Syringomyelia Is Associated with a Reduction in Spinal Canal Compliance, Venous Outflow Dilatation and Glymphatic Fluid Obstruction
Abstract
:1. Introduction
2. Discussion
2.1. Compliance and Venous Pressure in Hydrocephalus, Multiple Sclerosis and Syringomyelia
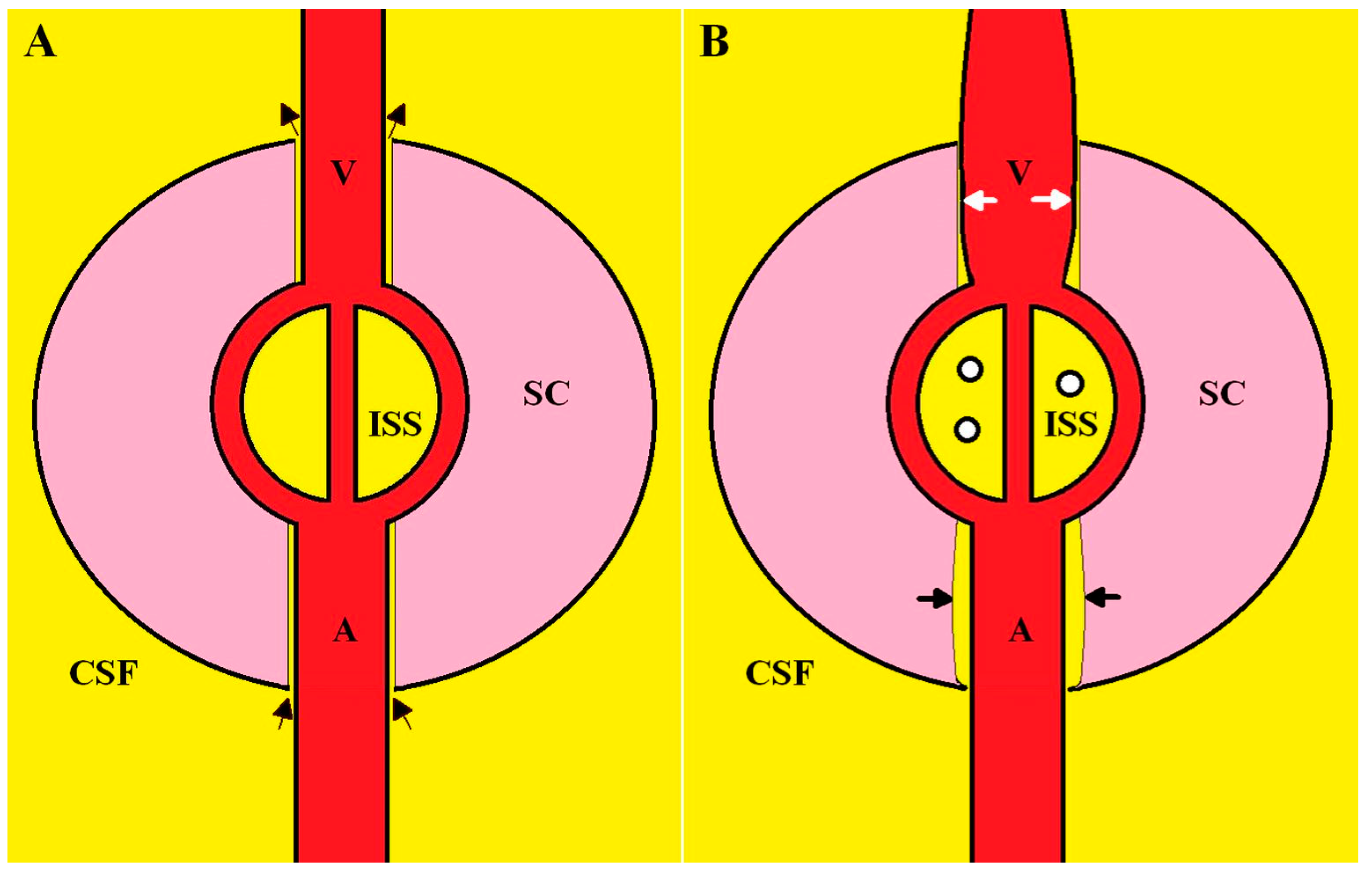
2.2. Dilated Outflow Veins and the Effect on Glymphatic Flow
2.3. The Complete Physiology of Syrinx Formation
2.4. Implications and Future Research Directions
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Levine, D.N. The pathogenesis of syringomyelia associated with lesions at the foramen magnum: A critical review of existing theories and proposal of a new hypothesis. J. Neurol. Sci. 2004, 220, 3–21. [Google Scholar] [CrossRef]
- Klekamp, J. Syringomyelia. In Practical Handbook of Neurosurgery: From Leading Neurosurgeons; Sindou, M., Ed.; Springer: Vienna, Austria, 2009; pp. 1260–1276. [Google Scholar]
- Elliott, N.S.J.; Bertram, C.D.; Martin, B.A.; Brodbelt, A.R. Syringomyelia: A review of the biomechanics. J. Fluids Struct. 2013, 40, 1–24. [Google Scholar] [CrossRef]
- Bateman, G.A. The role of altered impedance in the pathophysiology of normal pressure hydrocephalus, Alzheimer’s disease and syringomyelia. Med. Hypotheses 2004, 63, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G.A. Pulse wave myelopathy: An update of an hypothesis highlighting the similarities between syringomyelia and normal pressure hydrocephalus. Med. Hypotheses 2015, 85, 958–961. [Google Scholar] [CrossRef]
- Fischbein, N.J.; Dillon, W.P.; Cobbs, C.; Weinstein, P.R. The “presyrinx” state: Is there a reversible myelopathic condition that may precede syringomyelia? Neurosurg. Focus 2000, 8, E4. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G.A.; Bateman, A.R.; Subramanian, G.M. Dilatation of the bridging cerebral cortical veins in childhood hydrocephalus suggests a malfunction of venous impedance pumping. Sci. Rep. 2022, 12, 13045. [Google Scholar] [CrossRef]
- Tubbs, R.S.; McGirt, M.J.; Oakes, W.J. Surgical experience in 130 pediatric patients with Chiari I malformations. J. Neurosurg. 2003, 99, 291–296. [Google Scholar] [CrossRef]
- Weier, K.; Naegelin, Y.; Thoeni, A.; Hirsch, J.G.; Kappos, L.; Steinbrich, W.; Radue, E.W.; Gass, A. Non-communicating syringomyelia: A feature of spinal cord involvement in multiple sclerosis. Brain 2008, 131, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology, 10th ed.; Saunders: Philadelphia, PA, USA, 2000. [Google Scholar]
- Johnson, L.; Bartlett-Tomasetig, F.; Fok, S.; Whan, R.; Berliner, J.; Hemley, S.J.; Stoodley, M.A.; Bilston, L.E. A novel method to quantify perivascular space enlargement near the syrinx in a rodent model of post-traumatic syringomyelia. Sci. Rep. 2023, 13, 15043. [Google Scholar] [CrossRef]
- Bateman, G.A. The reversibility of reduced cortical vein compliance in normal-pressure hydrocephalus following shunt insertion. Neuroradiology 2003, 45, 65–70. [Google Scholar] [CrossRef]
- Saunders, H.M.; Burns, P.N.; Needleman, L.; Liu, J.B.; Boston, R.; Wortman, J.A.; Chan, L. Hemodynamic factors affecting uterine artery Doppler waveform pulsatility in sheep. J. Ultrasound Med. 1998, 17, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Hickerson, A.I.; Rinderknecht, D.; Gharib, M. Experimental study of the behavior of a valveless impedance pump. Exp. Fluids 2005, 38, 534–540. [Google Scholar] [CrossRef]
- Bateman, G.A.; Bateman, A.R.; Lechner-Scott, J. Dilatation of the bridging cerebral veins in multiple sclerosis correlates with fatigue and suggests an increase in pressure. Mult. Scler. Relat. Disord. 2023, 76, 104843. [Google Scholar] [CrossRef] [PubMed]
- Capel, C.; Lantonkpode, R.; Metanbou, S.; Peltier, J.; Balédent, O. Pathophysiology in Chiari Type 1 Malformations: Towards Understanding the Genesis of Syrinx. J. Clin. Med. 2023, 12, 5954. [Google Scholar] [CrossRef] [PubMed]
- Alperin, N.; Sivaramakrishnan, A.; Lichtor, T. Magnetic resonance imaging-based measurements of cerebrospinal fluid and blood flow as indicators of intracranial compliance in patients with Chiari malformation. J. Neurosurg. 2005, 103, 46–52. [Google Scholar] [CrossRef]
- Sivaramakrishnan, A.; Alperin, N.; Surapaneni, S.; Lichtor, T. Evaluating the effect of decompression surgery on cerebrospinal fluid flow and intracranial compliance in patients with chiari malformation with magnetic resonance imaging flow studies. Neurosurgery 2004, 55, 1344–1350; discussion 1341–1350. [Google Scholar] [CrossRef]
- Arnautovic, K.I.; Qaladize, B.F.; Pojskic, M.; Gienapp, A.J.; Splavski, B.; Boop, F.A. The 270 degrees Circumferential Microsurgical Decompression of the Foramen Magnum in Adult Chiari Malformation Type I: Single Surgeon Series of 130 Patients with Syringomyelia, Neurologic, and Headache Outcomes. World Neurosurg. 2021, 146, e1103–e1117. [Google Scholar] [CrossRef]
- Heiss, J.D.; Patronas, N.; DeVroom, H.L.; Shawker, T.; Ennis, R.; Kammerer, W.; Eidsath, A.; Talbot, T.; Morris, J.; Eskioglu, E.; et al. Elucidating the pathophysiology of syringomyelia. J. Neurosurg. 1999, 91, 553–562. [Google Scholar] [CrossRef]
- Yamada, H.; Yokota, A.; Haratake, J.; Horie, A. Morphological study of experimental syringomyelia with kaolin-induced hydrocephalus in a canine model. J. Neurosurg. 1996, 84, 999–1005. [Google Scholar] [CrossRef]
- Young, W.F.; Tuma, R.; O’Grady, T. Intraoperative measurement of spinal cord blood flow in syringomyelia. Clin. Neurol. Neurosurg. 2000, 102, 119–123. [Google Scholar] [CrossRef]
- Nedergaard, M. Neuroscience. Garbage truck of the brain. Science 2013, 340, 1529–1530. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Abrahao, A.; Heyn, C.C.; Bethune, A.J.; Huang, Y.; Pople, C.B.; Aubert, I.; Hamani, C.; Zinman, L.; Hynynen, K.; et al. Glymphatics Visualization after Focused Ultrasound-Induced Blood-Brain Barrier Opening in Humans. Ann. Neurol. 2019, 86, 975–980. [Google Scholar] [CrossRef]
- Bering, E.A., Jr. Water exchange of central nervous system and cerebrospinal fluid. J. Neurosurg. 1952, 9, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G.A.; Brown, K.M. The measurement of CSF flow through the aqueduct in normal and hydrocephalic children: From where does it come, to where does it go? Childs Nerv. Syst. 2012, 28, 55–63. [Google Scholar] [CrossRef]
- Lassek, A.M.; Rasmussen, G.L. A quantitative study of the newborn and adult spinal cords of man. J. Comp. Neurol. 1938, 69, 371–379. [Google Scholar] [CrossRef]
- Dekaban, A.S. Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Ann. Neurol. 1978, 4, 345–356. [Google Scholar] [CrossRef]
- Klostranec, J.M.; Vucevic, D.; Bhatia, K.D.; Kortman, H.G.J.; Krings, T.; Murphy, K.P.; terBrugge, K.G.; Mikulis, D.J. Current Concepts in Intracranial Interstitial Fluid Transport and the Glymphatic System: Part I-Anatomy and Physiology. Radiology 2021, 301, 502–514. [Google Scholar] [CrossRef]
- Carotenuto, A.; Cacciaguerra, L.; Pagani, E.; Preziosa, P.; Filippi, M.; Rocca, M.A. Glymphatic system impairment in multiple sclerosis: Relation with brain damage and disability. Brain 2022, 145, 2785–2795. [Google Scholar] [CrossRef]
- Georgiopoulos, C.; Tisell, A.; Holmgren, R.T.; Eleftheriou, A.; Rydja, J.; Lundin, F.; Tobieson, L. Noninvasive assessment of glymphatic dysfunction in idiopathic normal pressure hydrocephalus with diffusion tensor imaging. J. Neurosurg. 2023; publish before print. [Google Scholar] [CrossRef]
- Taoka, T.; Naganawa, S. Imaging for central nervous system (CNS) interstitial fluidopathy: Disorders with impaired interstitial fluid dynamics. Jpn. J. Radiol. 2021, 39, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Granberg, T.; Moridi, T.; Brand, J.S.; Neumann, S.; Hlavica, M.; Piehl, F.; Ineichen, B.V. Enlarged perivascular spaces in multiple sclerosis on magnetic resonance imaging: A systematic review and meta-analysis. J. Neurol. 2020, 267, 3199–3212. [Google Scholar] [CrossRef]
- Hu, H.Z.; Rusbridge, C.; Constantino-Casas, F.; Jeffery, N. Histopathological investigation of syringomyelia in the Cavalier King Charles spaniel. J. Comp. Pathol. 2012, 146, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Kato, K.; Guerrero, A.R.; Baba, H.; Yoshizawa, H. Experimental Syringohydromyelia Induced by Adhesive Arachnoiditis in the Rabbit: Changes in the Blood–Spinal Cord Barrier, Neuroinflammatory Foci, and Syrinx Formation. J. Neurotrauma 2012, 29, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Klekamp, J.; Volkel, K.; Bartels, C.J.; Samii, M. Disturbances of cerebrospinal fluid flow attributable to arachnoid scarring cause interstitial edema of the cat spinal cord. Neurosurgery 2001, 48, 174–185; discussion 176–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bilston, L.E.; Flores Rodriguez, N.; Wright, C.; McMullan, S.; Lloyd, R.; Stoodley, M.A.; Hemley, S.J. Changes in intrathoracic pressure, not arterial pulsations, exert the greatest effect on tracer influx in the spinal cord. Fluids Barriers CNS 2022, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.A.; Reymond, P.; Novy, J.; Baledent, O.; Stergiopulos, N. A coupled hydrodynamic model of the cardiovascular and cerebrospinal fluid system. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1492–H1509. [Google Scholar] [CrossRef]
- Ball, M.J.; Dayan, A.D. Pathogenesis of syringomyelia. Lancet 1972, 2, 799–801. [Google Scholar] [CrossRef]
- Peleggi, A.F.; Lovely, T.J. Treatment of delayed Chiari malformation and syringomyelia after lumboperitoneal shunt placement: Case report and treatment recommendations. Surg. Neurol. Int. 2012, 3, 101. [Google Scholar] [CrossRef]
- Kranz, P.G.; Viola, R.J.; Gray, L. Resolution of syringohydromyelia with targeted CT-guided epidural blood patching. J. Neurosurg. 2011, 115, 641–644. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bateman, G.A.; Bateman, A.R. Syringomyelia Is Associated with a Reduction in Spinal Canal Compliance, Venous Outflow Dilatation and Glymphatic Fluid Obstruction. J. Clin. Med. 2023, 12, 6646. https://doi.org/10.3390/jcm12206646
Bateman GA, Bateman AR. Syringomyelia Is Associated with a Reduction in Spinal Canal Compliance, Venous Outflow Dilatation and Glymphatic Fluid Obstruction. Journal of Clinical Medicine. 2023; 12(20):6646. https://doi.org/10.3390/jcm12206646
Chicago/Turabian StyleBateman, Grant Alexander, and Alexander Robert Bateman. 2023. "Syringomyelia Is Associated with a Reduction in Spinal Canal Compliance, Venous Outflow Dilatation and Glymphatic Fluid Obstruction" Journal of Clinical Medicine 12, no. 20: 6646. https://doi.org/10.3390/jcm12206646




