Hemodynamic Differences between Patients Hospitalized with Acutely Decompensated Chronic Heart Failure and De Novo Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Study Group Baseline Characteristics
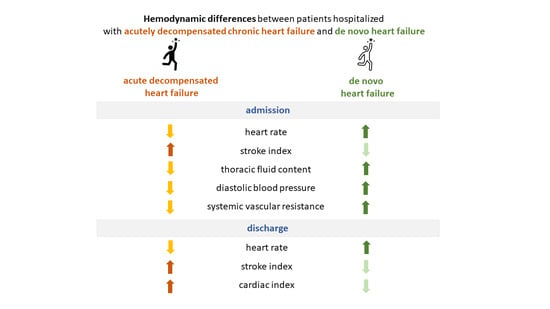
3.2. Comparison of Admission Characteristics of Patients Hospitalized with Acutely Decompensated CHF and De Novo HF
3.3. The Effect of Treatment in Patients Hospitalized with Acutely Decompensated CHF and De Novo HF
4. Discussion
4.1. Clinical Implications
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4091. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200, Erratum in Eur. Heart J. 2018, 39, 1206. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Butler, J. Acutely decompensated versus acute heart failure: Two different entities. Heart Fail. Rev. 2020, 25, 907–916. [Google Scholar] [CrossRef]
- Chapman, B.; DeVore, A.D. Clinical profiles in acute heart failure: An urgent need for a new approach. ESC Heart Fail. 2019, 6, 464–474. [Google Scholar] [CrossRef]
- Chaudhry, S.P.; Stewart, G.C. Advanced Heart Failure: Prevalence, Natural History, and Prognosis. Heart Fail. Clin. 2016, 12, 323–333. [Google Scholar] [CrossRef]
- Pranata, R.; Tondas, A.E. Differences in clinical characteristics and outcome of de novo heart failure compared to acutely decompensated chronic heart failure—Systematic review and meta-analysis. Acta Cardiol. 2021, 76, 410–420. [Google Scholar] [CrossRef]
- Nawrocka-Millward, S.; Biegus, J. Differences in the Biomarker Profile of De Novo Acute Heart Failure versus Decompensation of Chronic Heart Failure. Biomolecules 2021, 11, 1701. [Google Scholar] [CrossRef]
- López-Vilella, R.; Jover Pastor, P. Mortality After the First Hospital Admission for Acute Heart Failure, De Novo Versus Acutely Decompensated Heart Failure With Reduced Ejection Fraction. Am. J. Cardiol. 2023, 196, 59–66. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Zannad, F.; International Working Group on Acute Heart Failure Syndromes. Acute heart failure syndromes: Current state and framework for future research. Circulation 2005, 112, 3958–3968. [Google Scholar] [CrossRef]
- Raffaello, W.M.; Henrina, J. Clinical Characteristics of De Novo Heart Failure and Acute Decompensated Chronic Heart Failure: Are They Distinctive Phenotypes That Contribute to Different Outcomes? Card. Fail. Rev. 2021, 7, e02. [Google Scholar] [CrossRef]
- Galas, A.; Krzesiński, P. Complex assessment of patients with decompensated heart failure: The clinical value of impedance cardiography and N-terminal pro-brain natriuretic peptide. Heart Lung. 2019, 48, 294–301. [Google Scholar] [CrossRef]
- Krzesiński, P.; Jankowska, E.A. Effects of an outpatient intervention comprising nurse-led non-invasive assessments, telemedicine support and remote cardiologists’ decisions in patients with heart failure (AMULET study): A randomised controlled trial. Eur. J. Heart Fail. 2022, 24, 565–577. [Google Scholar] [CrossRef]
- Krzesiński, P.; Galas, A. Haemodynamic Effects of Anaemia in Patients with Acute Decompensated Heart Failure. Cardiol. Res. Pract. 2020, 2020, 9371967. [Google Scholar] [CrossRef]
- Whellan, D.J.; Droogan, C.J. Change in intrathoracic impedance measures during acute decompensated heart failure admission: Results from the Diagnostic Data for Discharge in Heart Failure Patients (3D-HF) Pilot Study. J. Card. Fail. 2012, 18, 107–112. [Google Scholar] [CrossRef]
- Peacock, W.F.I.V.; Albert, N.M. Bioimpedance monitoring: Better than chest x-ray for predicting abnormal pulmonary fluid? Congest. Heart Fail. 2000, 6, 86–89. [Google Scholar] [CrossRef]
- Packer, M.; Abraham, W.T. Prospective Evaluation and Identification of Cardiac Decompensation by ICG Test (PREDICT) Study Investigators and Coordinators. Utility of impedance cardiography for the identification of short-term risk of clinical decompensation in stable patients with chronic heart failure. J. Am. Coll. Cardiol. 2006, 47, 2245–2252. [Google Scholar] [CrossRef]
- Smilde, T.D.; van Veldhuisen, D.J. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation 2006, 114, 1572–1580. [Google Scholar] [CrossRef]
- Senni, M.; Gavazzi, A.; IN HF Outcome Investigators. In-hospital and 1-year outcomes of acute heart failure patients according to presentation (de novo vs. worsening) and ejection fraction. Results from IN-HF Outcome Registry. Int. J. Cardiol. 2014, 173, 163–169. [Google Scholar] [CrossRef]
- Nieminen, M.S.; Brutsaert, D.; Dickstein, K.; Drexler, H.; Follath, F.; Harjola, V.P.; Hochadel, M.; Komajda, M.; Lassus, J.; Lopez-Sendon, J.L.; et al. EuroHeart Failure Survey II (EHFS II): A survey on hospitalized acute heart failure patients: Description of population. Eur. Heart J. 2006, 27, 2725–2736. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, G.Y. Outcomes of de novo and acute decompensated heart failure patients according to ejection fraction. Heart 2018, 104, 525–532. [Google Scholar] [CrossRef]
- Rizzi, M.A.; Sarasola, A.G.; ICA-SEMES Research Group. Factors associated with in-hospital mortality and adverse outcomes during the vulnerable post-discharge phase after the first episode of acute heart failure: Results of the NOVICA-2 study. Clin. Res. Cardiol. 2021, 110, 993–1005, Erratum in Clin. Res. Cardiol. 2021, 110, 919. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Cho, D.H. Impact of NT-proBNP on prognosis of acute decompensated chronic heart failure versus de novo heart failure. Int. J. Cardiol. 2022, 363, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lassus, J.P.; Siirilä-Waris, K.; FINN-AKVA Study Group. Long-term survival after hospitalization for acute heart failure--differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int. J. Cardiol. 2013, 168, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Blázquez-Bermejo, Z.; Farré, N. Dose of furosemide before admission predicts diuretic efficiency and long-term prognosis in acute heart failure. ESC Heart Fail. 2022, 9, 656–666. [Google Scholar] [CrossRef]
- Greene, S.J.; Hernandez, A.F. Hospitalization for Recently Diagnosed Versus Worsening Chronic Heart Failure: From the ASCEND-HF Trial. J. Am. Coll. Cardiol. 2017, 69, 3029–3039. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Vignon, P.; ECHO-COVID Research Group. Echocardiography findings in COVID-19 patients admitted to intensive care units: A multi-national observational study (the ECHO-COVID study). Intensive Care Med. 2022, 48, 667–678. [Google Scholar] [CrossRef]
- Huang, S.; Vieillard-Baron, A.; ECHO-COVID Study Group. Echocardiography phenotypes of right ventricular involvement in COVID-19 ARDS patients and ICU mortality: Post-hoc (exploratory) analysis of repeated data from the ECHO-COVID study. Intensive Care Med. 2023, 49, 946–956. [Google Scholar] [CrossRef]

| Parameter | Study Group n = 102 |
|---|---|
| Age | 71.4 ± 12.5 |
| Males | 78 (76.5%) |
| HR (bpm) | 87 ± 24 |
| Systolic BP (mmHg) | 135 ± 27 |
| Diastolic BP (mmHg) | 82 ± 14 |
| De novo HF | 27 (26.5%) |
| Symptoms and signs, n (%) | |
| Dyspnea at rest | 41 (40.2%) |
| Dyspnea on effort | 100 (98.1%) |
| Orthopnea | 78 (77.2%) |
| Paroxysmal nocturnal dyspnea | 44 (43.1%) |
| Chest pain | 25 (24.5%) |
| Palpitations | 33 (32.4%) |
| Edema | 77 (75.5%) |
| Tachypnea | 21 (20.6%) |
| Ascites | 16 (15.7%) |
| Peripheral hypoperfusion | 10 (9.8%) |
| Hepatomegaly | 18 (17.6%) |
| Concomitant disease, n (%) | |
| Prior myocardial infarction | 42 (41.1%) |
| Hypertension | 68 (66.6%) |
| Atrial fibrillation | 54 (52.9%) |
| Previous stroke or transient ischemic attack | 9 (8.8%) |
| Diabetes mellitus (type 2) | 50 (49.0%) |
| Chronic obstructive pulmonary disease | 15 (14.7%) |
| Chronic kidney disease (stage ≥ 3) | 30 (29.4%) |
| Laboratory data on admission, mean ± SD | |
| NT-proBNP (pg/mL) | 6197 ± 7057 |
| Creatinine (mg/dL) | 1.31 ± 0.51 |
| eGFR (mL/min/1.73 m2) | 62.2 ± 23.9 |
| Hemoglobin (g/dL) | 12.6 ± 2.6 |
| Parameter | ADCHF Mean ± SD or Median (IQR)/n (%) | dnHF Mean ± SD or Median (IQR)/n (%) | p |
|---|---|---|---|
| Age (years) | 75 (65–83) | 68 (57–75) | 0.012 |
| Female | 19 (25.3%) | 5 (18.5%) | 0.474 |
| BMI (kg/m2) | 29.9 ± 5.8 | 30.6 ± 8.3 | 0.834 |
| Symptoms and signs | |||
| NYHA class | |||
| Class III | 52 (69.3%) | 14 (51.9%) | 0.103 |
| Class IV | 23 (30.7%) | 13 (48.1%) | |
| Dyspnea at rest | 26 (34.7%) | 15 (55.6%) | 0.058 |
| Dyspnea on effort | 75 (100%) | 25 (92.6%) | 0.017 |
| Orthopnea | 55 (74.3%) | 23 (85.2%) | 0.249 |
| Paroxysmal nocturnal dyspnea | 32 (42.7%) | 12 (44.4%) | 0.873 |
| Chest pain | 17 (21.3%) | 9 (33.3%) | 0.214 |
| Palpitations | 20 (26.7%) | 13 (48.2%) | 0.041 |
| Edema | 62 (82.7%) | 18 (66.7%) | 0.083 |
| Tachypnea | 16 (21.3%) | 5 (18.5%) | 0.756 |
| Ascites | 11 (14.7%) | 5 (18.5%) | 0.637 |
| Peripheral hypoperfusion | 4 (5.3%) | 6 (22.2%) | 0.011 |
| Hepatomegaly | 14 (18.7%) | 4 (14.8%) | 0.653 |
| Concomitant disease (in anamnesis) | |||
| Prior myocardial infarction | 34 (45.3%) | 8 (29.6%) | 0.155 |
| Hypertension | 52 (69.3%) | 16 (59.3%) | 0.341 |
| Atrial fibrillation | 45 (60.0%) | 9 (33.3%) | 0.017 |
| Previous stroke or transient ischemic attack | 6 (8.0%) | 3 (11.1%) | 0.625 |
| Diabetes mellitus (type 2) | 40 (53.3%) | 10 (37.0%) | 0.146 |
| Chronic obstructive pulmonary disease | 12 (16.0%) | 3 (11.1%) | 0.539 |
| Chronic kidney disease (stage ≥ 3) | 27 (36.0%) | 3 (11.1%) | 0.014 |
| Pharmacotherapy | |||
| ACE-I | 53 (71.6%) | 9 (34.6%) | 0.0008 |
| ARB | 7 (9.5%) | 3 (11.5%) | 0.761 |
| Beta-blockers | 66 (89.2%) | 12 (46.2%) | <0.0001 |
| MRA | 30 (40.5%) | 3 (11.5%) | 0.007 |
| Diuretics | 65 (87.8%) | 9 (34.6%) | <0.0001 |
| Laboratory tests | |||
| Creatinine (mg/dL) | 1.30 (1.00–1.60) | 1.10 (0.80–1.40) | 0.030 |
| eGFR, (mL/min/1.73 m2) | 56.4 (41.8–70.7) | 72.2 (53.2–90.2) | 0.007 |
| NTproBNP (pg/mL) | 3640 (1509–7102) | 4902 (2512–8563) | 0.258 |
| High-sensitivity troponin T (ng/L) | 38.8 (24.5–57.6) | 30.5 (19.3–67.3) | 0.371 |
| WBC (109/L) | 7.5 (6.4–9.1) | 8.5 (7.5–11.4) | 0.001 |
| Hemoglobin (g/dL) | 12.4 (10.9–13.5) | 14.0 (12.6–15.2) | 0.004 |
| Anemia | 49 (65.3%) | 8 (29.6%) | 0.001 |
| Bilirubin (mg/dL) | 1.00 (0.6–1.7) | 1.0 (0.8–1.5) | 0.683 |
| ALT (IU) | 31.0 (21–38) | 30.0 (25–52) | 0.142 |
| AST (IU) | 21 (15–34) | 33 (28–47) | 0.037 |
| Hospitalization | |||
| Days of hospitalization | 8 (6–13) | 8 (7–11) | 0.965 |
| Total IV dose of loop diuretics (mg) | 580 (340–900) | 380 (320–1000) | 0.442 |
| Total oral dose of loop diuretics (mg) | 280 (160–480) | 320 (120–400) | 0.806 |
| Hemodynamics at admission | |||
| LVEF (%) | 33 (25–50) | 35 (30–50) | 0.593 |
| HR (bpm) | 76 (65–86) | 87 (65–111) | 0.029 |
| Systolic BP (mmHg) | 118 (103–135) | 122 (114–149) | 0.183 |
| Diastolic BP (mmHg) | 70 (66–80) | 81 (72–85) | 0.029 |
| SI (mL*m−2) | 41.1 (33.0–47.8) | 36.0 (25.6–43.0) | 0.043 |
| CI (mL*m−2*min−1) | 3.0 (2.40–3.6) | 2.9 (2.6–3.3) | 0.377 |
| SVRI (dyn*s*cm−5*m2) | 2115 (1671–2603) | 2604 (2142–3044) | 0.013 |
| TFC (1*kΩ−1) | 33.6 (29.3–39.3) | 37.8 (31.3–41.9) | 0.194 |
| TFC ≥ 35.1*kΩ−1 | 33 (44.0%) | 15 (55.6%) | 0.302 |
| ADCHF Median (IQR); n (%) | dnHF Median (IQR); n (%) | p | |
|---|---|---|---|
| Change from admission to discharge | |||
| NTproBNP (pg/mL) | 1061 (216–2794) | 2393 (1245–4627) | 0.026 |
| TFC (1*kΩ−1) | 3.8 (0.8–8.8) | 7.8 (2.4–13.3) | 0.013 |
| Body mass (kg) | 3.8 (1.5–6.3) | 4.9 (3.4–9.6) | 0.228 |
| Hemodynamics at discharge | |||
| HR (bpm) | 72 (63–81) | 81 (73–103) | 0.002 |
| Systolic BP (mmHg) | 109 (94–122) | 107 (94–115) | 0.436 |
| Diastolic BP (mmHg) | 67 (60–74) | 68 (62–75) | 0.489 |
| SI (mL*m−2) | 40.5 (33.4–48.5) | 29.5 (27.0–37.0) | <0.0001 |
| CI (mL*m−2*min−1) | 2.9 (2.5–3.7) | 2.7 (2.2–3.0) | 0.023 |
| SVRI (dyn*s*cm−5*m2) | 2023 (1512–2390) | 2201 (1842–2596) | 0.087 |
| TFC (1*kΩ−1) | 29.6 (25.9–34.7) | 28.6 (23.1–32.8) | 0.358 |
| Pharmacotherapy | |||
| ACE-I | 62 (86.1) | 23 (85.2) | 0.906 |
| ARB | 4 (5.6) | 1 (3.7) | 0.708 |
| Beta-blockers | 70 (97.2) | 26 (96.3) | 0.811 |
| MRA | 41 (56.9) | 19 (70.4) | 0.223 |
| Diuretics | 71 (98.6) | 27 (100.0) | 0.538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galas, A.; Krzesiński, P.; Banak, M.; Gielerak, G. Hemodynamic Differences between Patients Hospitalized with Acutely Decompensated Chronic Heart Failure and De Novo Heart Failure. J. Clin. Med. 2023, 12, 6768. https://doi.org/10.3390/jcm12216768
Galas A, Krzesiński P, Banak M, Gielerak G. Hemodynamic Differences between Patients Hospitalized with Acutely Decompensated Chronic Heart Failure and De Novo Heart Failure. Journal of Clinical Medicine. 2023; 12(21):6768. https://doi.org/10.3390/jcm12216768
Chicago/Turabian StyleGalas, Agata, Paweł Krzesiński, Małgorzata Banak, and Grzegorz Gielerak. 2023. "Hemodynamic Differences between Patients Hospitalized with Acutely Decompensated Chronic Heart Failure and De Novo Heart Failure" Journal of Clinical Medicine 12, no. 21: 6768. https://doi.org/10.3390/jcm12216768
APA StyleGalas, A., Krzesiński, P., Banak, M., & Gielerak, G. (2023). Hemodynamic Differences between Patients Hospitalized with Acutely Decompensated Chronic Heart Failure and De Novo Heart Failure. Journal of Clinical Medicine, 12(21), 6768. https://doi.org/10.3390/jcm12216768