Association between Acute Respiratory Distress Syndrome Due to COVID-19 and Long-Term Sleep and Circadian Sleep–Wake Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Baseline Characteristics
2.2. Sleep Questionnaires
2.3. Home Sleep Apnea Test (HSAT)
2.4. Actigraphy
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics Four Months after Medical Discharge
3.2. Sleep and Circadian Rest–Activity Rhythm 12 Months after Hospital Discharge
3.3. Evolution of Sleep and Circadian Rest–Activity Rhythm between Four and Twelve Months after Hospital Discharge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Clinical Management of COVID-19: Living Guideline, 15 September 2022; World Health Organization: Geneva, Switzerland, 2022; Available online: https://iris.who.int/handle/10665/362783 (accessed on 18 September 2023).
- Azevedo, R.B.; Botelho, B.G.; de Hollanda, J.V.G.; Ferreira, L.V.L.; de Andrade, L.Z.J.; Oei, S.S.M.L.; Mello, T.d.S.; Muxfeldt, E.S. COVID-19 and the cardiovascular system: A comprehensive review. J. Hum. Hypertens. 2021, 35, 4–11. [Google Scholar] [CrossRef]
- La Via, L.; Dezio, V.; Santonocito, C.; Astuto, M.; Morelli, A.; Huang, S.; Vieillard-Baron, A.; Sanfilippo, F. Full and simplified assessment of left ventricular diastolic function in COVID-19 patients admitted to ICU: Feasibility, incidence, and association with mortality. Echocardiography 2022, 39, 1391–1400. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Martucci, G.; La Via, L.; Cuttone, G.; Dimarco, G.; Pulizzi, C.; Arcadipane, A.; Astuto, M. Hemoperfusion and blood purification strategies in patients with COVID-19: A systematic review. Artif. Organs 2021, 45, 1466–1476. [Google Scholar] [CrossRef]
- Karimi Shahri, M.; Niazkar, H.R.; Rad, F. COVID-19 and hematology findings based on the current evidences: A puzzle with many missing pieces. Int. J. Lab. Hematol. 2021, 43, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Karadağ, A.S.; Rowland-Payne, C.; Chiriac, A.; Lotti, T. Cutaneous signs in COVID-19 patients: A review. Dermatol. Ther. 2020, 33, e13549. [Google Scholar] [CrossRef]
- Hanson, S.W.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. A global systematic analysis of the occurrence, severity, and recovery pattern of long COVID in 2020 and 2021. medRxiv Prepr. Serv. Health Sci. 2022. [Google Scholar] [CrossRef]
- Yang, P.-L.; Chaytor, N.S.; Burr, R.L.; Kapur, V.K.; McCurry, S.M.; Vitiello, M.V.; Hough, C.L.; Parsons, E.C. Rest-Activity Rhythm Fragmentation and Weaker Circadian Strength Are Associated With Cognitive Impairment in Survivors of Acute Respiratory Failure. Biol. Res. Nurs. 2023, 25, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Beltrán, M.; Labarca, G.; Cigarroa, I.; Enos, D.; Lastra, J.; Nova-Lamperti, E.; Targa, A.; Barbe, F. Sleep health and the circadian rest-activity pattern four months after COVID-19. J. Bras. Pneumol. publicacao Of. da Soc. Bras. Pneumol. e Tisilogia 2022, 48, e20210398. [Google Scholar] [CrossRef] [PubMed]
- Benítez, I.D.; Moncusí-Moix, A.; Vaca, R.; Gort-Paniello, C.; Minguez, O.; Santisteve, S.; Carmona, P.; Torres, G.; Fagotti, J.; Labarca, G.; et al. Sleep and Circadian Health of Critical COVID-19 Survivors 3 Months After Hospital Discharge. Crit. Care Med. 2022, 50, 945–954. [Google Scholar] [CrossRef]
- Labarca, G.; Henriquez-beltran, M.; Llerena, F.; Erices, G.; Ormazabal, V.; Riffo, B.; Rubilar, D.; Sanhueza, R.; Vasquez, J.; Jorquera, J.; et al. Undiagnosed sleep disorder breathing as a risk factor for critical COVID-19 and pulmonary consequences at the midterm follow-up. Sleep Med. 2022, 91, 196–204. [Google Scholar] [CrossRef]
- Yang, P.L.; Ward, T.M.; Burr, R.L.; Kapur, V.K.; McCurry, S.M.; Vitiello, M.V.; Hough, C.L.; Parsons, E.C. Sleep and Circadian Rhythms in Survivors of Acute Respiratory Failure. Front. Neurol. 2020, 11, 94. [Google Scholar] [CrossRef]
- Goyal, A.; Saxena, K.; Kar, A.; Khurana, A.; Bhagtana, P.K.; Sridevi, C.S.K.R.; Pakhare, A. Obstructive sleep apnea is highly prevalent in COVID-19 related moderate to severe ARDS survivors: Findings of level I polysomnography in a tertiary care hospital. Sleep Med. 2022, 91, 226–230. [Google Scholar] [CrossRef]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.-M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Cai, J.; Lin, K.; Zhang, H.; Xue, Q.; Zhu, K.; Yuan, G.; Sun, Y.; Zhu, F.; Ai, J.; Wang, S.; et al. A one-year follow-up study of systematic impact of long COVID symptoms among patients post SARS-CoV-2 omicron variants infection in Shanghai, China. Emerg. Microbes Infect. 2023, 12, 2220578. [Google Scholar] [CrossRef]
- Bocchino, M.; Rea, G.; Capitelli, L.; Lieto, R.; Bruzzese, D. Chest CT Lung Abnormalities 1 Year after COVID-19: A Systematic Review and Meta-Analysis. Radiology 2023, 308, e230535. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Benítez, I.D.; Carmona, P.; Santisteve, S.; Monge, A.; Moncusí-Moix, A.; Gort-Paniello, C.; Pinilla, L.; Carratalá, A.; Zuil, M.; et al. Pulmonary Function and Radiologic Features in Survivors of Critical COVID-19: A 3-Month Prospective Cohort. Chest 2021, 160, 187–198. [Google Scholar] [CrossRef]
- Solverson, K.J.; Easton, P.A.; Doig, C.J. Assessment of sleep quality post-hospital discharge in survivors of critical illness. Respir. Med. 2016, 114, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qiao, L.; Bao, M.; Chao, J. Prevalence of Sleep Disorders among Survivors of Severe COVID-19 Infections: A Meta-Analysis. Asia-Pac. J. Public Health 2023, 35, 204–206. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of COVID-19; World Health Organization: Geneva, Switzerland, 2020; p. 62. Available online: https://apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf?sequence=1&isAllowed=y (accessed on 12 December 2020).
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: An American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J. Clin. Sleep Med. 2019, 14, 1209–1230, Erratum in 2019, 15, 531. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.S.B.; Adamowicz, T.; Louzada, F.M.; Moreno, C.R.; Araujo, J.F. A fresh look at the use of nonparametric analysis in actimetry. Sleep Med. Rev. 2015, 20, 84–91. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- Kong, H.; Xu, L.-M.; Wang, D.-X. Perioperative neurocognitive disorders: A narrative review focusing on diagnosis, prevention, and treatment. CNS Neurosci. Ther. 2022, 28, 1147–1167. [Google Scholar] [CrossRef]
- Aiello, G.; Cuocina, M.; La Via, L.; Messina, S.; Attaguile, G.A.; Cantarella, G.; Sanfilippo, F.; Bernardini, R. Melatonin or Ramelteon for Delirium Prevention in the Intensive Care Unit: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 435. [Google Scholar] [CrossRef]
- LaHue, S.C.; Escueta, D.P.; Guterman, E.L.; Patel, K.; Harrison, K.L.; Boscardin, W.J.; Douglas, V.C.; Newman, J.C. COVID-19 severity and age increase the odds of delirium in hospitalized adults with confirmed SARS-CoV-2 infection: A cohort study. BMC Psychiatry 2022, 22, 151. [Google Scholar] [CrossRef] [PubMed]
- McKenna, H.; van der Horst, G.T.J.; Reiss, I.; Martin, D. Clinical chronobiology: A timely consideration in critical care medicine. Crit. Care 2018, 22, 124. [Google Scholar] [CrossRef] [PubMed]
- Dhooria, S.; Sehgal, I.S.; Agrawal, A.K.; Agarwal, R.; Aggarwal, A.N.; Behera, D. Sleep after critical illness: Study of survivors of acute respiratory distress syndrome and systematic review of literature. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2016, 20, 323–331. [Google Scholar] [CrossRef]
- Altman, M.T.; Knauert, M.P.; Pisani, M.A. Sleep Disturbance after Hospitalization and Critical Illness: A Systematic Review. Ann. Am. Thorac. Soc. 2017, 14, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Bolaki, M.; Alexopoulou, C.; Georgopoulos, D. Sleep quality in survivors of critical illness: Practical shortcomings resolved. Sleep Breath. 2019, 23, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Park, H.Y.; Song, I.-A. Occurrence and Long-Term Prognosis of Insomnia Disorder among Survivors of Acute Respiratory Distress Syndrome in South Korea. Ann. Am. Thorac. Soc. 2022, 19, 1022–1029. [Google Scholar] [CrossRef]
- Targa, A.D.S.; Benítez, I.D.; González, J.; Torres, G.; Santisteve, S.; Vaca, R.; Minguez, O.; Aguilà, M.; Carmona, P.; Moncusí-Moix, A.; et al. Sleep and circadian health 6 months after critical COVID-19 disease. Respirology 2022, 27, 1083–1088. [Google Scholar] [CrossRef]
- Caruana, N.; McKinley, S.; Elliott, R.; Gholizadeh, L. Sleep Quality During and After Cardiothoracic Intensive Care and Psychological Health During Recovery. J. Cardiovasc. Nurs. 2018, 33, E40–E49. [Google Scholar] [CrossRef]
- McKinley, S.; Fien, M.; Elliott, R.; Elliott, D. Sleep and psychological health during early recovery from critical illness: An observational study. J. Psychosom. Res. 2013, 75, 539–545. [Google Scholar] [CrossRef]
- McSharry, D.; Lam, M.T.; Malhotra, A. OSA as a probable risk factor for severe COVID-19. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2020, 16, 1649. [Google Scholar] [CrossRef]
- Sweetman, A.; Lack, L.; Bastien, C. Co-Morbid Insomnia and Sleep Apnea (COMISA): Prevalence, Consequences, Methodological Considerations, and Recent Randomized Controlled Trials. Brain Sci. 2019, 9, 371. [Google Scholar] [CrossRef]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional disability 5 years after acute respiratory distress syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef]
- van Zanten, A.R.H.; De Waele, E.; Wischmeyer, P.E. Nutrition therapy and critical illness: Practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit. Care 2019, 23, 368. [Google Scholar] [CrossRef] [PubMed]
- Ridley, E.J.; Parke, R.L.; Davies, A.R.; Bailey, M.; Hodgson, C.; Deane, A.M.; McGuinness, S.; Cooper, D.J. What Happens to Nutrition Intake in the Post-Intensive Care Unit Hospitalization Period? An Observational Cohort Study in Critically Ill Adults. JPEN. J. Parenter. Enteral Nutr. 2019, 43, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, B.B.; Suri, R.; Suchyta, M.R.; Digrande, K.F.; Sherwood, K.D.; Colantuoni, E.; Dinglas, V.D.; Needham, D.M.; Hopkins, R.O. Return to work after critical illness: A systematic review and meta-analysis. Thorax 2020, 75, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Caccioppola, A.; Froio, S.; Formenti, P.; De Giorgis, V.; Galanti, V.; Consonni, D.; Chiumello, D. Effect of mechanical power on intensive care mortality in ARDS patients. Crit. Care 2020, 24, 246. [Google Scholar] [CrossRef] [PubMed]
- Merriweather, J.L.; Salisbury, L.G.; Walsh, T.S.; Smith, P. Nutritional care after critical illness: A qualitative study of patients’ experiences. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2016, 29, 127–136. [Google Scholar] [CrossRef]
- Herridge, M.S.; Cheung, A.M.; Tansey, C.M.; Matte-Martyn, A.; Diaz-Granados, N.; Al-Saidi, F.; Cooper, A.B.; Guest, C.B.; Mazer, C.D.; Mehta, S.; et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 2003, 348, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, A.; Reynolds, A.; Lack, L.C. Circadian factors in comorbid insomnia and sleep apnea. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2021, 17, 1959–1960. [Google Scholar] [CrossRef]
- Papagerakis, S.; Said, R.; Ketabat, F.; Mahmood, R.; Pundir, M.; Lobanova, L.; Guenther, G.; Pannone, G.; Lavender, K.; McAlpin, B.R.; et al. When the clock ticks wrong with COVID-19. Clin. Transl. Med. 2022, 12, e949. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Wu, Q.; Wang, X.; Qin, Z.; Wang, Y.; He, Y.; Wu, Q.; Li, L.; Chen, H. Plasma proteomic and metabolomic characterization of COVID-19 survivors 6 months after discharge. Cell Death Dis. 2022, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Musheyev, B.; Boparai, M.S.; Kimura, R.; Janowicz, R.; Pamlanye, S.; Hou, W.; Duong, T.Q. Longitudinal medical subspecialty follow-up of critically and non-critically ill hospitalized COVID-19 survivors up to 24 months after discharge. Intern. Emerg. Med. 2023, 18, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Curci, C.; Pisano, F.; Bonacci, E.; Camozzi, D.M.; Ceravolo, C.; Bergonzi, R.; De Franceschi, S.; Moro, P.; Guarnieri, R.; Ferrillo, M.; et al. Early rehabilitation in post-acute COVID-19 patients: Data from an Italian COVID-19 Rehabilitation Unit and proposal of a treatment protocol. Eur. J. Phys. Rehabil. Med. 2020, 56, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Fugazzaro, S.; Contri, A.; Esseroukh, O.; Kaleci, S.; Croci, S.; Massari, M.; Facciolongo, N.C.; Besutti, G.; Iori, M.; Salvarani, C.; et al. Rehabilitation Interventions for Post-Acute COVID-19 Syndrome: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5185. [Google Scholar] [CrossRef] [PubMed]
- Dillen, H.; Bekkering, G.; Gijsbers, S.; Vande Weygaerde, Y.; Van Herck, M.; Haesevoets, S.; Bos, D.A.G.; Li, A.; Janssens, W.; Gosselink, R.; et al. Clinical effectiveness of rehabilitation in ambulatory care for patients with persisting symptoms after COVID-19: A systematic review. BMC Infect. Dis. 2023, 23, 419. [Google Scholar] [CrossRef] [PubMed]

| Global | Non-ARDS | ARDS | ||
|---|---|---|---|---|
| (N = 52) | (N = 21) | (N = 31) | ||
| Median [p25;p75] or n(%) | Median [p25;p75] or n(%) | Median [p25;p75] or n(%) | p-Value | |
| Baseline characteristics | ||||
| Age, years | 49.0 [39.0;57.2] | 41.0 [34.0;49.0] | 54.0 [45.5;59.0] | 0.010 |
| Male | 28 (53.8%) | 7 (33.3%) | 21 (67.7%) | 0.031 |
| Smoking history | 0.094 | |||
| Non-smoker | 32 (61.5%) | 14 (66.7%) | 18 (58.1%) | |
| Current | 8 (15.4%) | 5 (23.8%) | 3 (9.68%) | |
| Former | 12 (23.1%) | 2 (9.52%) | 10 (32.3%) | |
| Alcohol | 0.481 | |||
| No | 22 (42.3%) | 8 (38.1%) | 14 (45.2%) | |
| Usually, | 28 (53.8%) | 13 (61.9%) | 15 (48.4%) | |
| Frequent | 2 (3.85%) | 0 (0.00%) | 2 (6.45%) | |
| BMI | 30.4 [28.0;33.5] | 29.0 [27.9;32.0] | 31.4 [28.7;35.4] | 0.088 |
| Neck circumference | 41.5 [39.0;45.2] | 39.0 [37.0;43.0] | 42.0 [41.0;46.0] | 0.011 |
| Years of education | 0.020 | |||
| <8 years | 21 (40.4%) | 4 (19.0%) | 17 (54.8%) | |
| 8–12 years | 10 (19.2%) | 4 (19.0%) | 6 (19.4%) | |
| >12 years | 21 (40.4%) | 13 (61.9%) | 8 (25.8%) | |
| Place of residence, rural area | 6 (11.5%) | 1 (4.76%) | 5 (16.1%) | 0.382 |
| Comorbidities | ||||
| Hypertension | 20 (38.5%) | 6 (28.6%) | 14 (45.2%) | 0.360 |
| Diabetes mellitus, | 7 (13.5%) | 4 (19.0%) | 3 (9.68%) | 0.420 |
| Insulin resistance | 11 (21.2%) | 1 (4.76%) | 10 (32.3%) | 0.034 |
| Global (N = 52) Mean ± SD or n (%) | Non-ARDS (N = 21) Mean ± SD or n (%) | ARDS (N = 31) Mean ± SD or n (%) | N | Unadjusted OR (95% CI) or Mean Difference (95%CI) | p-Value | Adjusted OR (95%CI) or Mean Difference (95%CI) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Sleep | ||||||||
| PSQI | 42 (91.3%) * | 10.0 (3.34) | 9.75 (4.64) | 46 | −0.25 (−2.73 to 2.23) | 0.84 | 1.13 (−2.16 to 4.42) | 0.50 |
| ESS | 23 (50.0%) * | 10.9 (7.09) | 10.8 (6.68) | 46 | −0.14 (−4.19 to 3.91) | 0.94 | 1.30 (−4.09 to 6.69) | 0.63 |
| ISI | 27 (58.7%) * | 10.0 (5.64) | 10.4 (7.74) | 46 | 0.43 (−3.72 to 4.57) | 0.84 | 2.51 (−3.06 to 8.08) | 0.38 |
| TST, (min) | 428 ± 92.1 | 454 (69.7) | 410 (101) | 46 | −44.08 (−97.69 to 9.53) | 0.11 | −55.01 (−128.09 to 18.06) | 0.14 |
| SE | 87.4% (5.15) | 86.8 (3.29) | 87.7 (6.09) | 46 | 0.84 (−2.23 to 3.92) | 0.59 | 0.30 (−3.71 to 4.30) | 0.88 |
| Circadian rhythms | ||||||||
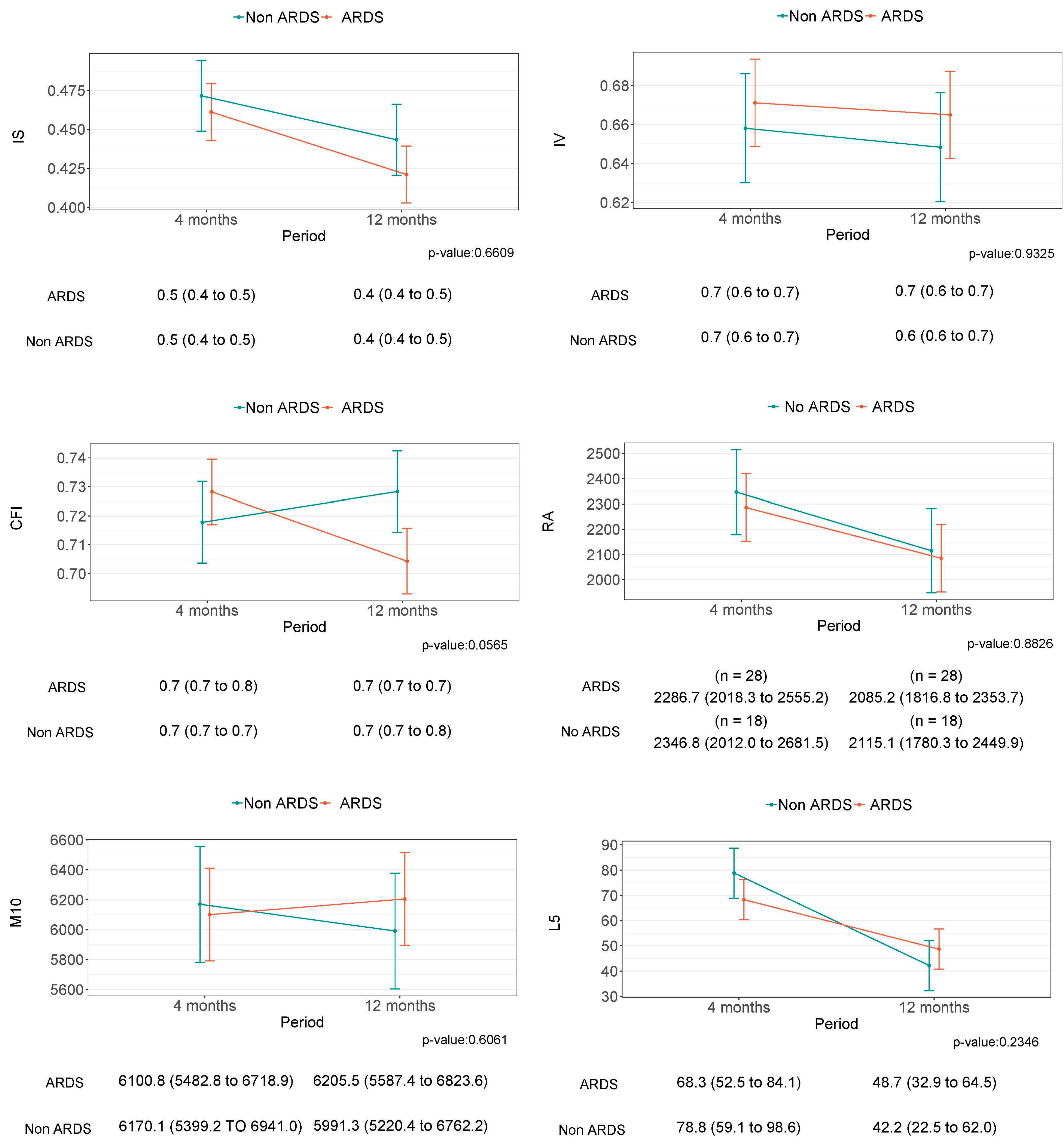
| IS | 0.43 (0.11) | 0.44 (0.10) | 0.42 (0.11) | 46 | −0.02 (−0.09 to 0.04) | 0.49 | −0.06 (−0.14 to 0.02) | 0.15 |
| IV | 0.66 (0.14) | 0.65 (0.12) | 0.66 (0.15) | 46 | 0.02 (−0.06 to 0.10) | 0.68 | 0.00 (−0.11 to 0.10) | 0.95 |
| CFI | 0.71 (0.07) | 0.73 (0.06) | 0.70 (0.07) | 46 | −0.02 (−0.06 to 0.02) | 0.23 | −0.04 (−0.09 to 0.01) | 0.15 |
| RA | 0.98 (0.02) | 0.98 (0.01) | 0.98 (0.02) | 46 | 0.00 (−0.01 to 0.01) | 0.54 | 0.00 (−0.01 to 0.02) | 0.60 |
| M10 | 6122 (1813) | 5991 (1681) | 6206 (1918) | 46 | 214.22 (−869.34 to 1297.78) | 0.70 | −40.73 (−1486.69 to 1405.23) | 0.95 |
| L5 | 46.2 (44.4) | 42.2 (38.1) | 48.7 (48.6) | 46 | 6.49 (−20.06 to 33.04) | 0.63 | −9.76 (−44.35 to 24.83) | 0.58 |
| Sleep Apnea | ||||||||
| RDI | 14.7 (15.8) | 5.57 (7.31) | 20.2 (17.1) | 45 | 14.61 (6.00 to 23.21) | 0.00 | 1.83 (−8.02 to 11.69) | 0.71 |
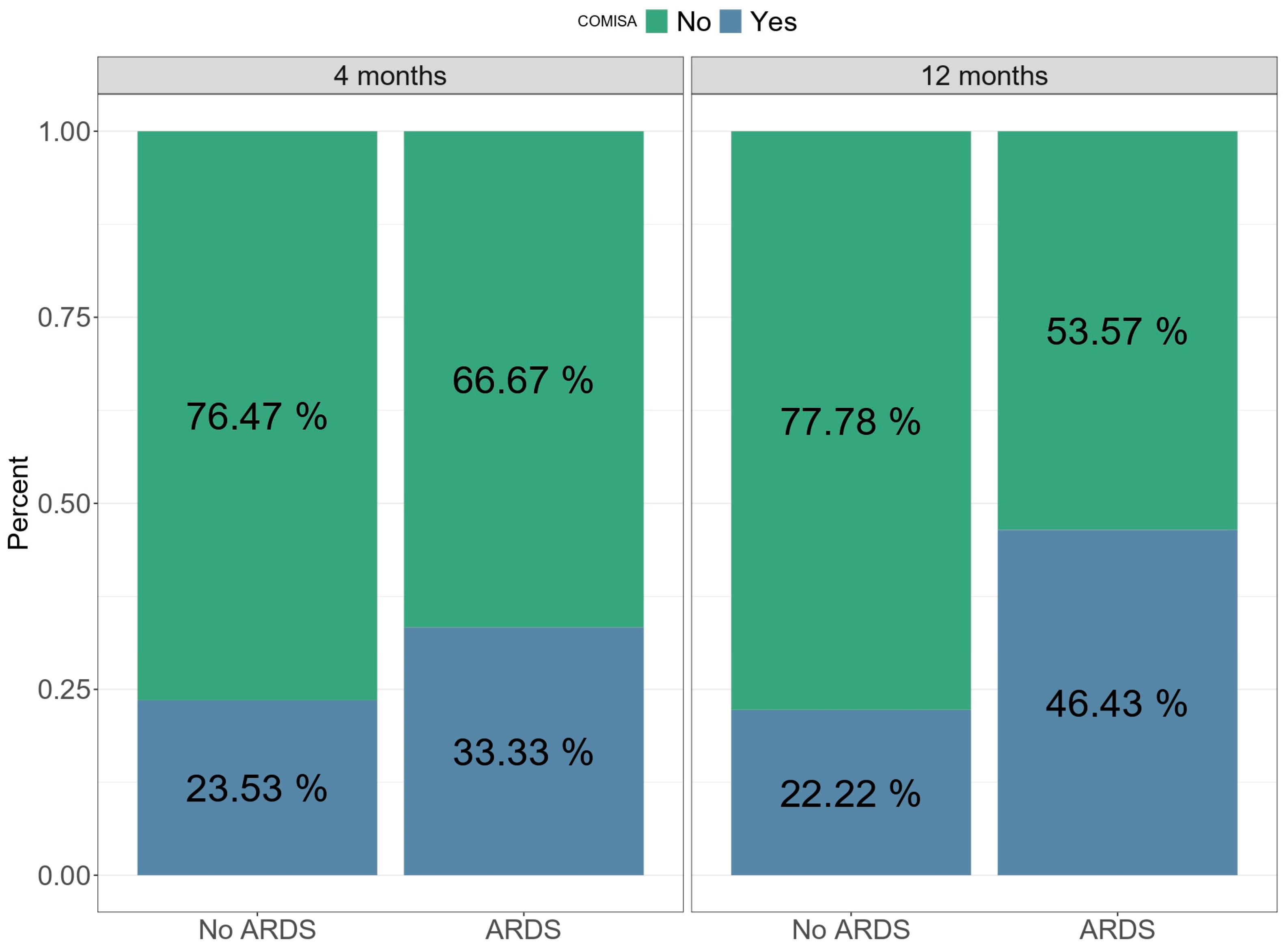
| Comisa | 17 (37.0%) | 4 (22.2%) | 13 (46.4%) | 46 | 3.03 (0.80 to 11.54) | 0.10 | 2.37 (0.43 to 13.06) | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henríquez-Beltrán, M.; Benítez, I.; Belmonte, T.; Jorquera, J.; Jorquera-Diaz, J.; Cigarroa, I.; Burgos, M.; Sanhueza, R.; Jeria, C.; Fernandez-Bussy, I.; et al. Association between Acute Respiratory Distress Syndrome Due to COVID-19 and Long-Term Sleep and Circadian Sleep–Wake Disorders. J. Clin. Med. 2023, 12, 6639. https://doi.org/10.3390/jcm12206639
Henríquez-Beltrán M, Benítez I, Belmonte T, Jorquera J, Jorquera-Diaz J, Cigarroa I, Burgos M, Sanhueza R, Jeria C, Fernandez-Bussy I, et al. Association between Acute Respiratory Distress Syndrome Due to COVID-19 and Long-Term Sleep and Circadian Sleep–Wake Disorders. Journal of Clinical Medicine. 2023; 12(20):6639. https://doi.org/10.3390/jcm12206639
Chicago/Turabian StyleHenríquez-Beltrán, Mario, Iván Benítez, Thalía Belmonte, Jorge Jorquera, Jorge Jorquera-Diaz, Igor Cigarroa, Matías Burgos, Rocio Sanhueza, Claudia Jeria, Isabel Fernandez-Bussy, and et al. 2023. "Association between Acute Respiratory Distress Syndrome Due to COVID-19 and Long-Term Sleep and Circadian Sleep–Wake Disorders" Journal of Clinical Medicine 12, no. 20: 6639. https://doi.org/10.3390/jcm12206639
APA StyleHenríquez-Beltrán, M., Benítez, I., Belmonte, T., Jorquera, J., Jorquera-Diaz, J., Cigarroa, I., Burgos, M., Sanhueza, R., Jeria, C., Fernandez-Bussy, I., Nova-Lamperti, E., Barbé, F., Targa, A., & Labarca, G. (2023). Association between Acute Respiratory Distress Syndrome Due to COVID-19 and Long-Term Sleep and Circadian Sleep–Wake Disorders. Journal of Clinical Medicine, 12(20), 6639. https://doi.org/10.3390/jcm12206639








