Criterion Validity and Responsiveness of Estimated Cardiorespiratory Fitness Models in Patients with Inflammatory Joint Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Setting
2.2. Outcome Assessment and Interim Period
2.3. Demographic Variables
2.4. Criterion Measurement of CRF
2.5. Estimated CRF
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. ICC Analysis
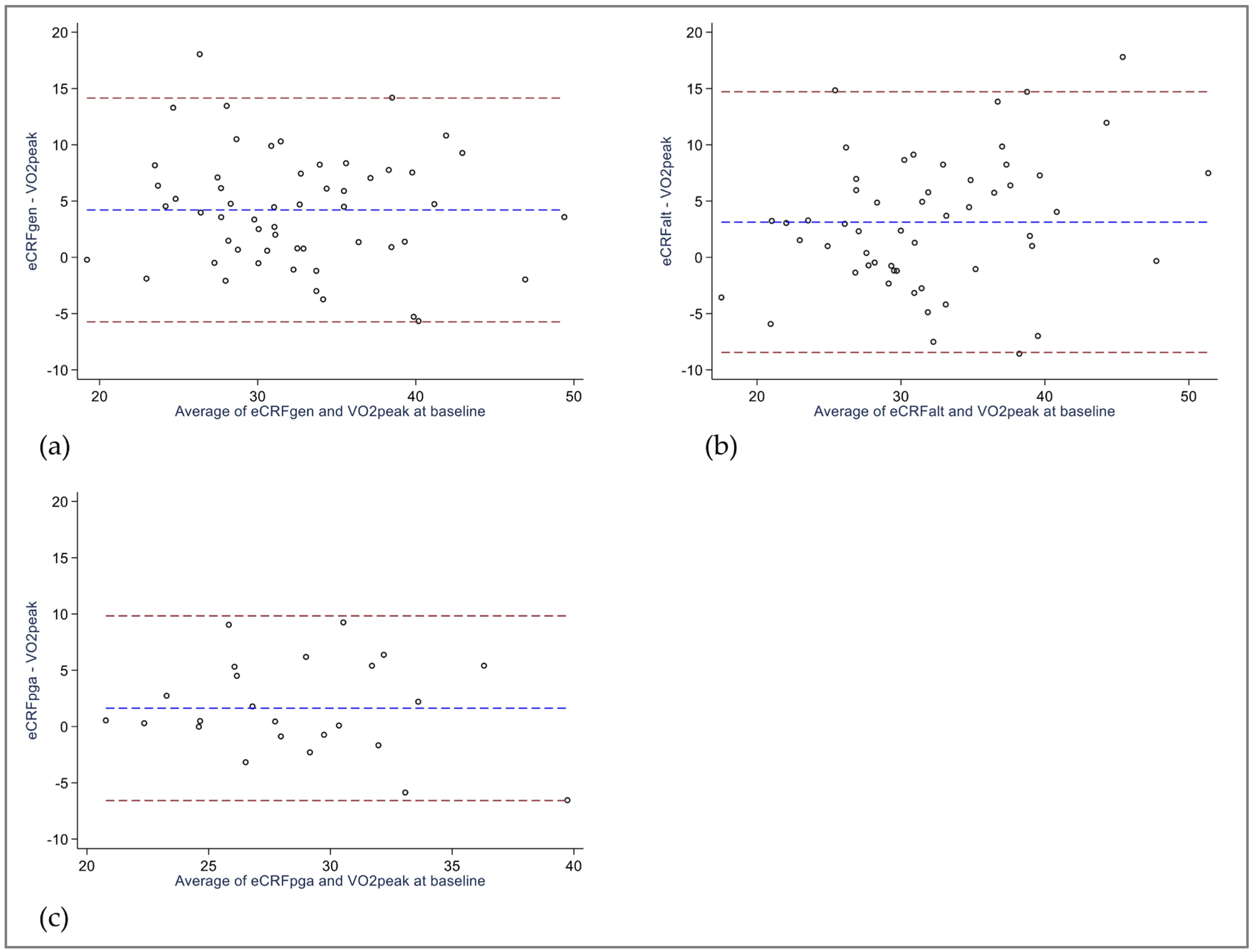
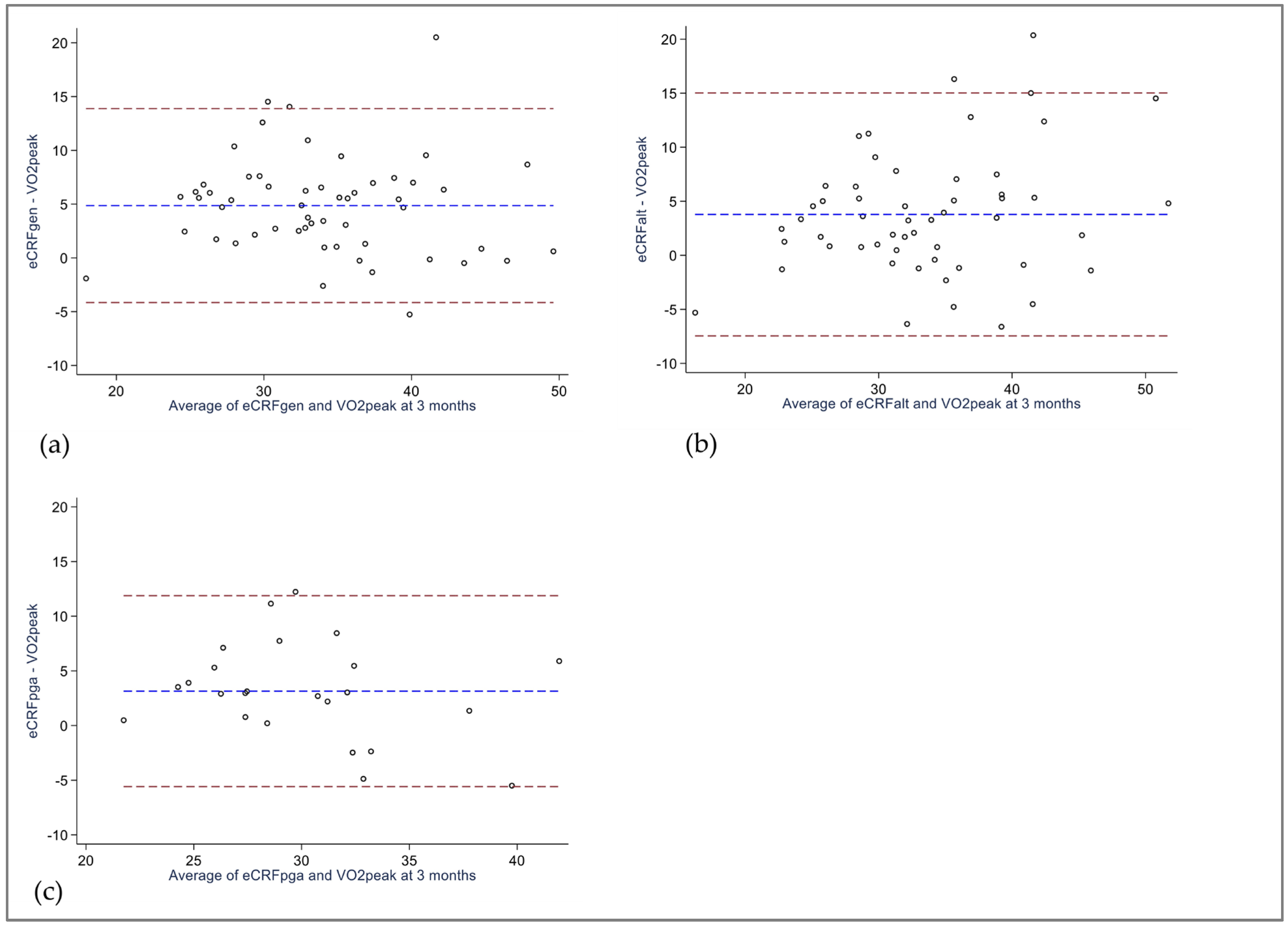
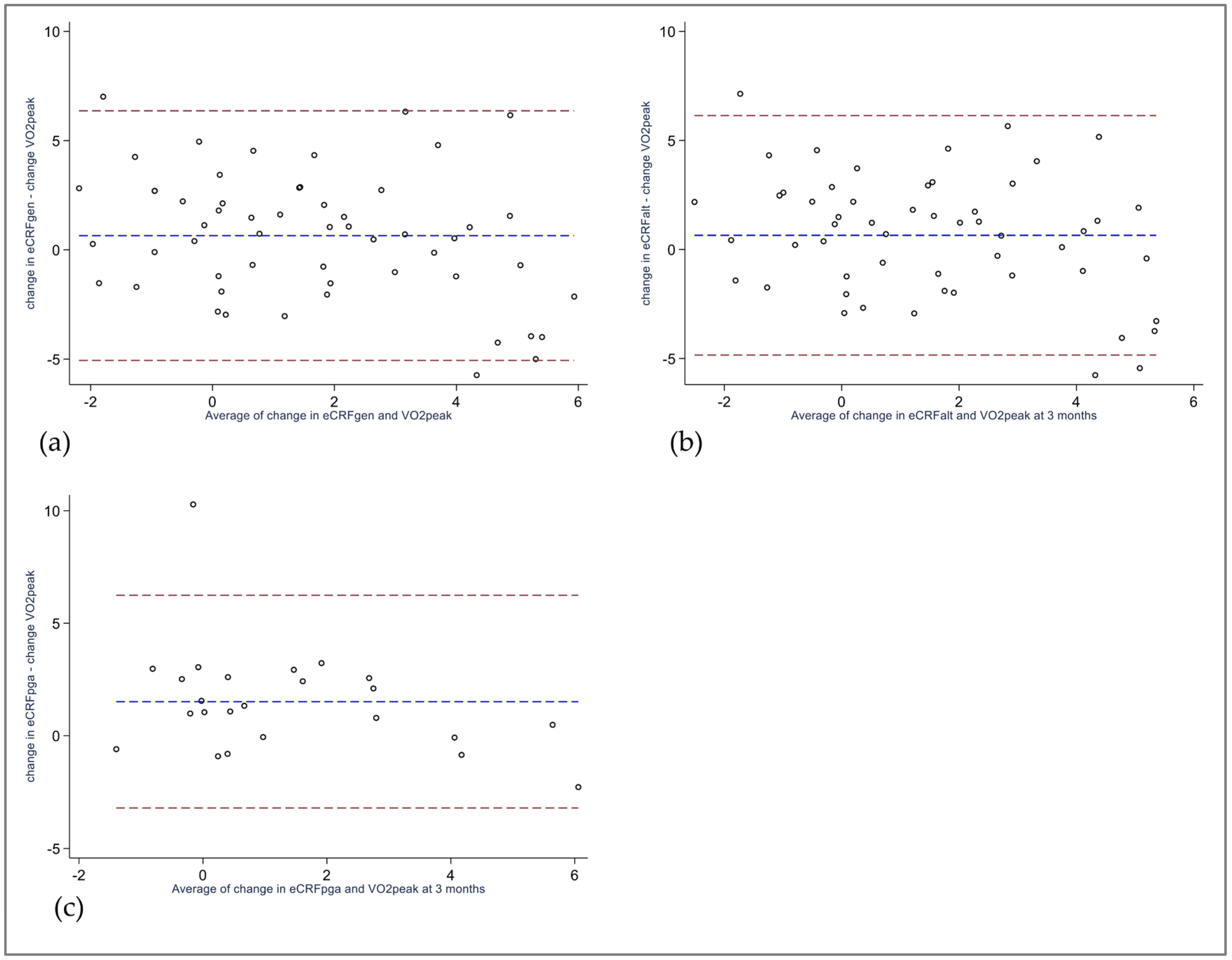
3.3. Bland–Altman Analysis
3.4. Area-under-the-Curve Analysis
4. Discussion
4.1. Clinical Implications and Future Research Avenues
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liguori, G.; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Han, M.; Qie, R.; Shi, X.; Yang, Y.; Lu, J.; Hu, F.; Zhang, M.; Zhang, Z.; Hu, D.; Zhao, Y. Cardiorespiratory fitness and mortality from all causes, cardiovascular disease and cancer: Dose-response meta-analysis of cohort studies. Br. J. Sports Med. 2022, 56, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Imboden, M.T.; Harber, M.P.; Whaley, M.H.; Finch, W.H.; Bishop, D.L.; Fleenor, B.S.; Kaminsky, L.A. The Association between the Change in Directly Measured Cardiorespiratory Fitness across Time and Mortality Risk. Prog. Cardiovasc. Dis. 2019, 62, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.T.; Di, C.; Bellettiere, J.; Lamonte, M.J.; Simonsick, E.M.; Parada, H., Jr.; Hooker, S.P.; Lacroix, A.Z. Validation, Recalibration, and Predictive Accuracy of Published VO 2max Prediction Equations for Adults Ages 50–96 Yr. Med. Sci. Sports Exerc. 2023, 55, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Despres, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, Itc1–Itc16. [Google Scholar] [CrossRef]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef]
- Conrad, N.; McInnes, I.B.; McMurray, J.J.V.; Sattar, N. Patients with a range of rheumatic diseases are at increased risk of cardiovascular disorders towards a re-evaluation of the European League against Rheumatism (EULAR)’s recommendations for cardiovascular risk management? Ann. Rheum. Dis. 2022, 82, 457–459. [Google Scholar] [CrossRef]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Metsios, G.S.; Koutedakis, Y.; Veldhuijzen van Zanten, J.J.; Stavropoulos-Kalinoglou, A.; Vitalis, P.; Duda, J.L.; Ntoumanis, N.; Rouse, P.C.; Kitas, G.D. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology 2015, 54, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Munsterman, T.; Takken, T.; Wittink, H. Are persons with rheumatoid arthritis deconditioned? A review of physical activity and aerobic capacity. BMC Musculoskelet. Disord. 2012, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, T.; O’Shea, F.; Wilson, F. Decreased physical activity and cardiorespiratory fitness in adults with ankylosing spondylitis: A cross-sectional controlled study. Rheumatol. Int. 2015, 35, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, S.; Vollestad, N.K.; Fongen, C.; Provan, S.A.; Semb, A.G.; Hagen, K.B.; Dagfinrud, H. Physical fitness in patients with ankylosing spondylitis: Comparison with population controls. Phys. Ther. 2012, 92, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Nordén, K.R.; Semb, A.G.; Dagfinrud, H.; Hisdal, J.; Ødegård, S.; Sexton, J.; Fongen, C.; Skandsen, J.; Blanck, T.; Metsios, G.S.; et al. Associations between cardiovascular risk factors, disease activity and cardiorespiratory fitness in patients with inflammatory joint disease: A cross-sectional analysis. BMC Sports Sci. Med. Rehabil. 2023, 15, 63. [Google Scholar] [CrossRef]
- Liff, M.H.; Hoff, M.; Wisloff, U.; Videm, V. Reduced cardiorespiratory fitness is a mediator of excess all-cause mortality in rheumatoid arthritis: The Trøndelag Health Study. RMD Open 2021, 7, e001545. [Google Scholar] [CrossRef]
- Videm, V.; Liff, M.H.; Hoff, M. Relative importance of inflammation and cardiorespiratory fitness for all-cause mortality risk in persons with rheumatoid arthritis: The population-based Trøndelag Health Study. RMD Open 2023, 9, e003194. [Google Scholar] [CrossRef]
- Nes, B.M.; Janszky, I.; Vatten, L.J.; Nilsen, T.I.; Aspenes, S.T.; Wisloff, U. Estimating V.O 2peak from a nonexercise prediction model: The HUNT Study, Norway. Med. Sci. Sports Exerc. 2011, 43, 2024–2030. [Google Scholar] [CrossRef]
- Nes, B.M.; Vatten, L.J.; Nauman, J.; Janszky, I.; Wisloff, U. A simple nonexercise model of cardiorespiratory fitness predicts long-term mortality. Med. Sci. Sports Exerc. 2014, 46, 1159–1165. [Google Scholar] [CrossRef]
- Peterman, J.E.; Whaley, M.H.; Harber, M.P.; Fleenor, B.S.; Imboden, M.T.; Myers, J.; Arena, R.; Kaminsky, L.A. Comparison of non-exercise cardiorespiratory fitness prediction equations in apparently healthy adults. Eur. J. Prev. Cardiol. 2021, 28, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Liff, M.H.; Hoff, M.; Fremo, T.; Wisløff, U.; Videm, V. An Estimation Model for Cardiorespiratory Fitness in Adults with Rheumatoid Arthritis. Med. Sci. Sports Exerc. 2020, 52, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Mokkink, L.; Terwee, C.; de Vet, H. Key concepts in clinical epidemiology: Responsiveness, the longitudinal aspect of validity. J. Clin. Epidemiol. 2021, 140, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Nordén, K.R.; Dagfinrud, H.; Semb, A.G.; Hisdal, J.; Viktil, K.K.; Sexton, J.; Fongen, C.; Skandsen, J.; Blanck, T.; Metsios, G.S.; et al. Effect of high-intensity exercise on cardiorespiratory fitness, cardiovascular disease risk and disease activity in patients with inflammatory joint disease: Protocol for the ExeHeart randomised controlled trial. BMJ Open 2022, 12, e058634. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Prinsen, C.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; De Vet, H.; Terwee, C.B. COSMIN Study Design Checklist for Patient-Reported Outcome Measurement Instruments. Available online: http://www.comsin.nl (accessed on 18 August 2023).
- Kottner, J.; Audigé, L.; Brorson, S.; Donner, A.; Gajewski, B.J.; Hróbjartsson, A.; Roberts, C.; Shoukri, M.; Streiner, D.L. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J. Clin. Epidemiol. 2011, 64, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Gagnier, J.J.; Lai, J.; Mokkink, L.B.; Terwee, C.B. COSMIN reporting guideline for studies on measurement properties of patient-reported outcome measures. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2021, 30, 2197–2218. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic, S.; American College of Chest, P. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Pritchard, A.; Burns, P.; Correia, J.; Jamieson, P.; Moxon, P.; Purvis, J.; Thomas, M.; Tighe, H.; Sylvester, K.P. ARTP statement on cardiopulmonary exercise testing 2021. BMJ Open Respir. Res. 2021, 8, e001121. [Google Scholar] [CrossRef]
- Balke, B.; Ware, R.W. An experimental study of physical fitness of Air Force personnel. U. S. Armed Forces Med. J. 1959, 10, 675–688. [Google Scholar]
- Edvardsen, E.; Hem, E.; Anderssen, S.A. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: A cross-sectional study. PLoS ONE 2014, 9, e85276. [Google Scholar] [CrossRef]
- Kurtze, N.; Rangul, V.; Hustvedt, B.E.; Flanders, W.D. Reliability and validity of self-reported physical activity in the Nord-Trondelag Health Study: HUNT 1. Scand. J. Public Health 2008, 36, 52–61. [Google Scholar] [CrossRef]
- Liljequist, D.; Elfving, B.; Skavberg Roaldsen, K. Intraclass correlation—A discussion and demonstration of basic features. PLoS ONE 2019, 14, e0219854. [Google Scholar] [CrossRef]
- Husted, J.A.; Cook, R.J.; Farewell, V.T.; Gladman, D.D. Methods for assessing responsiveness: A critical review and recommendations. J. Clin. Epidemiol. 2000, 53, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Correlation in restricted ranges of data. BMJ 2011, 342, d556. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [PubMed]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, J.A.; Isiozor, N.M.; Kunutsor, S.K. Objectively Assessed Cardiorespiratory Fitness and All-Cause Mortality Risk: An Updated Meta-analysis of 37 Cohort Studies Involving 2,258,029 Participants. Mayo Clin. Proc. 2022, 97, 1054–1073. [Google Scholar] [CrossRef] [PubMed]
- Letnes, J.M.; Dalen, H.; Vesterbekkmo, E.K.; Wisloff, U.; Nes, B.M. Peak oxygen uptake and incident coronary heart disease in a healthy population: The HUNT Fitness Study. Eur. Heart J. 2019, 40, 1633–1639. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesth. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- McAuley, E.; Szabo, A.N.; Mailey, E.L.; Erickson, K.I.; Voss, M.; White, S.M.; Wójcicki, T.R.; Gothe, N.; Olson, E.A.; Mullen, S.P.; et al. Non-Exercise Estimated Cardiorespiratory Fitness: Associations with Brain Structure, Cognition, and Memory Complaints in Older Adults. Ment. Health Phys. Act. 2011, 4, 5–11. [Google Scholar] [CrossRef]
- Mailey, E.L.; White, S.M.; Wójcicki, T.R.; Szabo, A.N.; Kramer, A.F.; McAuley, E. Construct validation of a non-exercise measure of cardiorespiratory fitness in older adults. BMC Public Health 2010, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Lannoy, L.; Ross, R. Nonexercise Equations for Determining Change in Cardiorespiratory Fitness. Med. Sci. Sports Exerc. 2020, 52, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Meyler, S.; Bottoms, L.; Muniz-Pumares, D. Biological and methodological factors affecting VO2max response variability to endurance training and the influence of exercise intensity prescription. Exp. Physiol. 2021, 106, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Houle, S.A.; Sui, X.; Blair, S.N.; Ross, R. Association Between Change in Nonexercise Estimated Cardiorespiratory Fitness and Mortality in Men. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, H.J.; Brage, S.; Warren, J.; Besson, H.; Ekelund, U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Bremander, A.; Malm, K.; Andersson, M.L. Physical activity in established rheumatoid arthritis and variables associated with maintenance of physical activity over a seven-year period—A longitudinal observational study. BMC Rheumatol. 2020, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, R.J.; Musa Yola, I.; Shrout, T.A.; Mitchell, G.F.; Vasan, R.S.; Xanthakis, V. Association of Estimated Cardiorespiratory Fitness in Midlife with Cardiometabolic Outcomes and Mortality. JAMA Netw. Open 2021, 4, e2131284. [Google Scholar] [CrossRef]
| Model | eCRF Equation |
|---|---|
| eCRFGEN [21] Female Male | 70.77 − (0.244 × age) − (0.749 × BMI) − (0.107 × resting heart rate) + (0.213 × physical activity index) 92.05 − (0.327 × age) − (0.933 × BMI) − (0.167 × resting heart rate) + (0.257 × physical activity index) |
eCRFALT [23] eCRFPGA [23] | (female = 0, male = 1; never smoked = 0, ever smoked = 1) 77.851 + (gender × 25.460) − (age × 0.381) − (age–gender interaction × 0.254) − (BMI × 0.743) − (resting heart rate × 0.115) − (smoking × 2.154) + (physical activity index × 0.209) 77.961 + (gender × 28.791) − (age × 0.358) − (age–gender interaction × 0.326) − (BMI × 0.700) − (resting heart rate × 0.125) − (smoking × 1.854) + (physical activity index × 0.211) − (PGA × 0.071) |
| Variable | |
|---|---|
| Age, years, median (IQR) | 59 (51–63) |
| Gender, female, n (%) | 31 (56) |
| Education > 12 years, n (%) | 42 (76) |
| Anthropometrics Height, cm, mean (SD) Weight, kg, median (IQR) BMI, median (IQR) | 173 (9) 74 (66–91) 25 (22–31) |
| Diagnosis Rheumatoid arthritis, n (%) Spondyloarthritis, n (%) Psoriatic arthritis, n (%) | 26 (47) 16 (29) 13 (24) |
| IJD disease duration, years, median (IQR) | 15 (7–30) |
| Disease activity categorized Remission, n (%) Low, n (%) Moderate, n (%) High, n (%) | 21 (38) 15 (27) 12 (22) 7 (13) |
| Comorbidity Diabetes, n (%) Chronic obstructive pulmonary disease, n (%) Inflammatory bowel disease, n (%) | 3 (5) 4 (7) 4 (7) |
| Ever smoker, yes, n (%) | 34 (62) |
| Resting heart rate, beats/min, mean (SD) | 68 (11) |
| Cardiopulmonary Exercise Test VO2peak, mL/kg/min, mean (SD) VO2peak, L/min, mean (SD) VO2 plateau at peak exercise, yes, n (%) Respiratory exchange ratio, VCO2/VO2, mean (SD) Borg RPE 0–10 (10 = maximal), median (IQR) Peak heart rate, beats/min, mean (SD) Percent of predicted peak heart rate (220-age), mean (SD) Post-exercise blood lactate, mmol/L, median (IQR) Ventilatory reserve, %, mean (SD) | 30.3 (6.9) 2.4 (0.7) 29 (53) 1.16 (0.1) 10 (9–10) 165 (14) 101 (7) 9.2 (7.3–11.7) § 25 (13) ^ |
| Physical activity index (0–45, 45 = best), median (IQR) | 0 (0–15) |
| Numerical Rating Scale (0–10), 0 = best Pain, median (IQR) Fatigue, median (IQR) | 2 (1–4) 3 (1–5) |
| Patient Global Assessment, 0–100 mm, 0 = best, mean (SD) | 29 (22) $ |
| Variable | Baseline (n = 55) | 3 Months (n = 55) | ∆Baseline to 3 Months (n = 55) |
|---|---|---|---|
| VO2peak, mL/kg/min | 30.3 (6.9) | 31.6 (7.0) | 1.3 (0.5 to 2.1) a |
| eCRFGEN, mL/kg/min ICC (95% CI) Linear regression, regression coefficient, (95% CI) R2 Difference eCRFGEN—VO2peak, mL/kg/min. (95% CI) 95% Limits of agreement | 34.5 (6.5) 0.60 (0.15 to 0.80) 4.2 (2.8 to 5.6) a −5.7 to 14.1 | 36.4 (6.7) 0.62 (0.03 to 0.84) 4.9 (3.6 to 6.1) a −4.2 to 13.9 | 2.0 (1.4 to 2.6) a 0.39 (0.15 to 0.59) 0.56 (0.22 to 0.90) 0.17 0.7 (−0.1 to 1.4) a −5.1 to 6.4 |
| eCRFALT, mL/kg/min ICC (95% CI) Linear regression, regression coefficient, (95% CI) R2 Difference eCRFALT—VO2peak, mL/kg/min, (95% CI) 95% Limits of agreement | 33.6 (8.3) 0.64 (0.38 to 0.79) 3.1 (1.5 to 4.7) a −8.4 to 14.7 | 35.5 (8.4) § 0.65 (0.31 to 0.82) § 3.8 (2.2 to 5.3) §a −7.5 to 15.0 § | 1.8 (1.2 to 2.4) §a 0.40 (0.16 to 0.60) § 0.58 (0.24 to 0.91) § 0.19 § 0.7 (−0.1 to 1.4) §a −4.8 to 6.1 § |
| Variable | Baseline (n = 24) | 3 months (n = 24) | ∆Baseline to 3 months (n = 24) |
| VO2peak, mL/kg/min | 27.9 (5.2) | 28.6 (5.8) | 0.6 (−0.5 to 1.8) a |
| eCRFPGA, mL/kg/min ICC (95% CI) Linear regression, regression coefficient, (95% CI) R2 Difference eCRFPGA—VO2peak, mL/kg/min, (95% CI) 95% Limits of agreement | 29.6 (4.6) 0.62 (0.30 to 0.81) 1.6 (−0.1 to 3.4) a −6.6 to 9.8 | 31.7 (4.8) 0.57 (0.13 to 0.81) 3.1 (1.3 to 5.0) a −5.6 to 11.9 | 2.1 (1.3 to 3.0) a 0.39 (0.01 to 0.68) 0.68 (0.06 to 1.29) b 0.24 1.5 (0.5 to 2.5) a −3.2 to 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordén, K.R.; Dagfinrud, H.; Semb, A.G.; Hisdal, J.; Metsios, G.S.; Sexton, J.; Fongen, C.; Bakke, E.A.; Tveter, A.T. Criterion Validity and Responsiveness of Estimated Cardiorespiratory Fitness Models in Patients with Inflammatory Joint Disease. J. Clin. Med. 2023, 12, 6753. https://doi.org/10.3390/jcm12216753
Nordén KR, Dagfinrud H, Semb AG, Hisdal J, Metsios GS, Sexton J, Fongen C, Bakke EA, Tveter AT. Criterion Validity and Responsiveness of Estimated Cardiorespiratory Fitness Models in Patients with Inflammatory Joint Disease. Journal of Clinical Medicine. 2023; 12(21):6753. https://doi.org/10.3390/jcm12216753
Chicago/Turabian StyleNordén, Kristine Røren, Hanne Dagfinrud, Anne Grete Semb, Jonny Hisdal, George S. Metsios, Joseph Sexton, Camilla Fongen, Emilie Andrea Bakke, and Anne Therese Tveter. 2023. "Criterion Validity and Responsiveness of Estimated Cardiorespiratory Fitness Models in Patients with Inflammatory Joint Disease" Journal of Clinical Medicine 12, no. 21: 6753. https://doi.org/10.3390/jcm12216753
APA StyleNordén, K. R., Dagfinrud, H., Semb, A. G., Hisdal, J., Metsios, G. S., Sexton, J., Fongen, C., Bakke, E. A., & Tveter, A. T. (2023). Criterion Validity and Responsiveness of Estimated Cardiorespiratory Fitness Models in Patients with Inflammatory Joint Disease. Journal of Clinical Medicine, 12(21), 6753. https://doi.org/10.3390/jcm12216753






