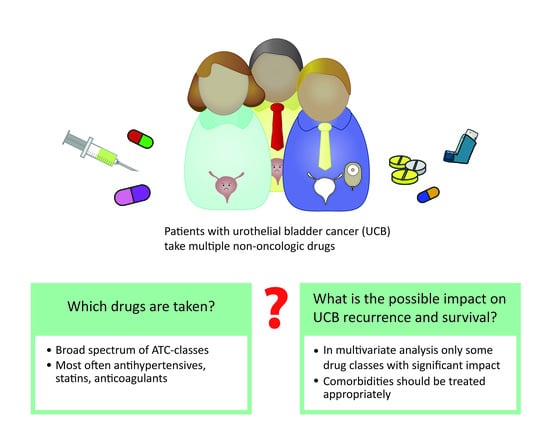
The Characterization of Non-oncologic Chronic Drug Therapy in Bladder Cancer Patients and the Impact on Recurrence-Free and Cancer-Specific Survival: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Non-oncologic Drugs Taken by UCB Patients
3.2. Univariate Analysis of the Influence of Non-oncologic Drugs on Survival of UCB Patients
3.3. Univariate Analysis on the Impact of Number of Non-oncologic Drugs on Survival in UCB Patients
3.4. Multivariate Analysis on the Impact of Non-oncologic Drugs and Additional Risk Factors on Survival in UCB Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Resaerch on Cancer. Cancer Today. Data Visualization Tools for Exploring the Global Cancer Burden in 2020. Available online: https://gco.iarc.fr (accessed on 12 February 2023).
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder cancer: Esmo clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 33, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Comperat, E.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; et al. EAU Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and CIS). European Association of Urology. Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Non-Muscle-Invasive-Bladder-Cancer-2022.pdf (accessed on 12 February 2023).
- AWMF Online: Leitlinienprogramm Onkologie. S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Harnblasenkarzinoms Langversion 2.0–März 2020 AWMF-Registernummer: 032/038OL. S3-Leitlinie Harnblasenkarzinom. Available online: https://www.leitlinienprogramm-onkologie.de (accessed on 12 February 2023).
- Halaseh, S.A.; Halaseh, S.; Alali, Y.; Ashour, M.E.; Alharayzah, M.J. A review on the etiology and epidemiology of bladder cancer: All you need to know. Curreus 2022, 14, e27330. [Google Scholar] [CrossRef] [PubMed]
- Knopf, H.; Grams, D. Medication use of adults in Germany: Results of the German health interview and examination survey for adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013, 56, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Kusi, M.; Vardy, J.L.; Bell, M.L.; Choi, B.M.; Axon, D.R. Comorbidities and perceived health status in persons with history of cancer in the USA. Support. Care Cancer 2022, 14, 16. [Google Scholar] [CrossRef]
- Roy, S.; Vallepu, S.; Barrios, C.; Hunter, K. Comparison of comorbid conditions between cancer survivors and age-matched patients without cancer. J. Clin. Med. Res. 2018, 10, 911–919. [Google Scholar] [CrossRef]
- Ahmadinezhad, M.; Arshadi, M.; Hesari, E.; Sharafoddin, M.; Azizi, H.; Khodamoradi, F. The relationship between metabolic syndrome and its components with bladder cancer: A systematic review and meta-analysis of cohort studies. Epidemiol. Health 2022, 44, e2022050. [Google Scholar] [CrossRef]
- Khajeh, N.R.; Khoyilar, C.; Wu, Y.; Spradling, K.; Zi, X.; Youssef, R.F. Bladder Cancer Chemopreventive Agents: Current Knowledge and Concepts. Mini Rev. Med. Chem. 2018, 18, 1143–1150. [Google Scholar] [CrossRef]
- Guercio, V.; Turati, F.; Bosetti, C.; Polesel, J.; Serraino, D.; Montella, M.; Libra, M.; Galfano, A.; La Vecchia, C.; Tavani, A. Bladder cancer risk in users of selected drugs for cardiovascular disease prevention. Eur. J. Cancer Prev. 2019, 28, 76–80. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, P.; Wang, M.; Zheng, Y.; Tian, T.; Yang, S.; Deng, Y.; Wu, Y.; Zhai, Z.; Hao, Q.; et al. Antihypertensive medications are associated with the risk of kidney and bladder cancer: A systematic review and meta-analysis. Aging 2020, 12, 1545–1562. [Google Scholar] [CrossRef]
- Blute, M.L., Jr.; Rushmer, T.J.; Shi, F.; Fuller, B.J.; Abel, E.J.; Jarrard, D.F.; Downs, T.M. Renin-Angiotensin Inhibitors Decrease Recurrence after Transurethral Resection of Bladder Tumor in Patients with Nonmuscle Invasive Bladder Cancer. J. Urol. 2015, 194, 1214–1219. [Google Scholar] [CrossRef]
- Dal Moro, F.; Bovo, A.; Crestani, A.; Vettor, R.; Gardiman, M.P.; Zattoni, F. Effect of hypertension on outcomes of high-risk patients after BCG-treated bladder cancer: A single-institution long follow-up cohort study. Medicine 2015, 94, e589. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kinoshita, H.; Fukui, K.; Matsuzaki, T.; Yoshida, K.; Mishima, T.; Yanishi, M.; Komai, Y.; Sugi, M.; Inoue, T.; et al. Prognostic Impact of Renin-Angiotensin Inhibitors in Patients with Bladder Cancer Undergoing Radical Cystectomy. Ann. Surg. Oncol. 2017, 24, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Yuge, K.; Miyajima, A.; Tanaka, N.; Shirotake, S.; Kosaka, T.; Kikuchi, E.; Oya, M. Prognostic value of renin-angiotensin system blockade in non-muscle-invasive bladder cancer. Ann. Surg. Oncol. 2012, 19, 3987–3993. [Google Scholar] [CrossRef] [PubMed]
- Haimerl, L.; Strobach, D.; Mannell, H.; Stief, C.G.; Buchner, A.; Karl, A.; Grimm, T. Retrospective evaluation of the impact of non-oncologic chronic drug therapy on the survival in patients with bladder cancer. Int. J. Clin. Pharm. 2022, 44, 339–347. [Google Scholar] [CrossRef]
- Santala, E.E.E.; Kotsar, A.; Veitonmäki, T.; Tammela, T.L.J.; Murtola, T.J. Risk of urothelial cancer death among people using antihypertensive drugs-a cohort study from Finland. Scand. J. Urol. 2019, 53, 185–192. [Google Scholar] [CrossRef]
- Guan, T.; Su, M.; Luo, Z.; Peng, W.; Zhou, R.; Lu, Z.; Feng, M.; Li, W.; Teng, Y.; Jiang, Y.; et al. Long-Term Cardiovascular Mortality among 80,042 Older Patients with Bladder Cancer. Cancers 2022, 14, 4572. [Google Scholar] [CrossRef]
- WHO The High5s Project—Standard Operating Protocol for Medication Reconciliation. Standard Implementation Protocol for Medication Reconciliation. Available online: https://www.who.int/publications/m/item/high5s-standard-operating-protocol-medication-reconciliation (accessed on 25 March 2023).
- Sylvester, R.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2023. Available online: www.whocc.no (accessed on 25 March 2023).
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 10, 230. [Google Scholar] [CrossRef]
- Da Silva, R.D.; Xylinas, E.; Kluth, L.; Crivelli, J.J.; Chrystal, J.; Chade, D.; Guglielmetti, G.B.; Pycha, A.; Lotan, Y.; Karakiewicz, P.I.; et al. Impact of statin use on oncologic outcomes in patients with urothelial carcinoma of the bladder treated with radical cystectomy. J. Urol. 2013, 190, 487–492. [Google Scholar] [CrossRef]
- Radkiewicz, C.; Edgren, G.; Johansson, A.L.V.; Jahnson, S.; Häggström, C.; Akre, O.; Lambe, M.; Dickman, P.W. Sex Differences in Urothelial Bladder Cancer Survival. Clin. Genitourin. Cancer 2020, 18, 26–34.e6. [Google Scholar] [CrossRef]
- Maffezzini, M.; Fontana, V.; Pacchetti, A.; Dotta, F.; Cerasuolo, M.; Chiappori, D.; Guano, G.; Mantica, G.; Terrone, C. Age above 70 years and Charlson Comorbidity Index higher than 3 are associated with reduced survival probabilities after radical cystectomy for bladder cancer. Data from a contemporary series of 334 consecutive patients. Arch. Ital. Urol. Androl. 2021, 93, 15–20. [Google Scholar] [CrossRef]
- Mateu, L.; García-Cruz, E.; Asiaín, I.; Castañeda, R.; Carrión, A.; Huguet, J.; Ribal, M.J.; Alcaraz, A. A higher Charlson comorbidity index is related to more aggressive characteristics in de novo vesical tumours. Actas Urol. Esp. 2016, 40, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.B.; Kamat, A.M.; Chamie, K.; Froehner, M.; Wirth, M.P.; Wiklund, P.N.; Black, P.C.; Steinberg, G.D.; Boorjian, S.A.; Daneshmand, S.; et al. Systematic Review of Comorbidity and Competing-risks Assessments for Bladder Cancer Patients. Eur. Urol. Oncol. 2018, 1, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Marrone, M.T.; Mondul, A.M.; Prizment, A.E.; Couper, D.; Barber, J.R.; Chappidi, M.R.; Joshu, C.E.; Platz, E.A. Lipid-Lowering Drug Use and Cancer Incidence and Mortality in the ARIC Study. JNCI Cancer Spectr. 2021, 5, pkab080. [Google Scholar] [CrossRef] [PubMed]
- Ortland, I.; Mendel Ott, M.; Kowar, M.; Sippel, C.; Ko, Y.D.; Jacobs, A.H.; Jaehde, U. Medication risks in older patients (70 +) with cancer and their association with therapy-related toxicity. BMC Geriatr. 2022, 22, 716. [Google Scholar] [CrossRef]
- Turner, J.P.; Shakib, S.; Singhal, N.; Hogan-Doran, J.; Prowse, R.; Johns, S.; Bell, J.S. Prevalence and factors associated with polypharmacy in older people with cancer. Support. Care Cancer 2014, 22, 1727–1734. [Google Scholar] [CrossRef]
- Mohamed, M.R.; Ramsdale, E.; Loh, K.P.; Arastu, A.; Xu, H.; Obrecht, S.; Castillo, D.; Sharma, M.; Holmes, H.M.; Nightingale, G.; et al. Associations of Polypharmacy and Inappropriate Medications with Adverse Outcomes in Older Adults with Cancer: A Systematic Review and Meta-Analysis. Oncologist 2020, 25, e94–e108. [Google Scholar] [CrossRef]
- Chen, L.J.; Nguyen, T.N.M.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H.; Schöttker, B. Association of Polypharmacy with Colorectal Cancer Survival among older patients. Oncologist 2021, 26, e2170–e2180. [Google Scholar] [CrossRef]
- Yeoh, T.T.; Tay, X.Y.; Si, P.; Chew, L. Drug-related problems in elderly patients with cancer receiving outpatient chemotherapy. J. Geriatr. Oncol. 2015, 6, 280–287. [Google Scholar] [CrossRef]
- Müller, G.; Butea-Bocu, M.; Brock, O.; Hanske, J.; Pucheril, D.; Noldus, J.; Otto, U. Association between Development of Metabolic Acidosis and Improvement of Urinary Continence after Ileal Neobladder Creation. J. Urol. 2020, 203, 585–590. [Google Scholar] [CrossRef]
- Barone, B.; Finati, M.; Cinelli, F.; Fanelli, A.; Del Giudice, F.; De Berardinis, E.; Sciarra, A.; Russo, G.; Mancini, V.; D’Altilia, N.; et al. Bladder Cancer and Risk Factors: Data from a Multi-Institutional Long-Term Analysis on Cardiovascular Disease and Cancer Incidence. J. Pers. Med. 2023, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Connaughton, M.; Dabagh, M. Association of Hypertension and Organ-Specific Cancer: A Meta-Analysis. Healthcare 2022, 10, 1074. [Google Scholar] [CrossRef] [PubMed]
- Motterle, G.; Morlacco, A.; Giovannini, G.; Vecchiato, E.; Iafrate, M.; Calpista, A.; Prayer-Galetti, T.; Martino, F.; Dal Moro, F.; Novara, G. Role of Renin-Angiotensin System Blockers on BCG Response in Nonmuscle Invasive, High Risk Bladder Cancer. Clin. Genitourin. Cancer 2022, 20, e303–e309. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Schooling, C.M.; Zhao, J.V. Genetic proxies for calcium-channel-blockers and cancer: A Mendelian randomization study. J. Hum. Hypertens. 2023, epub ahead of print. [Google Scholar] [CrossRef]
- Udumyan, R.; Botteri, E.; Jerlstrom, T.; Montgomery, S.; Smedby, K.E.; Fall, K. Beta-blocker use and urothelial bladder cancer survival: A Swedish register-based cohort study. Acta Oncol. 2022, 61, 922–930. [Google Scholar] [CrossRef]
- Wang, S.; Ge, C. High risk of non-cancer mortality in bladder cancer patients: Evidence from SEER-Medicaid. J. Cancer Res. Clin. Oncol. 2023, epub ahead of print. [Google Scholar] [CrossRef]
- Teleka, S.; Orho-Melander, M.; Liedberg, F.; Melander, O.; Jirström, K.; Stocks, T. Interaction between blood pressure and genetic risk score for bladder cancer, and risk of urothelial carcinoma in men. Sci. Rep. 2022, 12, 18336. [Google Scholar] [CrossRef]
- Ferro, M.; Marchioni, M.; Lucarelli, G.; Vartolomei, M.D.; Soria, F.; Terracciano, D.; Mistretta, F.A.; Luzzago, S.; Buonerba, C.; Cantiello, F.; et al. Association of statin use and oncological outcomes in patients with first diagnosis of T1 high grade non-muscle invasive urothelial bladder cancer: Results from a multicenter study. Minerva Urol. Nephrol. 2021, 73, 796–802. [Google Scholar] [CrossRef]
- Lundberg, E.; Hagberg, O.; Jahnson, S.; Ljungberg, B. Association between occurrence of urinary bladder cancer and treatment with statin medication. Turk. J. Urol. 2019, 45, 97–102. [Google Scholar] [CrossRef]
- Crivelli, J.J.; Xylinas, E.; Kluth, L.A.; da Silva, R.D.; Chrystal, J.; Novara, G.; Karakiewicz, P.I.; David, S.G.; Scherr, D.S.; Lotan, Y.; et al. Effect of statin use on outcomes of non-muscle-invasive bladder cancer. BJU Int. 2023, 112, E4–E12. [Google Scholar] [CrossRef]
- Fiala, O.; Buti, S.; Takeshita, H.; Okada, Y.; Massari, F.; Palacios, G.A.; Dionese, M.; Scagliarini, S.; Büttner, T.; Fornarini, G.; et al. Use of concomitant proton pump inhibitors, statins or metformin in patients treated with pembrolizumab for metastatic urothelial carcinoma: Data from the ARON-2 retrospective study. Cancer Immunol. Immunother. 2023, 72, 3665–3682. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Palleschi, G.; Fuschi, A.; Silvestri, L.; Al Salhi, Y.; Costantini, E.; Zucchi, A.; Petrozza, V.; de Nunzio, C.; Carbone, A. Can daily intake of aspirin and/or statins influence the behavior of non-muscle invasive bladder cancer? A retrospective study on a cohort of patients undergoing transurethral bladder resection. BMC Cancer 2015, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, C.R.; McDowell, R.D.; Hughes, C.M.; Hicks, B.; Murchie, P. Exposure to Ranitidine and Risk of Bladder Cancer: A Nested Case-Control Study. Am. J. Gastroenterol. 2021, 116, 1612–1619. [Google Scholar] [CrossRef]
- Lopes, S.; Pabst, L.; Dory, A.; Klotz, M.; Gourieux, B.; Michel, B.; Mascaux, C. Do proton pump inhibitors alter the response to immune checkpoint inhibitors in cancer patients? A meta-analysis. Front. Immunol. 2023, 14, 1070076. [Google Scholar] [CrossRef] [PubMed]
- Hotaling, J.M.; Wright, J.L.; Pocobelli, G.; Bhatti, P.; Porter, M.P.; White, E. Long-term use of supplemental vitamins and minerals does not reduce the risk of urothelial cell carcinoma of the bladder in the VITamins and Lifestyle study. J. Urol. 2021, 185, 1210–1215. [Google Scholar] [CrossRef]
- Wach, S.; Weigelt, K.; Michalke, B.; Lieb, V.; Stoehr, R.; Keck, B.; Hartmann, A.; Wullich, B.; Taubert, H.; Chaudhri, A. Diagnostic potential of major and trace elements in the serum of bladder cancer patients. J. Trace Elem. Med. Biol. 2018, 46, 150–155. [Google Scholar] [CrossRef]


| Parameter | ||
|---|---|---|
| First Diagnosis Cohort (n = 52) | ||
| age [years] | median 74, IQR 63–79 | |
| Sex | ||
| male | 83% | 43 |
| female | 17% | 9 |
| Charlson comorbidity index | median 2, IQR 2–4 | |
| Tumour classification | ||
| pTa/is | 62% | 32 |
| pT1 | 25% | 13 |
| >pT1 | 13% | 7 |
| Tumour grade | ||
| low grade | 37% | 19 |
| high grade | 63% | 33 |
| Radical cystectomy cohort (n = 78) | ||
| age [years] | median 72, IQR 63–78 | |
| Gender | ||
| male | 82% | 64 |
| female | 18% | 14 |
| Charlson comorbidity index | median 2, IQR 2–4 | |
| Sylvester recurrence index | median 6, IQR 3–7 | |
| Tumour classification | ||
| pT0 | 17% | 13 |
| pTa/is | 4% | 3 |
| pT1 | 15% | 12 |
| pT2 | 28% | 22 |
| pT3 | 6% | 5 |
| pT4 | 29% | 23 |
| Lymph node status | ||
| pN0 | 81% | 63 |
| pN+ | 10% | 8 |
| pNX | 9% | 7 |
| M0 | 99% | 77 |
| M1 | 1% | 1 |
| Tumour grade (only pT ≠ 0) | ||
| low grade | 5% | 3 |
| high grade | 95% | 62 |
| ATC | ntot a (of 114) | nFD (of 52) | nRC (of 78) | Drug |
|---|---|---|---|---|
| A02AH | 42 | 10 | 41 | Sodium bicarbonate |
| C10AA | 34 | 16 | 19 | Statins |
| B01AC | 33 | 13 | 21 | Antiplatelet drugs |
| C07AB | 32 | 15 | 21 | Beta-blockers |
| C09AA | 30 | 15 | 22 | Angiotensin-converting enzyme inhibitors |
| C08CA | 29 | 12 | 19 | Calcium channel blockers |
| C09CA | 29 | 13 | 19 | Angiotensin receptor blockers |
| C03AA | 23 | 9 | 18 | Thiazide diuretics |
| A11CC | 23 | 12 | 13 | Vitamin D |
| M04AA | 21 | 7 | 14 | Allopurinol/febuxostat |
| B01AF | 18 | 10 | 10 | Direct acting oral anticoagulants |
| C03CA | 18 | 6 | 12 | Sulfonamides (diuretics) |
| H03AA | 17 | 9 | 12 | Thyroid hormones |
| A02BC | 16 | 8 | 9 | Proton-pump-inhibitors |
| B03BA | 14 | 3 | 11 | Vitamin B12 |
| G04CA | 13 | 11 | 3 | Alpha-blockers |
| R03AC | 12 | 3 | 9 | Inhalative beta-2-agonists |
| A12CC | 12 | 6 | 8 | Magnesium |
| R03BA | 10 | 3 | 7 | Inhalative glucocorticoides |
| ATC | Drug | p-Value | |||
|---|---|---|---|---|---|
| RFS | CSS | ||||
| First Diagnosis | RC | First Diagnosis | RC | ||
| A02AH | Sodium bicarbonate | 0.930 | 0.253 | 0.943 | 0.173 |
| C10AA | Statins | 0.025 | 0.390 | 0.566 | 0.742 |
| B01AC | Antiplatelet drugs | 0.111 | 0.069 | 0.992 | 0.149 |
| C07AB | Beta-blockers | 0.540 | 0.099 | 0.151 | 0.183 |
| C09AA | ACE inhibitors | 0.665 | 0.082 | 0.008 | 0.294 |
| C08CA | Calcium channel blockers | 0.711 | 0.046 | 0.393 | 0.124 |
| C09CA | Angiotensin receptor blockers | 0.444 | 0.892 | 0.582 | 0.627 |
| C03AA | Thiazide diuretics | 0.895 | 0.051 | 0.183 | 0.450 |
| A11CC | Vitamin D | 0.856 | 0.269 | 0.333 | 0.635 |
| M04AA | Allopurinol/febuxostat | 0.456 | 0.574 | 0.471 | 0.767 |
| B01AF | DOACs | 0.517 | 0.480 | 0.328 | 0.368 |
| C03CA | Sulfonamides | 0.214 | 0.050 | 0.475 | 0.494 |
| H03AA | Thyroid hormones | 0.908 | 0.126 | 0.279 | 0.256 |
| A02BC | Proton-pump-inhibitors | 0.913 | 0.015 | 0.637 | 0.386 |
| B03BA | Vitamin B12 | 0.408 | 0.774 | 0.616 | 0.298 |
| G04CA | Alpha-blockers | 0.539 | 0.115 | 0.783 | 0.062 |
| R03AC | Inhalative beta-2-agonists | 0.364 | 0.459 | 0.602 | 0.212 |
| A12CC | Magnesium | 0.084 | 0.289 | 0.042 | 0.412 |
| R03BA | Inhalative glucocorticoides | 0.364 | 0.743 | 0.602 | 0.282 |
| Parameter | HR | 95% Ci of HR | p Value |
|---|---|---|---|
| FD patients—RFS | |||
| gender female | 4.67 | 1.32–16.48 | 0.017 |
| CCI | 1.49 | 1.01–2.18 | 0.043 |
| Statins | 0.12 | 0.01–0.97 | 0.047 |
| variables not included in the final model: age, pT ≥ 1, high grade | |||
| FD patients—CSS | |||
| CCI | 2.06 | 1.08–3.95 | 0.029 |
| Magnesium | 22.87 | 1.57–333.81 | 0.022 |
| ACEI | 15.20 | 1.30–177.67 | 0.030 |
| variables not included in the final model: age, gender, pT ≥ 1, high grade | |||
| RC patients—RFS | |||
| CCI | 1.54 | 1.17–2.01 | 0.002 |
| pT3-4 | 2.38 | 0.94–6.02 | 0.067 |
| variables not included in the final model: age, gender, PPI, CCB | |||
| RC patients—CSS | |||
| no multivariate model (no significant drugs in univariate analysis) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strobach, D.; Haimerl, L.; Mannell, H.; Stief, C.G.; Karl, A.; Grimm, T.; Buchner, A. The Characterization of Non-oncologic Chronic Drug Therapy in Bladder Cancer Patients and the Impact on Recurrence-Free and Cancer-Specific Survival: A Prospective Study. J. Clin. Med. 2023, 12, 6749. https://doi.org/10.3390/jcm12216749
Strobach D, Haimerl L, Mannell H, Stief CG, Karl A, Grimm T, Buchner A. The Characterization of Non-oncologic Chronic Drug Therapy in Bladder Cancer Patients and the Impact on Recurrence-Free and Cancer-Specific Survival: A Prospective Study. Journal of Clinical Medicine. 2023; 12(21):6749. https://doi.org/10.3390/jcm12216749
Chicago/Turabian StyleStrobach, Dorothea, Lisa Haimerl, Hanna Mannell, Christian G. Stief, Alexander Karl, Tobias Grimm, and Alexander Buchner. 2023. "The Characterization of Non-oncologic Chronic Drug Therapy in Bladder Cancer Patients and the Impact on Recurrence-Free and Cancer-Specific Survival: A Prospective Study" Journal of Clinical Medicine 12, no. 21: 6749. https://doi.org/10.3390/jcm12216749
APA StyleStrobach, D., Haimerl, L., Mannell, H., Stief, C. G., Karl, A., Grimm, T., & Buchner, A. (2023). The Characterization of Non-oncologic Chronic Drug Therapy in Bladder Cancer Patients and the Impact on Recurrence-Free and Cancer-Specific Survival: A Prospective Study. Journal of Clinical Medicine, 12(21), 6749. https://doi.org/10.3390/jcm12216749