Congestive heart failure, n (%) | 4 (4) | 2 (11) | 2 (2) | 0.15 |
| Vascular disease, n (%) | 17 (16) | 3 (16) | 14 (17) | 0.94 |
| Characteristics | All (n = 104) | Atrial Fibrillation (n = 20) | No Atrial Fibrillation (n = 84) | p-Value |
|---|---|---|---|---|
| ECG parameters | ||||
| P-Wave duration, ms, median (IQR) | 110 (100–120) | 110 (100–120) | 110 (100–120) | 0.68 |
| P-Wave dispersion, ms, median (IQR) | 20 (10–30) | 10 (10–20) | 20 (10–30) | 0.08 |
| P-Wave amplitude, mV, median (IQR) | 0.1 (0.08–0.12) | 0.1 (0.08–0.12) | 0.08 (0.08–0.12) | 0.76 |
| Dur. neg. P in V1, ms, median (IQR) | 60 (60–80) | 60 (50–70) | 60 (60–80) | 0.10 |
| Amp. neg. P in V1, mV, median (IQR) | 0.1 (0.05–0.1) | 0.1 (0.05–0.1) | 0.1 (0.05–0.1) | 0.42 |
| P wave biphasic in II, n (%) | 11 (10) | 2 (13) | 9 (10.1) | 0.97 |
| Premature atrial contractions present, n (%) | 10 (10) | 8 (40) | 12 (2) | <0.001 |
| Echocardiographic parameters | ||||
| LA diameter plax, mm, median (IQR) | 38 (35–41) | 39 (36–42) | 38 (35–41) | 0.15 |
| LA area ap4, cm2, median (IQR) | 18 (17–22) | 18 (16–24) | 18 (17–22) | 0.33 |
| LA area ap2, cm2, median (IQR) | 19 (16–22) | 20 (17–23) | 19 (16–22) | 0.17 |
| LA length, mm, median (IQR) | 52 (48–55) | 52 (47–57) | 52 (48–55) | 0.45 |
| LA volume, mL, median (IQR) | 56 (48–75) | 58 (49–80) | 56 (47–75) | 0.20 |
| LA volume index, mL/m2, median (IQR) | 29 (25–37) | 31 (24–36) | 29 (25–37) | 0.09 |
| Characteristics | Multivariate p-Value | Exp (β) |
|---|---|---|
| Baseline characteristics | ||
| Age | 0.01 | 1.09 |
| Sex | 0.48 | 1.56 |
| CHA2DS2-Vasc Score | 0.34 | 0.75 |
| Congestive heart failure | 0.33 | 4.30 |
| ECG parameters | ||
| Premature atrial contractions present, n (%) | <0.001 | 27.83 |
| Echocardiographic parameters | ||
| LA volume index | 0.74 | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höwel, D.; Leitz, P.; Frommeyer, G.; Ritter, M.A.; Reinke, F.; Füting, A.; Reinsch, N.; Eckardt, L.; Kochhäuser, S.; Dechering, D.G. Predictors of Atrial Fibrillation in Patients with Embolic Stroke of Unknown Etiology and Implantable Loop Recorders—Further Insights of the TRACK AF Study on the Role of ECG and Echocardiography. J. Clin. Med. 2023, 12, 6566. https://doi.org/10.3390/jcm12206566
Höwel D, Leitz P, Frommeyer G, Ritter MA, Reinke F, Füting A, Reinsch N, Eckardt L, Kochhäuser S, Dechering DG. Predictors of Atrial Fibrillation in Patients with Embolic Stroke of Unknown Etiology and Implantable Loop Recorders—Further Insights of the TRACK AF Study on the Role of ECG and Echocardiography. Journal of Clinical Medicine. 2023; 12(20):6566. https://doi.org/10.3390/jcm12206566
Chicago/Turabian StyleHöwel, Dennis, Patrick Leitz, Gerrit Frommeyer, Martin A. Ritter, Florian Reinke, Anna Füting, Nico Reinsch, Lars Eckardt, Simon Kochhäuser, and Dirk G. Dechering. 2023. "Predictors of Atrial Fibrillation in Patients with Embolic Stroke of Unknown Etiology and Implantable Loop Recorders—Further Insights of the TRACK AF Study on the Role of ECG and Echocardiography" Journal of Clinical Medicine 12, no. 20: 6566. https://doi.org/10.3390/jcm12206566
APA StyleHöwel, D., Leitz, P., Frommeyer, G., Ritter, M. A., Reinke, F., Füting, A., Reinsch, N., Eckardt, L., Kochhäuser, S., & Dechering, D. G. (2023). Predictors of Atrial Fibrillation in Patients with Embolic Stroke of Unknown Etiology and Implantable Loop Recorders—Further Insights of the TRACK AF Study on the Role of ECG and Echocardiography. Journal of Clinical Medicine, 12(20), 6566. https://doi.org/10.3390/jcm12206566






