PIPAC for Gastrointestinal Malignancies
Abstract
:1. Introduction
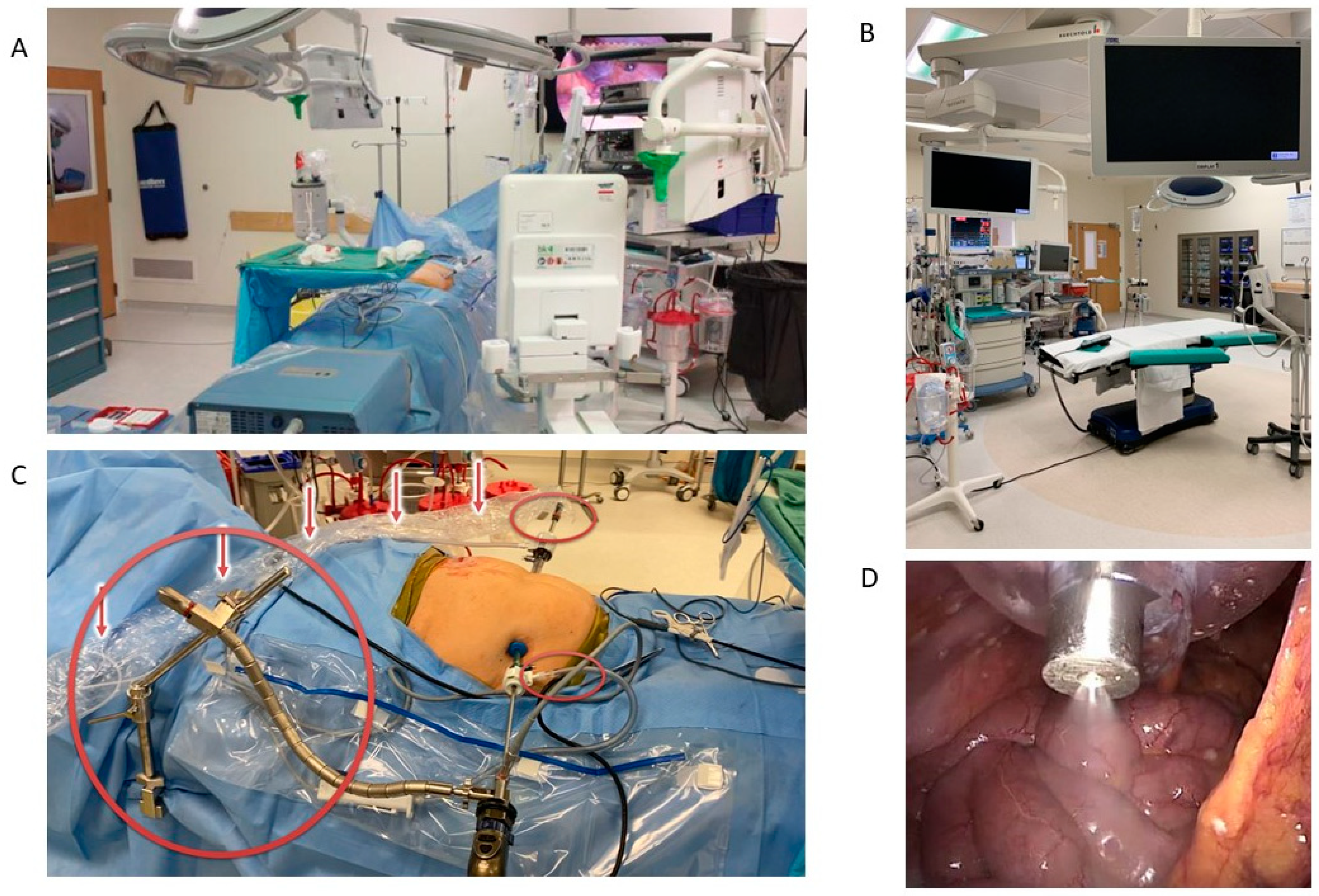
1.1. Surgical Technique
1.2. Drug Selection
1.3. Comparison of PIPAC to NIPEC and HIPEC
1.4. Complications with PIPAC
2. Clinical Data
2.1. Gastric
2.2. Colorectal and Appendiceal
2.3. Pancreatobiliary
3. Future Directions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarela, A.I.; Miner, T.J.; Karpeh, M.S.; Coit, D.G.; Jaques, D.P.; Brennan, M.F. Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann. Surg. 2006, 243, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.A.; Wiseman, J. Palliative Management of Peritoneal Metastases. Ann. Surg. Oncol. 2018, 25, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Bootsma, S.; Bijlsma, M.F.; Vermeulen, L. The molecular biology of peritoneal metastatic disease. EMBO Mol. Med. 2023, 15, e15914. [Google Scholar] [CrossRef]
- Sandoval, P.; Jiménez-Heffernan, J.A.; Rynne-Vidal, Á.; Pérez-Lozano, M.L.; Gilsanz, Á.; Ruiz-Carpio, V.; Reyes, R.; García-Bordas, J.; Stamatakis, K.; Dotor, J.; et al. Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J. Pathol. 2013, 231, 517–531. [Google Scholar] [CrossRef]
- Li, J.; Guo, T. Role of Peritoneal Mesothelial Cells in the Progression of Peritoneal Metastases. Cancers 2022, 14, 2856. [Google Scholar] [CrossRef]
- Dedrick, R.L.; Myers, C.E.; Bungay, P.M.; DeVita, V.T., Jr. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat. Rep. 1978, 62, 1–11. [Google Scholar]
- Flessner, M.F. Small-solute transport across specific peritoneal tissue surfaces in the rat. J. Am. Soc. Nephrol. 1996, 7, 225–233. [Google Scholar] [CrossRef]
- Spratt, J.S.; Adcock, R.A.; Muskovin, M.; Sherrill, W.; McKeown, J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980, 40, 256–260. [Google Scholar]
- Harper, M.M.; Kim, J.; Pandalai, P.K. Current Trends in Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Disease from Appendiceal and Colorectal Malignancies. J. Clin. Med. 2022, 11, 2840. [Google Scholar] [CrossRef]
- Kusamura, S.; Dominique, E.; Baratti, D.; Younan, R.; Deraco, M. Drugs carrier solutions temperature in hyperthermic intraperitoneal chemotherapy. J. Surg. Oncol. 2008, 98, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Normothermic intraperitoneal chemotherapy long term (NIPEC-LT) in the management of peritoneal surface malignancy, an overview. Pleura Perit. 2017, 2, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Intraperitoneal paclitaxel: Pharmacology, clinical results and future prospects. J. Gastrointest. Oncol. 2021, 12 (Suppl. S1), S231–S239. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [PubMed]
- Solass, W.; Herbette, A.; Schwarz, T.; Hetzel, A.; Sun, J.S.; Dutreix, M.; Reymond, M.A. Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: Proof of concept. Surg. Endosc. 2012, 26, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Khosrawipour, V.; Khosrawipour, T.; Kern, A.J.; Osma, A.; Kabakci, B.; Diaz-Carballo, D.; Förster, E.; Zieren, J.; Fakhrian, K. Distribution pattern and penetration depth of doxorubicin after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a postmortem swine model. J. Cancer Res. Clin. Oncol. 2016, 142, 2275–2280. [Google Scholar] [CrossRef]
- Esquis, P.; Consolo, D.; Magnin, G.; Pointaire, P.; Moretto, P.; Ynsa, M.D.; Beltramo, J.L.; Drogoul, C.; Simonet, M.; Benoit, L.; et al. High intra-abdominal pressure enhances the penetration and antitumor effect of intraperitoneal cisplatin on experimental peritoneal carcinomatosis. Ann. Surg. 2006, 244, 106–112. [Google Scholar] [CrossRef]
- Jacquet, P.; Stuart, O.A.; Chang, D.; Sugarbaker, P.H. Effects of intra-abdominal pressure on pharmacokinetics and tissue distribution of doxorubicin after intraperitoneal administration. Anticancer Drugs 1996, 7, 596–603. [Google Scholar] [CrossRef]
- Facy, O.; Al Samman, S.; Magnin, G.; Ghiringhelli, F.; Ladoire, S.; Chauffert, B.; Rat, P.; Ortega-Deballon, P. High pressure enhances the effect of hyperthermia in intraperitoneal chemotherapy with oxaliplatin: An experimental study. Ann. Surg. 2012, 256, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Shree, V.; Lim, T.J.; Lean, L.L.; So, B.Y.J.; Kim, G. Anaesthesia considerations and techniques for Pressurised IntraPeritoneal Aerosol Chemotherapy (PIPAC). Pleura Perit. 2020, 5, 20190013. [Google Scholar] [CrossRef]
- Robella, M.; Vaira, M.; De Simone, M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: An innovative approach to treat peritoneal carcinomatosis. World J. Surg. Oncol. 2016, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Giger-Pabst, U.; Tempfer, C.B. How to Perform Safe and Technically Optimized Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): Experience After a Consecutive Series of 1200 Procedures. J. Gastrointest. Surg. 2018, 22, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Vaira, M.; Robella, M.; Borsano, A.; De Simone, M. Single-port access for Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC): Technique, feasibility and safety. Pleura Perit. 2016, 1, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Robella, M.; Hubner, M.; Sgarbura, O.; Reymond, M.; Khomiakov, V.; di Giorgio, A.; Bhatt, A.; Bakrin, N.; Willaert, W.; Alyami, M.; et al. Feasibility and safety of PIPAC combined with additional surgical procedures: PLUS study. Eur. J. Surg. Oncol. 2022, 48, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Tabchouri, N.; Buggisch, J.; Demtröder, C.R.; Thiery, J.; Rezniczek, G.; Tempfer, C.B.; Fischer, B.; Dogan, C.; Lecomte, T.; Ouaissi, M.; et al. Pressurized Intraperitoneal Aerosol Chemotherapy for Colorectal Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 5275–5286. [Google Scholar] [CrossRef]
- Jansen-Winkeln, B.; Eberth, J.; Moulla, Y.; Mehdorn, M.; Niebisch, S.; Schierle, K.; Bläker, H.; Lordick, F.; Gockel, I.; Thieme, R. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with peritoneal surface malignancies (PSM): A prospective single-center registry study. J. Cancer Res. Clin. Oncol. 2023, 149, 1331–1341. [Google Scholar] [CrossRef]
- Reymond, M.A.; Konigsrainer, A. Chapter 10—Optimizing intraperitoneal drug delivery: Pressurized intraperitoneal aerosol chemotherapy (PIPAC). In Drug Delivery Trends; Expectations and Realities of Multifunctional Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2020; Volume 3, pp. 197–214. [Google Scholar]
- Davigo, A.; Passot, G.; Vassal, O.; Bost, M.; Tavernier, C.; Decullier, E.; Bakrin, N.; Alyami, M.; Bonnet, J.M.; Louzier, V.; et al. PIPAC versus HIPEC: Cisplatin spatial distribution and diffusion in a swine model. Int. J. Hyperth. 2020, 37, 144–150. [Google Scholar] [CrossRef]
- Khosrawipour, V.; Khosrawipour, T.; Diaz-Carballo, D.; Förster, E.; Zieren, J.; Giger-Pabst, U. Exploring the Spatial Drug Distribution Pattern of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Ann. Surg. Oncol. 2016, 23, 1220–1224. [Google Scholar] [CrossRef]
- Mimouni, M.; Richard, C.; Adenot, P.; Letheule, M.; Tarrade, A.; Sandra, O.; Dahirel, M.; Lilin, T.; Lecuelle, B.; Gélin, V.; et al. Pressurized intra-peritoneal aerosol chemotherapy (PIPAC): Increased intraperitoneal pressure does not affect distribution patterns but leads to deeper penetration depth of doxorubicin in a sheep model. BMC Cancer 2021, 21, 461. [Google Scholar] [CrossRef]
- Taibi, A.; Teixeira Farinha, H.; Durand Fontanier, S.; Sayedalamin, Z.; Hübner, M.; Sgarbura, O. Pressurized Intraperitoneal Aerosol Chemotherapy Enhanced by Electrostatic Precipitation (ePIPAC) for Patients with Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 3852–3860. [Google Scholar] [CrossRef]
- Robella, M.; De Simone, M.; Berchialla, P.; Argenziano, M.; Borsano, A.; Ansari, S.; Abollino, O.; Ficiarà, E.; Cinquegrana, A.; Cavalli, R.; et al. A Phase I Dose Escalation Study of Oxaliplatin, Cisplatin and Doxorubicin Applied as PIPAC in Patients with Peritoneal Carcinomatosis. Cancers 2021, 13, 1060. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Tan, H.L.; Sundar, R.; Lieske, B.; Chee, C.E.; Ho, J.; Shabbir, A.; Babak, M.V.; Ang, W.H.; Goh, B.C.; et al. PIPAC-OX: A Phase I Study of Oxaliplatin-Based Pressurized Intraperitoneal Aerosol Chemotherapy in Patients with Peritoneal Metastases. Clin. Cancer Res. 2021, 27, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Graversen, M.; Lundell, L.; Fristrup, C.; Pfeiffer, P.; Mortensen, M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) as an outpatient procedure. Pleura Perit. 2018, 3, 20180128. [Google Scholar] [CrossRef]
- Tidadini, F.; Abba, J.; Quesada, J.L.; Villeneuve, L.; Foote, A.; Baudrant, M.; Bonne, A.; Glehen, O.; Trilling, B.; Faucheron, J.L.; et al. Assessment of postoperative pain after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the treatment of peritoneal metastasis. Int. J. Color. Dis. 2022, 37, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Graversen, M.; Detlefsen, S.; Pfeiffer, P.; Lundell, L.; Mortensen, M.B. Severe peritoneal sclerosis after repeated pressurized intraperitoneal aerosol chemotherapy with oxaliplatin (PIPAC OX): Report of two cases and literature survey. Clin. Exp. Metastasis 2018, 35, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Sánchez, A.; Espinosa-Redondo, E.; Gutiérrez-Calvo, A.; Segura-Sampedro, J.J.; Pérez-Viejo, E.; Concepción-Martín, V.; Sánchez-García, S.; García-Fadrique, A.; Prieto-Nieto, I.; Barrios-Sanchez, P.; et al. Efficacy and Safety of Intraoperative Hyperthermic Intraperitoneal Chemotherapy for Locally Advanced Colon Cancer: A Phase 3 Randomized Clinical Trial. JAMA Surg. 2023, 158, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.; Khosrawipour, V.; Chaudhry, H.; Arafkas, M.; Knoefel, W.T.; Pigazzi, A.; Khosrawipour, T. Comparing the cytotoxicity of taurolidine, mitomycin C, and oxaliplatin on the proliferation of in vitro colon carcinoma cells following pressurized intra-peritoneal aerosol chemotherapy (PIPAC). World J. Surg. Oncol. 2019, 17, 93. [Google Scholar] [CrossRef]
- Alyami, M.; Gagniere, J.; Sgarbura, O.; Cabelguenne, D.; Villeneuve, L.; Pezet, D.; Quenet, F.; Glehen, O.; Bakrin, N.; Passot, G. Multicentric initial experience with the use of the pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the management of unresectable peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2017, 43, 2178–2183. [Google Scholar] [CrossRef]
- Raoof, M.; Sullivan, K.M.; Frankel, P.H.; Fakih, M.; Synold, T.W.; Lim, D.; Woo, Y.; Paz, I.B.; Fong, Y.; Thomas, R.M.; et al. Multicenter dose-escalation Phase I trial of mitomycin C pressurized intraperitoneal aerosolized chemotherapy in combination with systemic chemotherapy for appendiceal and colorectal peritoneal metastases: Rationale and design. Pleura Perit. 2022, 7, 169–177. [Google Scholar] [CrossRef]
- Mehta, S.; Kammar, P.; Patel, A.; Goswami, G.; Shaikh, S.; Sukumar, V.; Trivedi, E.; Bhatt, A. Feasibility and Safety of Taxane-PIPAC in Patients with Peritoneal Malignancies—A Retrospective Bi-institutional Study. Indian J. Surg. Oncol. 2022, 14 (Suppl. S1), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van De Sande, L.; Graversen, M.; Hubner, M.; Pocard, M.; Reymond, M.; Vaira, M.; Cosyns, S.; Willaert, W.; Ceelen, W. Intraperitoneal aerosolization of albumin-stabilized paclitaxel nanoparticles (Abraxane™) for peritoneal carcinomatosis—A phase I first-in-human study. Pleura Perit. 2018, 3, 20180112. [Google Scholar] [CrossRef]
- Lang, N.; Diciola, A.; Labidi-Galy, I.; Ris, F.; Di Marco, M.; Mach, N.; Petignat, P.; Toso, C.; Undurraga, M.; Hubner, M. Nab-PIPAC: A phase IB study protocol of intraperitoneal cisplatin and nab-paclitaxel administered by pressurised intraperitoneal aerosol chemotherapy (PIPAC) in the treatment of advanced malignancies confined to the peritoneal cavity. BMJ Open 2023, 13, e067691. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, C.; Passot, G.; Vassal, O.; Allaouchiche, B.; Decullier, E.; Bakrin, N.; Alyami, M.; Davigo, A.; Bonnet, J.M.; Louzier, V.; et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) might increase the risk of anastomotic leakage compared to HIPEC: An experimental study. Surg. Endosc. 2020, 34, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Ametsbichler, P.; Böhlandt, A.; Nowak, D.; Schierl, R. Occupational exposure to cisplatin/oxaliplatin during Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)? Eur. J. Surg. Oncol. 2018, 44, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Giger-Pabst, U.; Bucur, P.; Roger, S.; Falkenstein, T.A.; Tabchouri, N.; Le Pape, A.; Lerondel, S.; Demtröder, C.; Salamé, E.; Ouaissi, M. Comparison of Tissue and Blood Concentrations of Oxaliplatin Administrated by Different Modalities of Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2019, 26, 4445–4451. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, A.C.; Lee, R.M.; Turgeon, M.K.; Zaidi, M.Y.; Kimbrough, C.W.; Grotz, T.E.; Leiting, J.; Fournier, K.; Lee, A.J.; Dineen, S.P.; et al. Implications of Postoperative Complications for Survival after Cytoreductive Surgery and HIPEC: A Multi-Institutional Analysis of the US HIPEC Collaborative. Ann. Surg. Oncol. 2020, 27, 4980–4995. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, N.L.; Koh, C.E.; Ansari, N.; Munoz, P.A.; McNamara, S.G.; Steffens, D. Preoperative cardiopulmonary exercise testing improves risk assessment of morbidity and length of stay following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesth. Intensive Care 2022, 50, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Schena, C.A.; El Halabieh, M.A.; Abatini, C.; Vita, E.; Strippoli, A.; Inzani, F.; Rodolfino, E.; Romanò, B.; Pacelli, F.; et al. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): A bidirectional approach for gastric cancer peritoneal metastasis. Surg. Oncol. 2020, 34, 270–275. [Google Scholar] [CrossRef]
- Siebert, M.; Alyami, M.; Mercier, F.; Gallice, C.; Villeneuve, L.; Laplace, N.; Passot, G.; Bakrin, N.; Glehen, O.; Kepenekian, V. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in association with systemic chemotherapy and bevacizumab, evaluation of safety and feasibility. A single center comparative study. Eur. J. Surg. Oncol. 2021, 47, 139–142. [Google Scholar] [CrossRef]
- Graversen, M.; Rouvelas, I.; Ainsworth, A.P.; Bjarnesen, A.P.; Detlefsen, S.; Ellebaek, S.B.; Fristrup, C.W.; Liljefors, M.G.; Lundell, L.; Nilsson, M.; et al. Feasibility and Safety of Laparoscopic D2 Gastrectomy in Combination with Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in Patients with Gastric Cancer at High Risk of Recurrence—The PIPAC-OPC4 Study. Ann. Surg. Oncol. 2023, 30, 4433–4441. [Google Scholar] [CrossRef] [PubMed]
- Girshally, R.; Demtröder, C.; Albayrak, N.; Zieren, J.; Tempfer, C.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J. Surg. Oncol. 2016, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Tidadini, F.; Abba, J.; Quesada, J.L.; Trilling, B.; Bonne, A.; Foote, A.; Faucheron, J.L.; Arvieux, C. Oncological Outcomes After Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in the Treatment of Peritoneal Carcinomatosis. J. Gastrointest. Cancer 2023, 54, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Mercier, F.; Siebert, M.; Bonnot, P.E.; Laplace, N.; Villeneuve, L.; Passot, G.; Glehen, O.; Bakrin, N.; Kepenekian, V. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2021, 47, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, N.; Chen, H.C.; Wang, X.; Blum, M.; Estrella, J.S.; Fournier, K.; Mansfield, P.; Ajani, J.; Badgwell, B.D. Patterns of Initial Recurrence in Gastric Adenocarcinoma in the Era of Preoperative Therapy. Ann. Surg. Oncol. 2017, 24, 2679–2687. [Google Scholar] [CrossRef] [PubMed]
- Diniz, T.P.; da Costa, W.L., Jr.; Gomes, C.C.; de Jesus, V.H.F.; Felismino, T.C.; Torres, S.M.; Ribeiro, H.S.C.; Diniz, A.L.; de Godoy, A.L.; de Farias, I.C.; et al. Symptomatic Recurrence and Survival Outcomes After Curative Treatment of Gastric Cancer: Does Intensive Follow-up Evaluation Improve Survival? Ann. Surg. Oncol. 2022, 29, 274–284. [Google Scholar] [CrossRef]
- Green, B.L.; Blumenthaler, A.N.; Gamble, L.A.; McDonald, J.D.; Robinson, K.; Connolly, M.; Epstein, M.; Hernandez, J.M.; Blakely, A.M.; Badgwell, B.D.; et al. Cytoreduction and HIPEC for Gastric Carcinomatosis: Multi-institutional Analysis of Two Phase II Clinical Trials. Ann. Surg. Oncol. 2023, 30, 1852–1860. [Google Scholar] [CrossRef]
- Ji, Z.H.; Yu, Y.; Liu, G.; Zhang, Y.B.; An, S.L.; Li, B.; Li, X.B.; Yan, G.J.; Li, Y. Peritoneal cancer index (PCI) based patient selecting strategy for complete cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy in gastric cancer with peritoneal metastasis: A single-center retrospective analysis of 125 patients. Eur. J. Surg. Oncol. 2021, 47, 1411–1419. [Google Scholar] [CrossRef]
- Hotopp, T. HIPEC and CRS in peritoneal metastatic gastric cancer—Who really benefits? Surg. Oncol. 2019, 28, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Khomyakov, V.; Ryabov, A.; Ivanov, A.; Bolotina, L.; Utkina, A.; Volchenko, N.; Kaprin, A. Bidirectional chemotherapy in gastric cancer with peritoneal metastasis combining intravenous XELOX with intraperitoneal chemotherapy with low-dose cisplatin and Doxorubicin administered as a pressurized aerosol: An open-label, Phase-2 study (PIPAC-GA2). Pleura Perit. 2016, 1, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Nadiradze, G.; Giger-Pabst, U.; Zieren, J.; Strumberg, D.; Solass, W.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J. Gastrointest. Surg. 2016, 20, 367–373. [Google Scholar] [CrossRef]
- Gockel, I.; Jansen-Winkeln, B.; Haase, L.; Rhode, P.; Mehdorn, M.; Niebisch, S.; Moulla, Y.; Lyros, O.; Lordick, F.; Schierle, K.; et al. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in Gastric Cancer Patients with Peritoneal Metastasis (PM): Results of a Single-Center Experience and Register Study. J. Gastric Cancer 2018, 18, 379–391. [Google Scholar] [CrossRef]
- Struller, F.; Horvath, P.; Solass, W.; Weinreich, F.J.; Strumberg, D.; Kokkalis, M.K.; Fischer, I.; Meisner, C.; Königsrainer, A.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: A phase II study. Ther. Adv. Med. Oncol. 2019, 11, 1758835919846402. [Google Scholar] [CrossRef] [PubMed]
- Ellebæk, S.B.; Graversen, M.; Detlefsen, S.; Lundell, L.; Fristrup, C.W.; Pfeiffer, P.; Mortensen, M.B. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) of peritoneal metastasis from gastric cancer: A descriptive cohort study. Clin. Exp. Metastasis 2020, 37, 325–332. [Google Scholar] [CrossRef]
- Alyami, M.; Bonnot, P.E.; Mercier, F.; Laplace, N.; Villeneuve, L.; Passot, G.; Bakrin, N.; Kepenekian, V.; Glehen, O. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable peritoneal metastasis from gastric cancer. Eur. J. Surg. Oncol. 2021, 47, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Sindayigaya, R.; Dogan, C.; Demtröder, C.R.; Fischer, B.; Karam, E.; Buggisch, J.R.; Tempfer, C.B.; Lecomte, T.; Ouaissi, M.; Giger-Pabst, U. Clinical Outcome for Patients Managed with Low-Dose Cisplatin and Doxorubicin Delivered as Pressurized Intraperitoneal Aerosol Chemotherapy for Unresectable Peritoneal Metastases of Gastric Cancer. Ann. Surg. Oncol. 2022, 29, 112–123. [Google Scholar] [CrossRef]
- Tidadini, F.; Abba, J.; Quesada, J.L.; Baudrant, M.; Bonne, A.; Foote, A.; Faucheron, J.L.; Glehen, O.; Villeneuve, L.; Arvieux, C. Effect of Pressurized Intraperitoneal Aerosol Chemotherapy on the Survival Rate of Patients with Peritoneal Carcinomatosis of Gastric Origin. J. Gastrointest. Cancer 2022, 53, 971–979. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 1139–1167. [Google Scholar] [CrossRef] [PubMed]
- Segelman, J.; Granath, F.; Holm, T.; Machado, M.; Mahteme, H.; Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2012, 99, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Sánchez, A.; Barrios, P.; Boldo-Roda, E.; Camps, B.; Carrasco-Campos, J.; Concepción Martín, V.; García-Fadrique, A.; Gutiérrez-Calvo, A.; Morales, R.; Ortega-Pérez, G.; et al. HIPECT4: Multicentre, randomized clinical trial to evaluate safety and efficacy of Hyperthermic intra-peritoneal chemotherapy (HIPEC) with Mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma. BMC Cancer 2018, 18, 183. [Google Scholar] [CrossRef]
- Shaib, W.L.; Assi, R.; Shamseddine, A.; Alese, O.B.; Staley, C., 3rd; Memis, B.; Adsay, V.; Bekaii-Saab, T.; El-Rayes, B.F. Appendiceal Mucinous Neoplasms: Diagnosis and Management. Oncologist 2017, 22, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.H., IV; Shen, P.; Russell, G.B.; Bradley, R.F.; Hundley, J.C.; Loggie, B.L.; Geisinger, K.R.; Levine, E.A. Appendiceal neoplasms with peritoneal dissemination: Outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann. Surg. Oncol. 2006, 13, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Shaib, W.L.; Martin, L.K.; Choi, M.; Chen, Z.; Krishna, K.; Kim, S.; Brutcher, E.; Staley, C., 3rd; Maithel, S.K.; Philip, P.; et al. Hyperthermic Intraperitoneal Chemotherapy Following Cytoreductive Surgery Improves Outcome in Patients with Primary Appendiceal Mucinous Adenocarcinoma: A Pooled Analysis from Three Tertiary Care Centers. Oncologist 2015, 20, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T.; Yamanaka, K. Repeat cytoreduction and hyperthermic intraperitoneal chemotherapy for recurrent peritoneal carcinomatosis of appendiceal origin. Int. J. Clin. Oncol. 2018, 23, 298–304. [Google Scholar] [CrossRef]
- Mercier, F.; Passot, G.; Bonnot, P.E.; Cashin, P.; Ceelen, W.; Decullier, E.; Villeneuve, L.; Walter, T.; Levine, E.A.; Glehen, O.; et al. An International Registry of Peritoneal Carcinomatosis from Appendiceal Goblet Cell Carcinoma Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. World J. Surg. 2022, 46, 1336–1343. [Google Scholar] [CrossRef]
- Moaven, O.; Votanopoulos, K.I.; Shen, P.; Mansfield, P.; Bartlett, D.L.; Russell, G.; McQuellon, R.; Stewart, J.H.; Levine, E.A. Health-Related Quality of Life After Cytoreductive Surgery/HIPEC for Mucinous Appendiceal Cancer: Results of a Multicenter Randomized Trial Comparing Oxaliplatin and Mitomycin. Ann. Surg. Oncol. 2020, 27, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Demtröder, C.; Solass, W.; Zieren, J.; Strumberg, D.; Giger-Pabst, U.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Color. Dis. 2016, 18, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Ellebæk, S.B.; Graversen, M.; Detlefsen, S.; Lundell, L.; Fristrup, C.W.; Pfeiffer, P.; Mortensen, M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC)-directed treatment of peritoneal metastasis in end-stage colo-rectal cancer patients. Pleura Perit. 2020, 5, 20200109. [Google Scholar] [CrossRef] [PubMed]
- Rovers, K.P.; Wassenaar, E.C.E.; Lurvink, R.J.; Creemers, G.M.; Burger, J.W.A.; Los, M.; Huysentruyt, C.J.R.; van Lijnschoten, G.; Nederend, J.; Lahaye, M.J.; et al. Pressurized Intraperitoneal Aerosol Chemotherapy (Oxaliplatin) for Unresectable Colorectal Peritoneal Metastases: A Multicenter, Single-Arm, Phase II Trial (CRC-PIPAC). Ann. Surg. Oncol. 2021, 28, 5311–5326. [Google Scholar] [CrossRef]
- Somashekhar, S.P.; Abba, J.; Sgarbura, O.; Alyami, M.; Teixeira Farinha, H.; Rao, R.G.; Willaert, W.; Hübner, M.; PIPAC Study Group. Assessment of Treatment Response after Pressurized Intra-Peritoneal Aerosol Chemotherapy (PIPAC) for Appendiceal Peritoneal Metastases. Cancers 2022, 14, 4998. [Google Scholar] [CrossRef] [PubMed]
- Raoof, M.; Whelan, R.L.; Sullivan, K.M.; Ruel, C.; Frankel, P.H.; Cole, S.E.; Tinsley, R.; Eng, M.; Fakih, M.; Chao, J.; et al. Safety and Efficacy of Oxaliplatin Pressurized Intraperitoneal Aerosolized Chemotherapy (PIPAC) in Colorectal and Appendiceal Cancer with Peritoneal Metastases: Results of a Multicenter Phase I Trial in the USA. Ann. Surg. Oncol. 2023, 30, 7814–7824. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; D’Angelica, M.I.; Abrams, T.; Abbott, D.E.; Ahmed, A.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Binder, D.; et al. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023. J. Natl. Compr. Cancer Netw. 2023, 21, 694–704. [Google Scholar] [CrossRef]
- Thomassen, I.; Lemmens, V.E.; Nienhuijs, S.W.; Luyer, M.D.; Klaver, Y.L.; de Hingh, I.H. Incidence, prognosis, and possible treatment strategies of peritoneal carcinomatosis of pancreatic origin: A population-based study. Pancreas 2013, 42, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Takano, K.; Shimazu, M.; Okihara, M.; Sano, T.; Chiba, N.; Kawachi, S. Long-term survival of a recurrent gallbladder carcinoma patient with lymph node and peritoneal metastases after multidisciplinary treatments: A case report. Surg. Case Rep. 2016, 2, 12. [Google Scholar] [CrossRef]
- Amblard, I.; Mercier, F.; Bartlett, D.L.; Ahrendt, S.A.; Lee, K.W.; Zeh, H.J.; Levine, E.A.; Baratti, D.; Deraco, M.; Piso, P.; et al. Cytoreductive surgery and HIPEC improve survival compared to palliative chemotherapy for biliary carcinoma with peritoneal metastasis: A multi-institutional cohort from PSOGI and BIG RENAPE groups. Eur. J. Surg. Oncol. 2018, 44, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Tentes, A.K. Hyperthermic intra-operative intraperitoneal chemotherapy as an adjuvant to pancreatic cancer resection. J. Gastrointest. Oncol. 2021, 12 (Suppl. S1), S91–S98. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Stuart, O.A. Intraperitoneal gemcitabine chemotherapy is safe for patients with resected pancreatic cancer: Final clinical and pharmacologic data from a phase II protocol and recommended future directions. J. Gastrointest. Oncol. 2021, 12 (Suppl. S1), S99–S109. [Google Scholar] [CrossRef] [PubMed]
- Grotz, T.E.; Yonkus, J.A.; Thiels, C.A.; Warner, S.G.; McWilliams, R.R.; Mahipal, A.; Bekaii-Saab, T.S.; Cleary, S.P.; Kendrick, M.L.; Truty, M.J. Cytoreduction with Hyperthermic Intraperitoneal Chemoperfusion for Pancreatic Cancer with Low-Volume Peritoneal Metastasis: Results from a Prospective Pilot Study. Ann. Surg. Oncol. 2023, 30, 395–403. [Google Scholar] [CrossRef]
- Padilla-Valverde, D.; García-Santos, E.; Sanchez, S.; Manzanares, C.; Rodriguez, M.; González, L.; Ambrós, A.; Cano, J.M.; Serrano, L.; Bodoque, R.; et al. Safety of perioperative hyperthermic intraperitoneal chemotherapy with gemcitabine in patients with resected pancreatic adenocarcinoma: A pilot study of the clinical trial EudraCT 2016-004298-41. J. Gastrointest. Oncol. 2021, 12 (Suppl. S1), S80–S90. [Google Scholar] [CrossRef] [PubMed]
- Yurttas, C.; Horvath, P.; Fischer, I.; Meisner, C.; Nadalin, S.; Königsrainer, I.; Königsrainer, A.; Beckert, S.; Löffler, M.W. A Prospective, Phase I/II, Open-Label Pilot Trial to Assess the Safety of Hyperthermic Intraperitoneal Chemotherapy after Oncological Resection of Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2021, 28, 9086–9095. [Google Scholar] [CrossRef] [PubMed]
- Graversen, M.; Detlefsen, S.; Bjerregaard, J.K.; Pfeiffer, P.; Mortensen, M.B. Peritoneal metastasis from pancreatic cancer treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Clin. Exp. Metastasis 2017, 34, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein, T.A.; Götze, T.O.; Ouaissi, M.; Tempfer, C.B.; Giger-Pabst, U.; Demtröder, C. First Clinical Data of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) as Salvage Therapy for Peritoneal Metastatic Biliary Tract Cancer. Anticancer Res. 2018, 38, 373–378. [Google Scholar] [PubMed]
- Di Giorgio, A.; Sgarbura, O.; Rotolo, S.; Schena, C.A.; Bagalà, C.; Inzani, F.; Russo, A.; Chiantera, V.; Pacelli, F. Pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin or oxaliplatin for peritoneal metastasis from pancreatic adenocarcinoma and cholangiocarcinoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920940887. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Graversen, M.; Ellebæk, S.B.; Kristensen, T.K.; Fristrup, C.; Pfeiffer, P.; Mortensen, M.B.; Detlefsen, S. Next-generation sequencing and histological response assessment in peritoneal metastasis from pancreatic cancer treated with PIPAC. J. Clin. Pathol. 2021, 74, 19–24. [Google Scholar] [CrossRef]
- Teixeira Farinha, H.; Grass, F.; Kefleyesus, A.; Achtari, C.; Romain, B.; Montemurro, M.; Demartines, N.; Hübner, M. Impact of Pressurized Intraperitoneal Aerosol Chemotherapy on Quality of Life and Symptoms in Patients with Peritoneal Carcinomatosis: A Retrospective Cohort Study. Gastroenterol. Res. Pract. 2017, 2017, 4596176. [Google Scholar] [CrossRef]
- Dumont, F.; Passot, C.; Raoul, J.L.; Kepenekian, V.; Lelièvre, B.; Boisdron-Celle, M.; Hiret, S.; Senellart, H.; Pein, F.; Blanc-Lapierre, A.; et al. A phase I dose-escalation study of oxaliplatin delivered via a laparoscopic approach using pressurised intraperitoneal aerosol chemotherapy for advanced peritoneal metastases of gastrointestinal tract cancers. Eur. J. Cancer 2020, 140, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lurvink, R.J.; Rovers, K.P.; Wassenaar, E.C.E.; Bakkers, C.; Burger, J.W.A.; Creemers, G.M.; Los, M.; Mols, F.; Wiezer, M.J.; Nienhuijs, S.W.; et al. Patient-reported outcomes during repetitive oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy for isolated unresectable colorectal peritoneal metastases in a multicenter, single-arm, phase 2 trial (CRC-PIPAC). Surg. Endosc. 2022, 36, 4486–4498. [Google Scholar] [CrossRef]
- Odendahl, K.; Solass, W.; Demtröder, C.; Giger-Pabst, U.; Zieren, J.; Tempfer, C.; Reymond, M.A. Quality of life of patients with end-stage peritoneal metastasis treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC). Eur. J. Surg. Oncol. 2015, 41, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, S.; Di Giorgio, A.; Cintoni, M.; Rinninella, E.; Palombaro, M.; Pulcini, G.; Schena, C.A.; Chiantera, V.; Vizzielli, G.; Gasbarrini, A.; et al. Body composition and immunonutritional status in patients treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) for gastrointestinal peritoneal metastases: A prospective single-center analysis. Pleura Perit. 2022, 7, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Tidadini, F.; Ezanno, A.C.; Trilling, B.; Aime, A.; Abba, J.; Quesada, J.L.; Foote, A.; Chevallier, T.; Glehen, O.; Faucheron, J.L.; et al. Hospitalization cost of Pressurized Intraperitoneal Aerosol chemotherapy (PIPAC). Eur. J. Surg. Oncol. 2023, 49, 165–172. [Google Scholar] [CrossRef]
- Javanbakht, M.; Mashayekhi, A.; Branagan-Harris, M.; Horvath, P.; Königsrainer, A.; Reymond, M.A.; Yaghoubi, M. Cost-effectiveness analysis of pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with gastric cancer and peritoneal metastasis. Eur. J. Surg. Oncol. 2022, 48, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Willaert, W.; Van de Sande, L.; Van Daele, E.; Van De Putte, D.; Van Nieuwenhove, Y.; Pattyn, P.; Ceelen, W. Safety and preliminary efficacy of electrostatic precipitation during pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable carcinomatosis. Eur. J. Surg. Oncol. 2019, 45, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
| Trial Number | Country | Disease Site | PIPAC Drug | Systemic Treatment |
|---|---|---|---|---|
| NCT05644249 | Lithuania | Gastric | Cisplatin/Doxorubicin | FOLFOX |
| NCT05303714 | Italy | Gastric | Cisplatin/Doxorubicin | FOLFOX |
| NCT05318794 | England | Gastric | Cisplatin/Doxorubicin | Not specified |
| NCT04595929 | Russia | Gastric | Cisplatin/Doxorubicin | FLOT |
| NCT03304210 | Belgium | Gastric/Pancreas | Paclitaxel | Not specified |
| NCT04475159 | Israel | Colorectal | Not specified | Not specified |
| NCT03280511 | Denmark | Colorectal | Oxaliplatin | Not specified |
| NCT03868228 | England | Colorectal | Oxaliplatin | Not specified |
| NCT04734691 | Switzerland | Colorectal | Oxaliplatin | Not specified |
| NCT04956068 | Singapore | Any | Cisplatin/Doxorubicin or Oxaliplatin | Not specified |
| NCT05395910 | Singapore | Any | Paclitaxel | Not specified |
| NCT04000906 | Switzerland | Any | Cisplatin/Paclitaxel | Not specified |
| NCT03172416 | Singapore | Any | Oxaliplatin | Nivolumab |
| NCT05277766 | Belgium | Any | Irinotecan | Not specified |
| NCT05431907 | Israel | Any | Allocetra-OTS | Not specified |
| NCT04329494 | USA | Any | Not specified | Not specified |
| Year | First Author [Reference] | # PIPAC Patients (1/2/3/4+ Treatments (Total)) | Median # Treatments | Median Initial PCI | % Previous Surgery | % Signet Ring Histology | % Extraperitoneal Disease | Concurrent Systemic Treatment Regimen | % Initial Access Failure | % PRGS Grade 1/2 w/2+ Treatments | OS (Months) | % Subsequent CRS | LOS (Days) | % Major Morbidity (CTCAE or CD 3–4) | % Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | Khomyakov [63] | 16/7/6/2 (31) | 1.5 | 16 | 97% | 0% | XELOX | 60% | 13 | 3 | 3.2% | 0% | |||
| 2016 | Nadiradze [64] | 7/7/3/7 (24) | 2 | 16 | 63% | 75% | 17% | Not specified | 4% | 50% | 15.4 | 37.5% | 8.3% | ||
| 2018 | Gockel [65] | 9/6/5/4 (24) | 2 | 14 | 54% | 21% | Not specified | 18% | 7 | 0% | 0% | ||||
| 2019 | Struller [66] | 7/12/6/0 (25) | 2 | 15.3 | 60% | 88% | None | 83% | 6.7 | 12% | |||||
| 2020 | Di Giorgio [50] | 8/13/7/0 (28) | 2 | 20 | 57% | 75% | 21% | Not specified | 4% | 12.3 | 3.5% | 2 | 4% | 4% | |
| 2020 | Ellebæk [67] | 6/4/10 (20) | 2.5 | 10.5 | 25% | 45% | Not specified | 0% | 40% | 4.7 | 10% | 0% | |||
| 2021 | Alyami [68] | 8/4/17/13 (42) | 3 | 17 | 79% | Not Specified | 19.1 | 14% | 3 | 3% | 4.7% | ||||
| 2022 | Sindayigaya [69] | 52/32/24/21 (131) | 2 | 15 | Not specified | 8% | 73% | 10 | 7% | 5 | 5% | 1.4% | |||
| 2022 | Tidadini [70] | (17) | 2 | 18 | 12% | 0% | Not specified | 12.8 | 11.8% | 11.8% | 0.0% | ||||
| 2023 | Graversen [52] | (21) | 0 | Not specified | 0% | 6 | 9.5% | 0.0% |
| Year | First Author [Reference] | # PIPAC Patients (1/2/3/4+ Treatments (Total)) | Median # Treatments | Median Initial PCI | % Previous Surgery | % Signet Ring Histology | Concurrent Systemic Treatment Regimen | % Initial Access Failure | % PRGS Grade 1/2 w/2+ Treatments | OS after Initial PIPAC (Months) | % Subsequent CRS | % Major Morbidity (CTCAE or CD 3–4) | % Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | Demtroder [83] | 3/5/3/6 (17) | 2 | 16 | 100% | Not specified | 0% | 79% | 15.7 | 11.7% | 23% | 0% | |
| 2020 | Ellebaek [84] | 5/4/15/0 (24) | 3 | 11 | 4.2% | Not specified | 0% | 67% | 20.5 | 0% | 8.3% | 0% | |
| 2021 | Tabchouri [25] | 26/20/17/17 (102) | 2 | 14 | 96% | Not specified | 21.6% | 74% | 14 | 5.9% | 1% | ||
| 2021 | Rovers [85] | 4/3/7/6 (20) | 3 | 29 | 55% | 45% | 5-FU + Leucovorin | 5% | 56% | 8 | 15% | 0% | |
| 2022 | Somashekhar [86] | 25/14/19/19 (77) | 2 | 23 | 35% | Not specified | 5.1% | 49% | |||||
| 2023 | Raoof [87] | 4/2/6/0 (12) | 2.5 | 28 | 42% | 25% | 5-FU + Leucovorin | 7.7% | 42% | 12 | 17% | 16.7% | 0% |
| Year | First Author [Reference] | # PIPAC Patients (1/2/3/4+ Treatments (Total)) | Median # Treatments | Median Initial PCI | % Previous Surgery | Drugs/Dosage | Concurrent Systemic Treatment | % Initial Access Failure | % PRGS Grade 1/2 w/2+ Treatments | OS (Months) | % Major Morbidity (CTCAE or CD 3–4) | % Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | Graversen [97] | 0/2/3/2 (5) | 3 | 40% | cisplatin 7.5 mg/m2 and doxorubicin 1.5 mg/m2 | Not specified | 0% | 80% | 6 | 0% | ||
| 2018 | Falkenstein [98] | 7/5/1/0 (13) | 2 | 20 | 69% | cisplatin 7.5 mg/m2 and doxorubicin 1.5 mg/m2 or oxaliplatin 92 mg/m2 | None | 15.4% | 80% | 2.8 | 0% | 0% |
| 2020 | Di Giorgio [99] | 9/4/3/4 (20) | 2 | 18 | 60% | cisplatin 7.5 mg/m2 and doxorubicin 1.5 mg/m2 or oxaliplatin 92 mg/m2 | Not specified | 0% | 50% | 10 | 0% | 0% |
| 2021 | Nielsen [100] | 3/7/1/5 (16) | 3 | cisplatin 7.5 mg/m2 and doxorubicin 1.5 mg/m2 or oxaliplatin 92 mg/m2 | Not specified | 0% | 61% | 9.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel, S.K.; Sun, B.J.; Lee, B. PIPAC for Gastrointestinal Malignancies. J. Clin. Med. 2023, 12, 6799. https://doi.org/10.3390/jcm12216799
Daniel SK, Sun BJ, Lee B. PIPAC for Gastrointestinal Malignancies. Journal of Clinical Medicine. 2023; 12(21):6799. https://doi.org/10.3390/jcm12216799
Chicago/Turabian StyleDaniel, Sara K., Beatrice J. Sun, and Byrne Lee. 2023. "PIPAC for Gastrointestinal Malignancies" Journal of Clinical Medicine 12, no. 21: 6799. https://doi.org/10.3390/jcm12216799




