The Influence of Food Intake and Preload Augmentation on Cardiac Functional Parameters: A Study Using Both Cardiac Magnetic Resonance and Echocardiography
Abstract
:1. Introduction
2. Materials and Methods
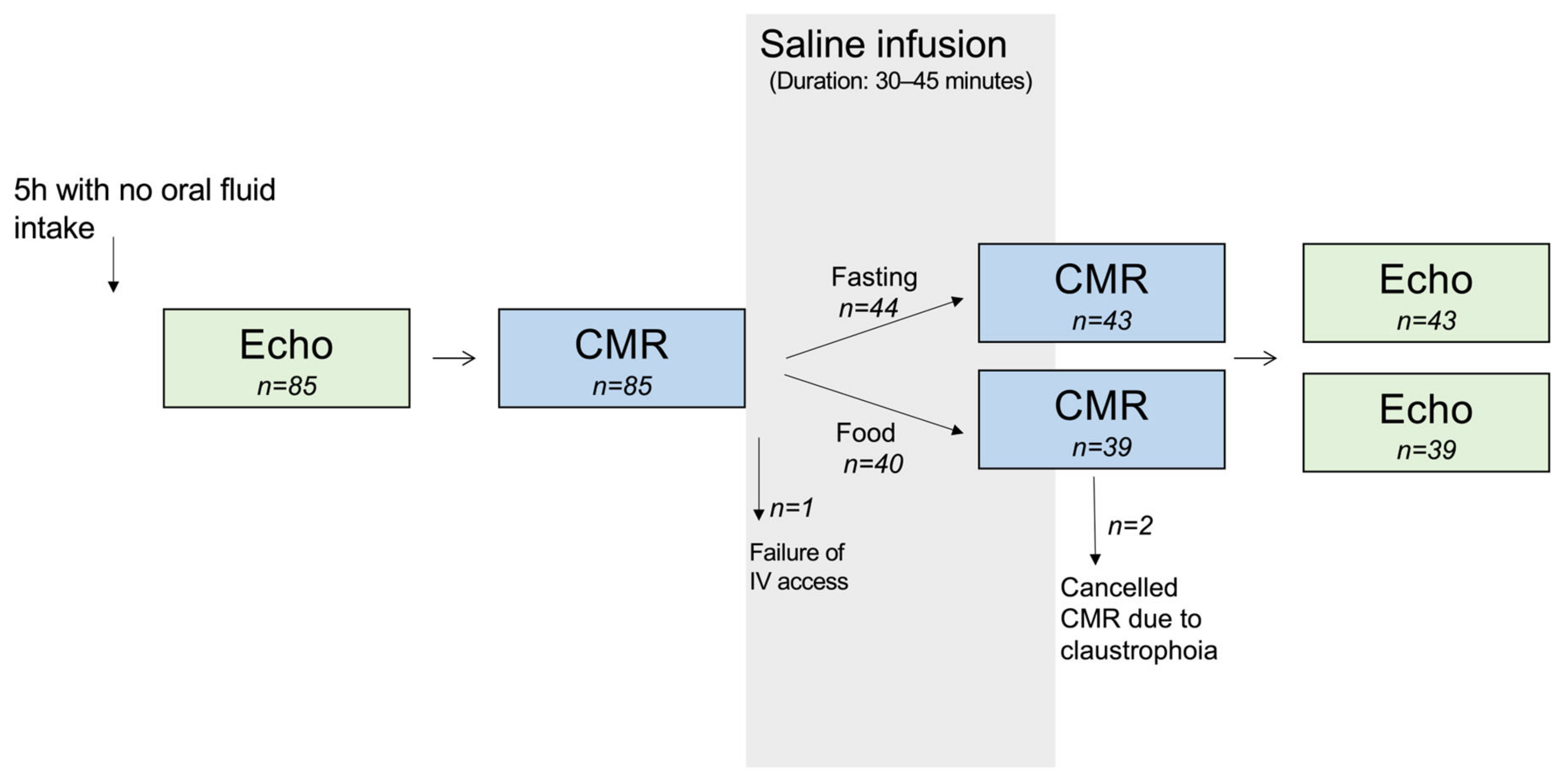
2.1. Study Design
2.2. CMR Acquisition and Analysis
2.3. Echocardiographic Acquisition and Analysis
2.4. Statistics
3. Results
3.1. Study Population
3.2. Left Ventricular Volumes and Ejection Fraction
3.3. Right Ventricular Volumes and Ejection Fraction
3.4. Left Ventricular Strain and Feature Tracking
3.5. Right Ventricular Strain and Feature Tracking
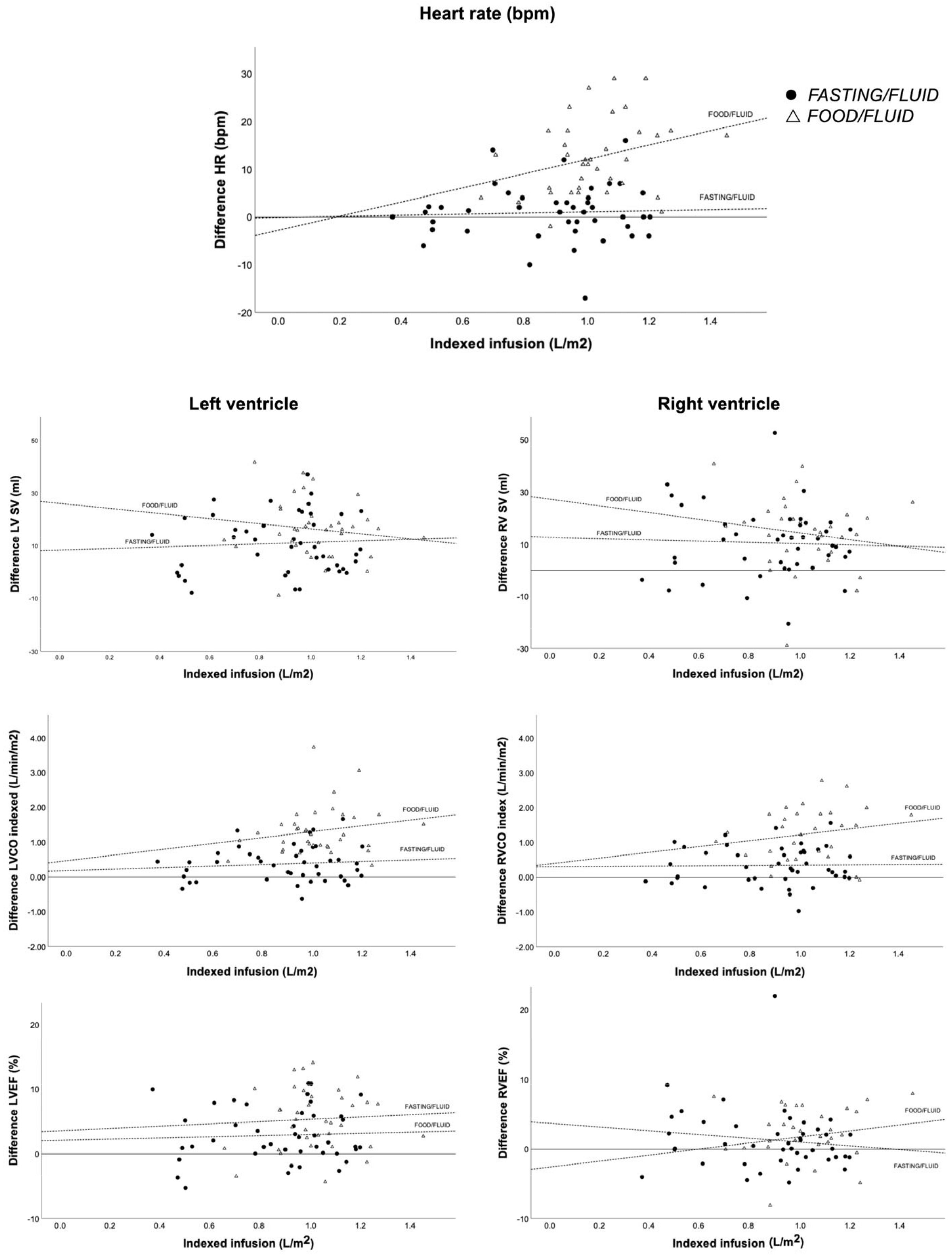
3.6. Heart Rate
3.7. Global Longitudinal Strain Deterioration According to Current Cardio-Oncology Guidelines
4. Discussion
4.1. Our Most Important Findings Are
- (1)
- CO and LV contractility (measured as LVEF, GLS, and CMR-FT) are significantly increased by preload augmentation and further increased by food intake.
- (2)
- Fluid infusion and food intake may falsely improve GLS, which may affect follow-up.
- (3)
- The LV and RV seem to act through different mechanisms when increasing CO: the LV through increased contractility and the RV through augmented EDV and ESV, which are indirectly driven by the effects of the LV.
4.2. Food Intake Recommendations before Cardiac Assessment
4.3. Mechanisms to Increase Cardiac Output—Differences between LV and RV
4.4. The Clinical Impact of Variations in Global Longitudinal Strain in Follow-Up Patients
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hollenberg, S.M. Hemodynamic monitoring. Chest 2013, 143, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Bleasdale, R.A.; Frenneaux, M.P. Prognostic importance of right ventricular dysfunction. Heart 2002, 88, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Mogelvang, R.; Sogaard, P.; Pedersen, S.A.; Olsen, N.T.; Marott, J.L.; Schnohr, P.; Goetze, J.P.; Jensen, J.S. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation 2009, 119, 2679–2685. [Google Scholar] [CrossRef]
- De Groote, P.; Millaire, A.; Foucher-Hossein, C.; Nugue, O.; Marchandise, X.; Ducloux, G.; Lablanche, J.M. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J. Am. Coll. Cardiol. 1998, 32, 948–954. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution o. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Waaler, B.A.; Eriksen, M.; Toska, K. The effect of meal size on postprandial increase in cardiac output. Acta Physiol. Scand. 1991, 142, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hlebowicz, J.; Lindstedt, S.; Björgell, O.; Dencker, M. Relationship between postprandial changes in cardiac left ventricular function, glucose and insulin concentrations, gastric emptying, and satiety in healthy subjects. Nutr. J. 2011, 10, 26. [Google Scholar] [CrossRef]
- Waaler, B.A.; Eriksen, M. Post-prandial cardiovascular responses in man after ingestion of carbohydrate, protein or fat. Acta Physiol. Scand. 1992, 146, 321–327. [Google Scholar]
- Fagan, T.C.; Sawyer, P.R.; Gourley, L.A.; Lee, J.T.; Gaffney, T.E. Postprandial alterations in hemodynamics and blood pressure in normal subjects. Am. J. Cardiol. 1986, 58, 636–641. [Google Scholar] [CrossRef]
- Ishimine, M.; Takamoto, T.; Nitta, M.; Marumo, F.; Murakami, K.; Takasu, N. Postprandial hemodynamic changes evaluated by a Doppler echocardiographic method. Jpn. Heart J. 1994, 35, 35–42. [Google Scholar] [CrossRef]
- Dencker, M.; Björgell, O.; Hlebowicz, J. Effect of food intake on commonly used pulsed Doppler and tissue Doppler measurements. Echocardiography 2011, 28, 843–847. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography. JACC Cardiovasc. Imaging 2019, 11, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, H.M.; Boubertakh, R.; Miquel, M.E.; Petersen, S.E. Myocardial deformation assessment using cardiovascular magnetic resonance-feature tracking technique. Br. J. Radiol. 2017, 90, 20170072. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Staszewsky, L.; Latini, R.; Barlera, S.; Glazer, R.; Aknay, N.; Hester, A.; Anand, I.; Cohn, J.N. Severity of left ventricular remodeling defines outcomes and response to therapy in heart failure: Valsartan heart failure trial (Val-HeFT) echocardiographic data. J. Am. Coll. Cardiol. 2004, 43, 2022–2027. [Google Scholar] [CrossRef]
- Toma, M.; Ezekowitz, J.A.; Bakal, J.A.; O’Connor, C.M.; Hernandez, A.F.; Sardar, M.R.; Zolty, R.; Massie, B.M.; Swedberg, K.; Armstrong, P.W.; et al. The relationship between left ventricular ejection fraction and mortality in patients with acute heart failure: Insights from the ASCEND-HF Trial. Eur. J. Heart Fail. 2014, 16, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.E.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020, 9, e018403. [Google Scholar] [CrossRef] [PubMed]
- Nagre, A. Focus-assessed transthoracic echocardiography: Implications in perioperative and intensive care. Ann. Card. Anaesth. 2019, 22, 302–308. [Google Scholar] [CrossRef] [PubMed]
- La Via, L.; Dezio, V.; Santonocito, C.; Astuto, M.; Morelli, A.; Huang, S.; Vieillard-Baron, A.; Sanfilippo, F. Full and simplified assessment of left ventricular diastolic function in COVID-19 patients admitted to ICU: Feasibility, incidence, and association with mortality. Echocardiography 2022, 39, 1391–1400. [Google Scholar] [CrossRef]
- Sanfilippo, F.; La Via, L.; Dezio, V.; Amelio, P.; Genoese, G.; Franchi, F.; Messina, A.; Robba, C.; Noto, A. Inferior vena cava distensibility from subcostal and trans-hepatic imaging using both M-mode or artificial intelligence: A prospective study on mechanically ventilated patients. Intensive Care Med. Exp. 2023, 11, 40. [Google Scholar] [CrossRef]
- Mohsin, M.; Farooq, M.U.; Akhtar, W.; Mustafa, W.; Rehman, T.U.; Malik, J.; Zahid, T. Echocardiography in a critical care unit: A contemporary review. Expert. Rev. Cardiovasc. Ther. 2022, 20, 55–63. [Google Scholar] [CrossRef]
- Papathanasiou, G.; Georgakopoulos, D.; Papageorgiou, E.; Zerva, E.; Michalis, L.; Kalfakakou, V.; Evangelou, A. Effects of Smoking on Heart Rate at Rest and During Exercise, and on Heart Rate Recovery, in Young Adults. Hell. J. Cardiol. 2013, 54, 168–177. [Google Scholar]
- Ramakrishnan, S.; Bhatt, K.; Dubey, A.K.; Roy, A.; Singh, S.; Naik, N.; Seth, S.; Bhargava, B. Acute Electrocardiographic Changes during Smoking: An Observational Study. BMJ Open 2013, 3, 2486. Available online: http://bmjopen.bmj.com (accessed on 3 October 2023). [CrossRef] [PubMed]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 87. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Kelbaek, H. Acute effects of alcohol and food intake on cardiac performance. Prog. Cardiovasc. Dis. 1990, 32, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Dieden, A.; Gårdinger, Y.; Hlebowicz, J.; Björgell, O.; Dencker, M. Effect of food intake on left and right ventricular systolic tissue Doppler measurements. Clin. Physiol. Funct. Imaging 2016, 36, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Lakatos, B.; Tokodi, M.; Merkely, B. Right ventricular mechanical pattern in health and disease: Beyond longitudinal shortening. Heart Fail. Rev. 2019, 24, 511–520. [Google Scholar] [CrossRef]
- Buckberg, G.; Hoffman, J.I.E. Evolving Technology/Basic Science Right ventricular architecture responsible for mechanical performance: Unifying role of ventricular septum. J. Thorac. Cardiovasc. Surg. 2014, 148, 3166–3171.e4. [Google Scholar] [CrossRef]
- Kukulski, T.; Hübbert, L.; Arnold, M.; Wranne, B.; Hatle, L.; Sutherland, G.R. Normal regional right ventricular function and its change with age: A Doppler myocardial imaging study. J. Am. Soc. Echocardiogr. 2000, 13, 194–204. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Austin, P.C.; Lee, D.S.; Amir, E.; Tu, J.V.; Thavendiranathan, P.; Fung, K.; Anderson, G.M. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol. 2017, 2, 88–93. [Google Scholar] [CrossRef]
- Armenian, S.H.; Xu, L.; Ky, B.; Sun, C.; Farol, L.T.; Pal, S.K.; Douglas, P.S.; Bhatia, S.; Chao, C. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J. Clin. Oncol. 2016, 34, 1122–1130. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Virani, S.A.; Dent, S.; Brezden-Masley, C.; Clarke, B.; Davis, M.K.; Jassal, D.S.; Johnson, C.; Lemieux, J.; Paterson, I.; Sebag, I.A.; et al. Canadian Cardiovascular Society Guidelines for Evaluation and Management of Cardiovascular Complications of Cancer Therapy. Can. J. Cardiol. 2016, 32, 831–841. [Google Scholar] [CrossRef] [PubMed]

| Fasting/Fluid (n = 43) | Food/Fluid (n = 39) | p-Value | |
|---|---|---|---|
| Age (years) | 42 ± 15 | 45 ± 13 | 0.35 |
| Male sex, n (%) | 30 (70) | 27 (70) | 1.00 |
| Height (m) | 179 ± 9 | 178 ± 10 | 0.77 |
| Weight (kg) | 80 ± 16 | 82 ± 16 | 0.66 |
| BMI (kg/m2) | 25 ± 4 | 26 ± 4 | 0.47 |
| BSA (m2) | 2.0 ± 0.2 | 2.0 ± 0.2 | 0.82 |
| Saline infusion (L) | 1.7 ± 0.4 | 2.0 ± 0.3 | <0.01 |
| Saline infusion index (L/m2) | 0.9 ± 0.2 | 1.0 ± 0.2 | <0.01 |
| Cardiac disease, n (%) | 23 (54) | 17 (44) | 0.29 |
| Risk factors | |||
| Diabetes, n (%) | 1 (2) | 2 (5) | 0.51 |
| Hypertension, n (%) | 8 (19) | 9 (23) | 0.62 |
| Stroke or TIA, n (%) | 1 (3) | 0 | 0.30 |
| Previous/current smoker, n (%) | 21 (49) | 14 (36) | 0.24 |
| Left ventricular geometry | |||
| Normal, n (%) | 15 (35) | 16 (41) | 0.57 |
| Dilated, n (%) | 13 (30) | 10 (26) | 0.65 |
| Hypertrophied, n (%) | 8 (19) | 5 (13) | 0.48 |
| Dilated + Hypertrophied, n (%) | 7 (16) | 8 (21) | 0.63 |
| Fasting/Fluid n = 43 | Food/Fluid n = 39 | Pre vs. Pre | Diff (Post–Pre) | Diff vs. Diff | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p | Pre | Post | p | p | Fasting/Fluid | Food/Fluid | p | |
| SBP (mmHg) | 124 ± 18 | 127 ± 19 | <0.05 | 129 ± 16 | 130 ± 17 | NS | NS | 3 ± 9 | 1 ± 10 | NS |
| DBP (mmHg) | 73 ± 12 | 76 ± 12 | <0.01 | 78 ± 13 | 79 ± 11 | NS | <0.05 | 3 ± 6 | 2 ± 9 | NS |
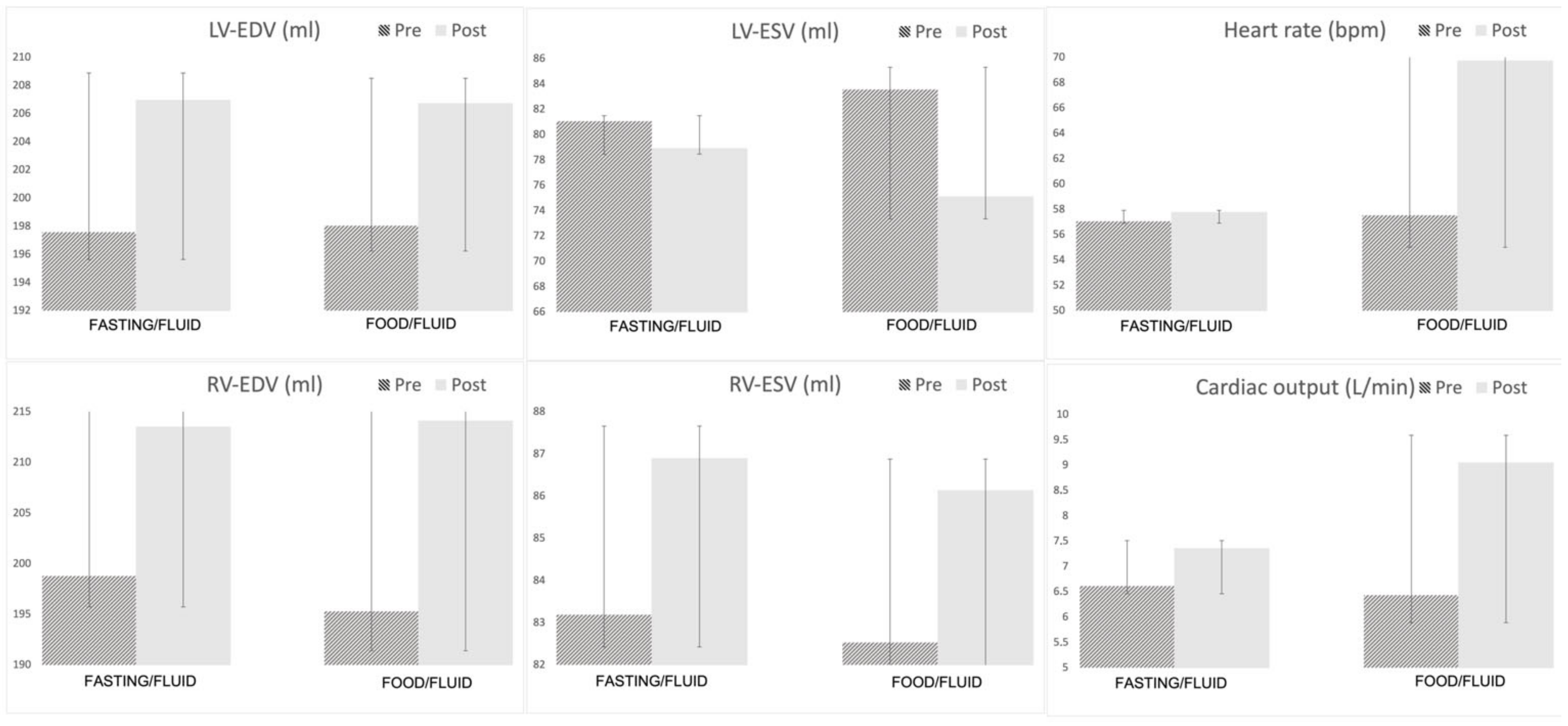
| HR (bpm) | 57 ± 10 | 57 ± 11 | NS | 57 ± 10 | 70 ± 11 | <0.001 | NS | 1 ± 6 | 12 ± 8 | <0.001 |
| LV-EDV (mL) | 198 ± 59 | 207 ± 57 | <0.001 | 198 ± 64 | 207 ± 58 | <0.001 | NS | 9 ± 13 | 9 ± 12 | NS |
| RV-EDV (mL) | 200 ± 47 | 214 ± 50 | <0.001 | 195 ± 43 | 212 ± 45 | <0.001 | NS | 14 ± 16 | 18 ± 14 | NS |
| LV-ESV (mL) | 81 ± 29 | 79 ± 28 | NS | 82 ± 35 | 75 ± 32 | <0.001 | NS | −2 ± 10 | −7 ± 11 | <0.05 |
| RV-ESV (mL) | 84 ± 26 | 87 ± 31 | NS | 82 ± 23 | 86 ± 25 | NS | NS | 3 ± 13 | 4 ± 11 | NS |
| LV-SV (mL) | 117 ± 34 | 128 ± 33 | <0.001 | 115 ± 33 | 132 ± 32 | <0.001 | NS | 11 ± 11 | 16 ± 11 | <0.05 |
| RV-SV (mL) | 116 ± 25 | 127 ± 26 | <0.001 | 113 ± 24 | 127 ± 26 | <0.001 | NS | 11 ± 13 | 14 ± 14 | NS |
| LV-CO (L/min) | 6.6 ± 2.1 | 7.4 ± 2.1 | <0.001 | 6.5 ± 1.4 | 9.0 ± 2.3 | <0.001 | NS | 0.7 ± 1.0 | 2.6 ± 1.3 | <0.001 |
| RV-CO (L/min) | 6.6 ± 1.9 | 7.3 ± 1.8 | <0.001 | 6.3 ± 1.4 | 8.8 ± 1.7 | <0.001 | NS | 0.7 ± 1.1 | 2.4 ± 1.4 | <0.001 |
| LV-CO index (L/min/m2) | 3.3 ± 0.9 | 3.7 ± 1.0 | <0.001 | 3.3 ± 0.6 | 4.6 ± 1.1 | <0.001 | NS | 0.4 ± 0.5 | 1.3 ± 0.7 | <0.001 |
| RV-CO-index (L/min/m2) | 3.3 ± 0.8 | 3.7 ± 0.8 | <0.001 | 3.2 ± 0.6 | 4.5 ± 0.8 | <0.001 | NS | 0.3 ± 0.5 | 1.2 ± 0.8 | <0.001 |
| LVEF(%) | 59 ± 6 | 62 ± 5 | <0.001 | 59 ± 6 | 64 ± 6 | <0.001 | NS | 3 ± 4 | 5 ± 5 | <0.05 |
| RVEF(%) | 59 ± 6 | 60 ± 6 | NS | 58 ± 5 | 60 ± 5 | <0.05 | NS | 1 ± 5 | 2 ± 5 | NS |
| Model | Food Intake | Indexed Fluid Infusion (L/m2) | |||
|---|---|---|---|---|---|
| p | B | p | B | p | |
| LV-CO (L/min) | <0.001 | 1.8 | <0.001 | 0.5 | NS |
| LV-CO index (L/min/m2) | <0.001 | 0.9 | <0.001 | 0.4 | NS |
| RV-CO-index (L/min/m2) | <0.001 | 1.7 | <0.001 | 0.4 | NS |
| RV-CO-index (L/min/m2) | <0.001 | 0.9 | <0.001 | 0.3 | NS |
| LV-FT (%) | <0.001 | −1.1 | <0.01 | −1.9 | NS |
| GLS (%) | <0.05 | −0.3 | NS | −2.3 | <0.05 |
| GLSbasal (%) | <0.05 | −0.3 | NS | −2.0 | <0.05 |
| GLSmid (%) | <0.05 | −0.1 | NS | −1.8 | <0.05 |
| GLSapical (%) | NS | −0.6 | NS | −3.4 | NS |
| RV-FT (%) | NS | 0.9 | NS | 1.3 | NS |
| RV-strainall (%) | <0.05 | −2.9 | <0.01 | 1.2 | NS |
| RV-strainfreewall (%) | NS | −1.1 | NS | −2.1 | NS |
| Fasting/Fluid n = 43 | Food/Fluid n = 39 | Pre vs. Pre | Diff (Post–Pre) | Diff vs. Diff | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p | Pre | Post | p | p | Fasting/Fluid | Food/Fluid | p | |
| LV-FT (%) | −19.0 ± 2.9 | −19.6 ± 3.5 | <0.01 | −19.1 ± 2.5 | −21.0 ± 3.5 | <0.001 | NS | −0.6 ± 1.3 | −2.0 ± 2.3 | <0.01 |
| GLS (%) | −18.8 ± 3.6 | −19.7 ± 4.0 | <0.001 | −18.9 ± 3.0 | −20.4 ± 3.4 | <0.001 | NS | −0.9 ± 1.5 | −1.5 ± 1.8 | NS |
| GLSbasal (%) | −17.9 ± 2.8 | −18.3 ± 3.0 | 0.10 | −17.4 ± 3.1 | −18.3 ± 3.6 | <0.001 | NS | −0.4 ± 1.7 | −1.0 ± 1.7 | NS |
| GLSmid (%) | −18.5 ± 3.6 | −19.5 ± 4.0 | <0.001 | −18.8 ± 3.0 | −20.0 ± 3.5 | <0.001 | NS | −1.0 ± 1.5 | −1.3 ± 1.4 | NS |
| GLSapical (%) | −20.1 ± 5.6 | −21.3 ± 6.0 | <0.01 | −20.8 ± 4.1 | −23.0 ± 4.5 | <0.001 | NS | −1.2 ± 2.6 | −2.2 ± 3.8 | NS |
| RV-FT (%) | −26.2 ± 3.9 | −26.9 ± 3.4 | NS | −26.0 ± 3.3 | −27.7 ± 3.1 | <0.01 | NS | −0.6 ± 3.0 | −1.7 ± 3.1 | NS |
| RV-strainall (%) | −21.9 ± 2.8 | −21.9 ± 2.9 | NS | −20.8 ± 2.5 | −23.5 ± 2.8 | <0.01 | <0.05 | 0.1 ± 2.0 | −2.7 ± 1.6 | <0.01 |
| RV-strainfreewall (%) | −27.4 ± 3.5 | −27.2 ± 3.8 | NS | −25.3 ± 3.7 | −26.3 ± 4.6 | NS | NS | 0.3 ± 2.9 | −1.0 ± 3.5 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visby, L.; Møgelvang, R.; Grund, F.F.; Myhr, K.A.; Hassager, C.; Vejlstrup, N.; Mattu, R.; Kristensen, C.B. The Influence of Food Intake and Preload Augmentation on Cardiac Functional Parameters: A Study Using Both Cardiac Magnetic Resonance and Echocardiography. J. Clin. Med. 2023, 12, 6781. https://doi.org/10.3390/jcm12216781
Visby L, Møgelvang R, Grund FF, Myhr KA, Hassager C, Vejlstrup N, Mattu R, Kristensen CB. The Influence of Food Intake and Preload Augmentation on Cardiac Functional Parameters: A Study Using Both Cardiac Magnetic Resonance and Echocardiography. Journal of Clinical Medicine. 2023; 12(21):6781. https://doi.org/10.3390/jcm12216781
Chicago/Turabian StyleVisby, Lasse, Rasmus Møgelvang, Frederik Fasth Grund, Katrine Aagaard Myhr, Christian Hassager, Niels Vejlstrup, Raj Mattu, and Charlotte Burup Kristensen. 2023. "The Influence of Food Intake and Preload Augmentation on Cardiac Functional Parameters: A Study Using Both Cardiac Magnetic Resonance and Echocardiography" Journal of Clinical Medicine 12, no. 21: 6781. https://doi.org/10.3390/jcm12216781
APA StyleVisby, L., Møgelvang, R., Grund, F. F., Myhr, K. A., Hassager, C., Vejlstrup, N., Mattu, R., & Kristensen, C. B. (2023). The Influence of Food Intake and Preload Augmentation on Cardiac Functional Parameters: A Study Using Both Cardiac Magnetic Resonance and Echocardiography. Journal of Clinical Medicine, 12(21), 6781. https://doi.org/10.3390/jcm12216781





